Abstract
Background
Shoulder pain (SP) was first reported after laparoscopic gynecological procedures. It is assumed to be multifactorial in nature. Several methods to reduce SP after laparoscopic cholecystectomy (LC) have been postulated. In this study, we have worked to decrease it using 2 approaches; lung recruitment maneuver and intraperitoneal local analgesic instillation.
Objectives
This study was designed to assess the clinical efficacy of ketamine as an adjunct to intraperitoneal bupivacaine for the relief of post-operative shoulder pain after LC.
Methods and material
This prospective, randomized, double-blinded study is comprised of 40 patients of either sex, with age range of 20–50 years, planned for elective LC. Just after inflating the pneumoperitoneum, the surgeon sprayed 50 mL of a blinded solution intraperitoneally. Patients were randomly allocated to: group B received a 50 mL solution of intraperitoneal bupivacaine 0.25% and group BK received 0.5 mg/kg ketamine mixed with bupivacaine 0.25%.
Results
This study showed that ketamine bupivacaine admixture had made dramatic decline in shoulder pain VAS scores specifically at the 24th hour; 15 patients in the BK group had either VAS score zero or 1 when compared to B group whom their lowest score at the 24th hour was 4. Also, there was more decrease in postoperative analgesic consumption in BK group. No psychomimetic side effects or sedation were noticed in both groups.
Conclusions
We conclude that intraperitoneal instillation of low dose ketamine to bupivacaine 0.25% in elective LC significantly reduced post-operative shoulder pain and analgesic requirement when compared to bupivacaine 0.25% alone.
1 Introduction
In view of less postoperative pain and earlier ambulation with return of normal activities, laparoscopic cholecystectomy (LC) had become the gold standard in gallbladder surgery. But it is not without undesirable adverse effects, of which shoulder tip pain is a troublesome symptom that might surpasses the pain at the incision site [Citation1]. Shoulder pain incidence ranges from 35 to 80% and it might pass unnoticed by the physicians and therefore not treated properly due to early discharge [Citation2].
After LC and during the 1st postoperative day, visceral pain predominates then soon subsides gradually on the first day. On the other hand, shoulder pain on the first day increases gradually and becomes significant on the next day. It most often affects the right shoulder but the left shoulder can also be affected as well [Citation3].
Different multimodal approaches have been attempted to decrease postoperative pain. These include parenteral analgesics (including opioids and non-steroidal anti-inflammatory drugs), local infiltration with local anesthetics as well as intraperitoneal routes that in turn has been explored using local anesthetics as bupivacaine. Also, various adjuvants to local anesthetics have been utilized to prolong the duration of analgesia including ketamine. Ketamine is a nonspecific N-methyl–D-aspartate (NMDA) receptor antagonist that can be administrated through different routes [Citation4]. It simply crosses through most tissue membranes that lead to easy absorption. It also has rapid onset of action and because of peripheral action at both opioid and NMDA receptor, its peripheral application has been evaluated in many studies [Citation5,Citation6]. NMDA receptor activation has a role in postoperative pain through peripheral and central effects [Citation7]. At small doses (0.1–0.5 mg/kg), ketamine has a noticeable analgesic action, which can be used as an adjuvant to local anesthetics without side effects. [Citation8].
On the other hand, because carbon dioxide retention is a key factor in laparoscopy-induced shoulder pain, removing residual carbon dioxide might help reduce the occurrence or severity of this pain in both the shoulder and upper abdomen. This can be done through applying lung recruitment maneuver that leads to increased intraperitoneal pressure so facilitating residual carbon dioxide removal. It is done at the end of the operation through opened trocars [Citation9].
The proposed study is designed to assess the clinical efficacy of sub-anesthetic ketamine as an adjunct to intraperitoneal bupivacaine for the relief of post-operative shoulder pain after laparoscopic cholecystectomy.
