?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of the present study intended mainly to investigate the interrelationships between the six studied taxa namely Abutilon theophrasti, Lavatera cretica, Hibiscus trionum, Hibiscus sabdariffa, Malva parviflora and Sida alba collected from ten different accessions in Egypt belonging to family Malvaceae. Biochemical studies include protein profile using SDS-PAGE technique and three isozymes (esterase, peroxidase and acid phosphatase). The electrophoretic analysis revealed the presence of eighteen bands of molecular weight ranging from 11.3 to 115.3 KD. The highest number of bands 15 was observed in H. sabdariffa (Hs1) collected from Menia el-Kamh district and the two accessions of M. parviflora where as the lowest number 11 bands were recorded in H. trionum collected from Talkha district. Four loci of peroxidase isozyme distinguished, three loci of acid phosphatase isozyme and two loci of esterase isozyme. In regarding to random amplified polymorphic DNA technique (RAPD), ten primers were used to differentiate between these accessions. Primer OPA-4 gave the highest percentage of polymorphism (100%), while primer OP-B6 produced the lowest percentage (50%).
1 Introduction
Malvaceae or the mallow family is the family of flowering plants containing over 200 genera with close to 2300 species. The largest genera Hibiscus (300 species), Streculia (250 species), Dombeya (225 species), Pavonia (200 species) and Sida (200 species). The principle economic use of Malvaceae plants is