2 Subjects and methods
2.1 Study design and setting
This is a prospective, randomized, double blind study that was conducted in Ain Sham University (El Demerdash) hospitals through the period of March 2018 to June 2018 in Assembled operating theatre
2.2 Study population
The present study comprised 40 patients of either sex, American Society of Anesthesiologists (ASA) I-II, with age >20 and <50 years, body weight from 70 to 80 kg, planned for elective laparoscopic cholecystectomy. An informed written consent from the patients and approval from local ethics committee was obtained which is in agreement with the ethical guidelines of the Declaration of Helsinki, 1975.
Patients with chronic pain diseases other than gallstone disease, opioids users and patients known to have amide local anesthetic allergy are all excluded. In addition, patients with acute cholecystitis, conversion to an open cholecystectomy, postoperative complications that increased postoperative pain, and those with psychological or nervous system diseases were also excluded.
2.3 Preoperative preparation
Preanesthetic check‑up was done the day preceding surgery and included a detailed history and complete general physical and systemic examination. Basic hemodynamic data were recorded; heart rate (HR) and mean arterial blood pressure (MBP). Routine investigations were revised.
In the preanesthetic room: an intravenous access was secured and all patients were premedicated just before surgery with midazolam 0.05 mg/kg, decadron 8 mg and ranitidine 50 mg. All were given intravenously, and the last 2 medications were diluted in 10 mL normal saline and were given very slowly. On entering the operating theatre, full monitors were applied.
2.4 Patients’ randomization, intraoperative interventions and management
Patients were randomly allocated to 1 of 2 groups (each of which consisted of 20 patients) with the help of computer-generated random number tables in opaque sealed envelopes prepared by an anesthesiologist not part of the study. The envelopes were opened by the staff nurse, and peritoneal solution was prepared according to group allocation by a surgical scrub nurse who is not involved in the study.
| – | Group 1 (Bupivacaine group = B group) received a 50 mL solution of bupivacaine 0.25% intraperitoneal instilled solution. | ||||
| – | Group 2 (Bupivacaine Ketamine Group = BK group) received 0.5 mg/kg ketamine mixed with bupivacaine 0.25% with a total volume of 50 mL intraperitoneal instilled solution. Bupivacaine dose was limited to a maximum dose of 2 mg/kg in all patients. | ||||
At the start of surgery and just after inflating the pneumoperitoneum and before any dissection, the surgeon sprayed 50 mL of a blinded solution (bupivacaine or bupivacaine ketamine) intraperitoneally in a standardized manner to the subdiaphragmatic space and gallbladder area guided by the camera and the patients were kept in Trendelenburg position for 5–10 min. Thereafter, all patients were positioned in the anti-Trendelenburg position (with their right shoulder raised) to start the surgery and the laparoscopic procedure was carried out in a standard fashion
Pre-oxygenation with 100% oxygen was done. General anesthesia was induced intravenously with propofol 2 mg/kg, fentanyl 1.5 µg/kg, and atracurium 0.5 mg/kg to be followed by orotracheal intubation after 3 min of manual ventilation.
Maintenance of anesthesia was done by isoflurane 1.2–2.5% in oxygen/air mixture. Increments of 10 mg of atracurium were administered repetitively every 20 min to achieve muscle relaxation and a bolus injection of fentanyl (0.5 µg/kg) every 30 min
Any increase or decrease of HR or blood pressure intraoperatively, was managed as required. For example, MAP rise of >20% above baseline was treated by administering a 0.5 μg/kg intravenous bolus of fentanyl. MAP drop of >20% below baseline was dealt with at first with reduction of the isoflurane concentration to 0.6% and 250 mL intravenous ringer solution given as bolus. If patient was still hypotensive, 6 mg ephedrine was given intravenously.
During laparoscopy, intraabdominal pressure was maintained between 10 mm Hg and 15 mm Hg. Minute ventilation volume was adjusted to keep endtidal PCO2 at 35 mm Hg to 40 mm Hg.
At the end of surgery, ondansetron 4 mg was given slowly intravenously. Also, CO2 was removed in both groups by lung recruitment maneuver. This maneuver requires placing the patients in the Trendelenburg position then doing five manual pulmonary inflations with a maximum pressure of 35 cm of H2O. The 5th positive pressure inflation was held for approximately 5 s. During these maneuvers, the trocar sleeve valve was fully open to allow the CO2 gas to escape. The patients were then placed back in the level position, the trocar was removed and the abdominal incisions were closed. Isoflurane was discontinued; FiO2 was increased to 100%. The residual neuromuscular blockade was reversed with a mixture of neostigmine 0.05 mg/kg and atropine 0.01 mg/kg. Extubation was done. The time of arrival in the postoperative unit is defined as 0 h postoperatively. All patients stayed in PACU after surgery for two hours before transferal to ward. A fixed dose of intravenous paracetamol 1 gm was given every 6 h to all patients in both groups starting from 0 h in PACU.
The following measures were assessed and recorded once reaching the recovery room, and at 1, 2, 6, 12, and 24 h postoperatively: Postoperative hemodynamics (heart rate, mean blood pressure, oxygen saturation), postoperative shoulder pain which was evaluated by visual analogue scale (VAS) score of 0 to 10 (0 = no pain, 10 = unendurable pain). In case of a pain score >4, a 25 mg pethidine was given intravenously. Also we assessed severity of postoperative abdominal pain which if was >4, a 25 mg pethidine was given intravenously, sedation using a four-point scale (0 = alert, 1 = quietly awake, 2 = asleep but easily aroused, and 3 = deep sleep) and finally postoperative nausea and vomiting (PONV) was rated on a 4-point scale (0 = no PONV, 1 = Mild nausea, 2 = Severe nausea, 3 = Vomiting). If PONV scale was 2 or more, ondansetron 4 mg was given intravenously (with a maximum total dose of 16 mg / day).
Also, we recorded: after how much hours patients requested their 1st analgesia after extubation, total dose of intravenous pethidine postoperatively (mg/24 h) and finally any complications such as cardiovascular, respiratory and neurological side‑effects in the postoperative period.
Primary outcome was the intensity of post-laparoscopic pain in shoulder at 24 h after surgery. Secondary outcomes were: abdominal pain intensity, postoperative amount of analgesics, nausea, and vomiting and sedation level.
2.5 Sample size determination and statistical analysis
Sample size was calculated using PASS 13, based on previous study (El Gaby et al 2017) [Citation10]. It was calculated that a sample size of 20 patients per group achieved 90% power that 4.3% at “Intraperitoneal saline group” has no pain at 12 h postoperative compared to all cases in “Intraperitoneal Ketamine group” assuming that 50% of cases will have no pain with a significance level (alpha) of 0.05000.
The statistical analysis was performed using a standard SPSS software package version 21 (Chicago, IL). independent Student’s t-test was used to compare normally distributed numerical data and are presented as mean ± SD, data not normally distributed were compared using Mann-Whitney test and are presented as median(IQR) and categorical variables were analyzed using the χ2 test or fisher exact test and are presented as number. P < 0.05 is considered statistically significant.
3 Results
40 patients (23 men and 17 women) were eligible and entered the study. The patients’ age was with age >20 and <50 years. None of the patients were excluded from the study. Both groups were comparable with respect to age, weight, sex, ASA physical status and mean duration of surgery (). As regards hemodynamic parameters, mean arterial pressure and heart rate were similar in the two groups. One can notice in trends of each group, mean arterial pressure and heart rate were significantly lower at 45th, 60th minute after pneumoperitoneum, and also after extubation by one hour till 24th hour when compared to baseline values (, ). On the other hand, O2 Saturation was non-significant in both groups ().
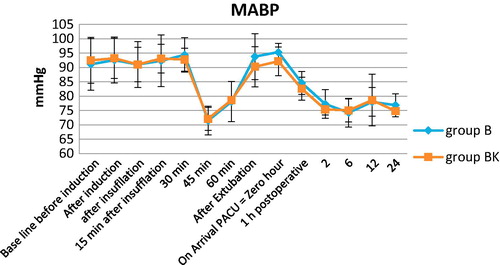
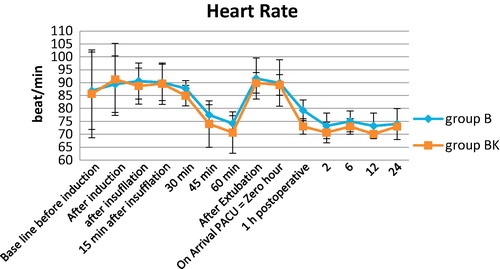
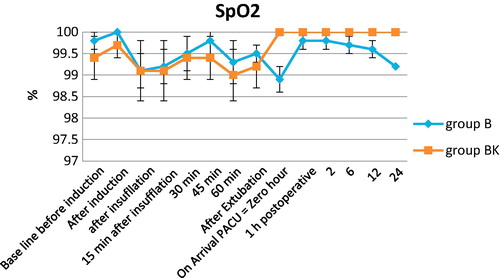
Table 1 Demographic and clinical data of all study participants (n = 40).
Regarding postoperative shoulder pain, Shoulder VAS scores are shown in . Group BK had significantly lower VAS scores than group B at 12 & 24th hours and the difference was statistically significant. Also, regarding postoperative abdominal VAS scores; One can notice that Group BK had significantly lower VAS scores (almost zero score) than group B at 12 & 24th hours and the difference was statistically significant as shown in .
Table 2 Shoulder pain VAS scores during 1st postoperative day.
Table 3 Abdominal pain VAS scores during 1st postoperative day.
Specifically at the 24th hour, VAS score was further analyzed and number of patients having each score of the VAS was counted and statistically analyzed as shown in . Obviously, “all” patients in BK group had VAS score <4 with 15 patients out of 20 had VAS score zero or 1. On the other hand, lowest VAS score in B group was VAS 4.
Table 4 Number of patients having each VAS score at the 24th hour in both groups.
Sedation scores were statistically non-significantly when compared between both groups during the first 24 postoperative hours (). Also, Overall analysis showed that postoperative complications such as nausea and vomiting were not statistically significant between the two study groups () except at the 6th hour but that was not clinically significant. No other side effects were reported.
Table 5 Sedation scores during 1st postoperative day.
Table 6 PONV scores during 1st postoperative day.
Time to first request of analgesia was longer in BK group (20.26 ± 0.8 h) as compared to B group (7.73 ± 1.9 h). Also, total pethidine consumption was also lower in BK group when compared to B group (11.58 ± 6 mg versus 53.75 ± 16.8 mg) and that difference was highly statistically significant as shown in .
Table 7 Postoperative analgesia data.
4 Discussion
Shoulder tip pain was almost unheard of in open cholecystectomy era and was first reported after laparoscopic gynecological procedures [Citation11]. Nowadays, SP is assumed to be multifactorial in nature. A proposed cause is direct damage and/or irritation of the diaphragmatic peritoneal nerves and so pain might occur due to carbonic acid produced from CO2 within the peritoneal cavity [Citation12]. Another etiology is peritoneal surface stretching leading to traction and tearing of microvascular structures with subsequent hemorrhage that may be microscopic or macroscopic. It causes pain due to the release of inflammatory mediators [Citation13]. Another possible theory is the loss of the ’suction’ effect between the liver and diaphragm allowing traction on the triangular and coronary ligaments of the liver that leads to sub diaphragmatic pain and SP [Citation14].
Several methods to reduce SP after laparoscopic cholecystectomy (LC) have been postulated. In this study, we have worked to decrease shoulder pain using 2 different approaches; the 1st one is lung recruitment maneuver aiming to remove as much as CO2 from the abdomen (so maintaining suction effect between liver and diaphragm plus decreasing amount of carbonic acid formed) in addition to bupivacaine instillation intraperitonealy with or without ketamine to decrease visceral pain and inflammatory reactions in the peritoneum that might result from the remaining carbonic acid and/or peritoneal hemorrhage. We chose bupivacaine for our study because of its potency and prolonged duration of action. The half-life of bupivacaine is between 5 and 16 h. And since shoulder pain usually peaks several hours postoperatively, we used ketamine as an adjunct to bupivacaine to prolong its analgesic effect.
This study showed that ketamine bupivacaine admixture when given intraperitonealy showed dramatic decline in shoulder pain VAS scores specifically at the 24th hour;15 patients in the BK group had either VAS score zero or 1 when compared to B group whom their lowest score at the 24th hour was 4. In addition to this, there was decreased postoperative analgesic consumption during first 24 h postoperatively in both groups but more in BK group (mean of 12 mg pethidine in BK group versus 54 mg in B group). No psychomimetic side effects or sedation was noticed in both groups with stable intra and postoperative hemodynamics.
The rationale for choosing the intraperitoneal route is to block the visceral afferent signaling and potentially modifying visceral nociception and provides analgesia [Citation15]. Analgesic efficacy of intraperitoneal bupivacaine was under research last years. Whether given before gall bladder removal [Citation16–Citation18] or after gall bladder removal [Citation19–Citation21], it proved an analgesic efficacy specifically for shoulder pain and this goes with our results. Different doses of intraperitoneal bupivacaine were tried in different studies to achieve an ideal safe dose for intraperitoneal instillation. For example, a dose of 125 mg bupivacaine was administered by Anand and his colleagues [Citation19] after surgical removal of the gall bladder. This dose was safe and provided analgesia up to 9 ± 2 h with reduced incidence and severity of shoulder pain and with a decrease in postoperative intramuscular tramadol in 24 h as an analgesic rescue. And this goes with our results that used the same dose of bupivacaine and showed even longer duration of postoperative shoulder analgesia reaching up to 12 h with VAS score < or equal to 4 in Group B. This longer duration might be contributed to lung recruitment maneuver used in all study groups. A dose of 100 mg bupivacaine was also used in different studies providing efficacy in reducing shoulder pain from 6 up to 24 h [Citation16,Citation17,Citation21,Citation22] associated with dramatic decrease in postoperative non-steroidal anti-inflammatory drugs use as rescue analgesic. Notably, those studies that recorded “No shoulder pain for up to 24 h”, has an 1:200,000 epinephrine added to bupivacaine as an adjunct [Citation16,Citation17]. Other studies used lower dose of bupivacaine 75 mg and 50 mg [Citation20,Citation23,Citation24]. They showed that bupivacaine decreased shoulder pain but only for 4 to 8 h postoperatively. This is of course related to the lesser dose used. There was also marked decrease in postoperative analgesic consumption in all of the previous studies.
Other studies confirmed our results regarding efficacy of bupivacaine in laparoscopic surgery for abdominal visceral pain analgesia for 8 h postoperatively [Citation25,Citation26]. A meta-analysis by Boddy et al. [Citation18] established the efficacy of intraperitoneal local anesthetics in reducing early post laparoscopic cholecystectomy pain score at 4 h. Subgroup analysis suggested that the effect was greater when the local anesthetic was given at the start of the operation compared with instillation at the end.
Surprisingly, Rademaker et al. [Citation27] failed to demonstrate any reduction in postoperative pain, which could be due to the fact that instillation of local anesthetic was done in the supine position preventing its flow over the phrenic nerve endings. Also; Schenin and his colleagues [Citation28], Raetzell and his colleagues [Citation29] and Joris and his colleagues [Citation30] showed no significant difference in pain scores between the interventional groups. This can be attributed to lower bupivacaine concentrations because it is the concentration that is important in intraperitoneal instillation rather than the volume.
Few studies were done on ketamine bupivacaine intraperitoneal admixture. For example; Abdelraouf and his colleagues [Citation31] in 2004 had shown that Intraperitoneal co-administration of ketamine (1 mg/kg) with 0.25% bupivacaine has been found to provide better pain relief than intraperitoneal bupivacaine (0.25%) alone. In their ketamine/bupivacaine group, patients reported shoulder pain after 18 h. Also, the severity of shoulder pain ranged from 2 to 4. Our study is obviously different; Shoulder & abdominal visceral pain were almost absent during whole first postoperative day with rescue analgesics of mean (12 ± 6) mg pethidine only. In Abdelraouf study [Citation31], 1st dose of rescue analgesics was after a mean of 2 h. Also, they injected their medications at the end of surgery after gall bladder removal while we did a preemptive approach by injecting our medications before any surgical manipulations. Finally, we added lung recruitment maneuver in all study groups which has an important role in attenuating or even vanishing postoperative shoulder pain.
Intraperitoneal ketamine alone was used many times in different studies all with a dose of 0.5 mg/kg [Citation10,Citation32,Citation33]. In those 3 studies, ketamine was injected at the end of surgery; also the effective postoperative analgesia was so obvious with very low VAS scores till 24 h postoperatively. Also, postoperative analgesic consumption was reduced dramatically. In a unique study done by Goma et al. [Citation6]; she concluded that intraperitoneal ketamine in a dose of 0.5 mg/kg may reduce the postoperative analgesic needs up to 24 h postoperatively in morbidly obese patients following bariatric surgery. In spite of all the encouraging previous results, Klimscha et al. [Citation34] also demonstrated that intraperitoneal ketamine did not have any antinociceptive effects after intraperitoneal administration in rats. Also, in a study done by Ahmed and his colleagues [Citation35], he demonstrated the lack of any analgesia enhancing effect of ketamine added to bupivacaine 0.5% in thoracic paravertebral block (TPVB). Finally, Lee et al. [Citation36] did not find any sensory or motor prolonging effect of ketamine when added to ropivacaine 0.5% in interscalene block. There are 2 explanations for the negative results of ketamine's analgesic effect in those 3 antagonising studies. 1st of all, they used low ketamine concentration. Secondly, it is assumed that ketamine exerts its local antinociception effect only when there is an inflammatory process at the site of injection.
Although, in the present study, the blood levels of administered drugs were not estimated, no major systemic side-effects were recorded especially that our dosage was limited to 2 mg/kg. Higher doses of bupivacaine have been used safely. Doses up to 150 mg of bupivacaine are presumed to be fairly safe [Citation37]. Regarding ketamine side effects, it is assumed that in sub-anesthetic doses, side effects are infrequent. In Shawky's study [Citation32], perioperative administration of 0.5 mg/kg ketamine, given intraperitoneally, provides effective pain relief without side effects in patients undergoing laparoscopic surgery and this goes with our study that showed absence of any side effects in BK group. On the other hand, a study by Lee and his colleagues [Citation36] have reported 44% incidence of psychomimetic side effects in patients who had received ketamine/ropivacaine in interscalene block. As regard postoperative sedation score; in this study all patients in both groups were fully conscious in PACU with no sedation at all. This could be attributed to the early administration of ketamine at the beginning of the operation. This differs from Shawky's results [Citation32] who administered ketamine at the end of operation. Their patients in ketamine group were sedated for 30 min after extubation. Finally, regarding patients' hemodynamics, in a study done by Shawky and his colleagues [Citation32], stable hemodynamics was noted in ketamine group throughout all postoperative 24 h. Of course, this is related to effective ketamine analgesia. And this goes with our study.
There are limitations to our study. First of all, a single dose of ketamine was studied, and further studies are needed to determine the most appropriate dose of ketamine intraperitonealy. Secondly, our study has a relatively smaller sample size. Thirdly, we didn't assess the blood levels of administered drugs. Lastly, we didn’t assess the recovery profile and extubation time that could be affected by the study medications.
5 Conclusion
We conclude that intraperitoneal instillation of low dose ketamine (0.5 mg/kg) as an adjunct to bupivacaine 0.25% in elective laparoscopic cholecystectomy significantly reduced post-operative shoulder pain and analgesic requirement in post-operative period as compared to bupivacaine 0.25% alone.
Support
There was no financial support.
Conflicts of interest
There were no conflicts of interest.
Notes
Peer review under responsibility of Egyptian Society of Anesthesiologists.
This study was done in the surgical department of Ain Shams university educational hospital, Cairo, Egypt.
References
- M.G.CunniffeO.J.McAnenaM.A.DarA prospective randomized trial of intraoperative bupivacaine irrigation for management of shoulder-tip pain following laparoscopyAm J Surg1761998258261
- J.B.DixonY.ReugenC.HalketP.E.O’BrienShoulder pain is a common problem following laparoscopic adjustable gastric band surgeryObes Surg15200511111117
- T.BisgaardH.KehletJ.RosenbergPain and convalescence after laparoscopic cholecystectomyEur J Surg16720018496
- K.LaskowskiA.StirlingW.P.McKayH.J.LimA systematic review of intravenous ketamine for postoperative analgesiaCan J Anaesth58102011911923
- R.S.MoharariA.NajafiM.R.KhajaviG.S.MoharariM.R.NikoobakhtIntraurethral instillation of ketamine for male rigid cystoscopyJ Endourol2412201020332036
- H.M.GomaB.A.ElhamidIntra peritoneal S ketamine reduces postoperative analgesic requirements in morbidly obese patient a controlled studyEnliven: J Anesthesiol Crit Care Med252015014
- J.A.McRobertsS.V.CoutinhoJ.C.MarvizónE.F.GradyM.TognettoJ.N.SenguptaRole of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in ratsGastroenterology1207200117371748
- R.KohrsDurieux M.KetamineTeaching an old drug new trick[4]Anaesth Analg87199811861193
- S.H.SharamiAbdollahzadeh MSharamiMBA.KeyvanRandomised clinical trial of the influence of pulmonary recruitment manoeuvre on reducing shoulder pain after laparoscopyJ Obstet Gynaecol3052010505510
- S.S.El-GabyS.S.MohamedIntraperitoneal ketamine attenuates the inflammatory reactivity associated with pneumoperitoneumRes Opin Anesth Intens Care432017149155
- L.M.RubinsteinT.B.LebherzV.KleinkopfLaparoscopic tubal sterilization: long-term postoperative follow-upContraception131976631638
- H.H.RiedelK.SemmThe post-laparoscopic pain syndrome (author’s syndrome)Geburtshilfe Frauenheilkunde401980635643
- W.G.MoutonJ.R.BessellK.T.OttenG.J.MaddernPain after laparoscopySurg Endosc131999445448
- C.P.PerryR.TombrelloEffect of fluid instillation on postlaparoscopy painJ Reprod Med381993786870
- S.S.LiuP.S.HodgsonLocal anaestheticsP.G.BarashB.F.CullenR.K.StoeltingClinical anaesthesia4th ed.2001Lippicott Williams and WilkinsPhiladelphia449469
- I.O.LeeS.H.KimM.H.KongM.K.LeeN.S.KimY.S.ChoiPain after laparoscopic cholecystectomy: the effect and timing of incisional and intraperitoneal bupivacaineCan J Anaesth482001545550
- P.NarchiD.BenhamouH.FernandezIntraperitoneal local anaesthetic for shoulder pain after day case laparoscopyLancet338199115691570
- A.P.BoddyS.MehtaM.RhodesThe effect of intraperitoneal local anesthesia in laparoscopic cholecystectomy: a systematic review and meta–analysisAnesth Analg1032006682688
- S.AnandS.S.BajwaB.B.KapoorM.JitenderaH.GuptaComparative evaluation of intraperitoneal bupivacaine, magnesium sulfate and their combination for postoperative analgesia in patients undergoing laparoscopic cholecystectomyNiger J Surg Sci2420144248
- M.UpadyaS.H.PushpavathiK.R.SeetharamComparison of intra-peritoneal bupivacaine and intravenous paracetamol for postoperative pain relief after laparoscopic cholecystectomyAnesth Essays Res9120153943
- S.I.Jabbour-KhouryA.S.DabbousF.G.GergesM.S.AzarC.M.AyoubG.S.KhouryIntraperitoneal and intravenous routes for pain relief in laparoscopic cholecystectomyJSLS92005316321
- N.BhardwajV.SharmaP.ChariIntraperitoneal bupivacaine instillation for post-operative pain relief after laparoscopic cholecystectomyIndian J Anesth4620024952
- J.Hernández-PalazónJ.A.TortosaV.Nuño de la RosaJ.Giménez-ViudesG.RamírezR.RoblesIntraperitoneal application of bupivacaine plus morphine for pain relief after laparoscopic cholecystectomyEur J Anaesthesiol202003891896
- T.ChundrigarA.R.HedgesR.MorrisJ.D.StamatakisIntraperitoneal bupivacaine for effective pain relief after laparoscopic cholecystectomyAnn R Coll Surg Engl751993437439
- S.GolubovićV.GolubovićM.Cindrić-StancinV.S.TokmadzićIntraperitoneal analgesia for laparoscopic cholecystectomy: bupivacaine versus bupivacaine with tramadolColl Antropol3312009299302
- N.MalhotraC.ChananaK.K.RoyS.KumarV.RewariJ.B.SharmaTo compare the efficacy of two doses of intraperitoneal bupivacaine for pain relief after operative laparoscopy in gynecologyArch Gynecol Obstet2762007323326
- B.M.RademakerC.J.KalkmanJ.A.OdoomL.de WitJ.RingersIntraperitoneal local anaesthetics after laparoscopic cholecystectomy: effects on postoperative pain, metabolic responses and lung functionBr J Anaesth721994263266
- B.ScheininI.KellokumpuL.LindgrenC.HaglundP.H.RosenbergEffect of intraperitoneal bupivacaine on pain after laparoscopic cholecystectomyActa Anaesthesiol Scand391995195198
- M.RaetzellC.MaierD.SchröderH.WulfIntraperitoneal application of bupivacaine during laparoscopic cholecystectomy – risk or benefit?Anesth Analg811995967972
- J.JorisE.ThiryI.ParisJ.WeertsM.LamyPain after laparoscopic cholecystectomy: characteristics and effect of intraperitoneal bupivacaineAnesth Analg811995379384
- M.Abdel-RaoufH.AmerPostoperative analgesic effects of intraperitoneal NMDA receptor antagonists (ketamine and magnesium) in patients undergoing laparoscopic cholecystectomyEgypt J Anaesth202004107111
- A.M.ShawkyA.EssaA.EmamEffect of intraperitoneal ketamine as postoperative analgesia in laparoscopic cholecystectomyEgypt J Hosp Med7220184224422910.21608/ejhm.2018.9142
- R.MoharariM.HadaviP.PourfakhrA.NajafiF.EtezadiM.KhajaviEvaluation of the postoperative analgesic efficacy of intraperitoneal ketamine compared with bupivacaine in laparoscopic cholecystectomyAACC212016146149
- W.KlimschaG.HorvathM.SzikszayI.DobosG.BenedekAntinociceptive effect of the S (1)-enantiomer of ketamine on carrageenan hyperalgesia after intraperitoneal administration in ratsAnesth Analg861998561565
- O.AhmedM.AbdelazizH.HishamO.H.AboushanabRole of ketamine and tramadol as adjuncts to bupivacaine 0.5% in paravertebral block for breast surgery: a randomized double-blind studyWorld Pumps27201110110510.1016/j.egja.2011.04.002
- I.O.LeeW.K.KimM.H.KongM.K.LeeN.S.KimY.S.ChoiNo enhancement of sensory and motor by ketamine added to ropivacaine interscalene brachial plexus blockadeActa Anaesthesiol Scand462002821826
- S.MøinicheH.JørgensenJ.WetterslevJ.B.DahlLocal anesthetic infiltration for postoperative pain relief after laparoscopy: a qualitative and quantitative systematic review of intraperitoneal, port–site infiltration and mesosalpinx blockAnesth Analg902000899912
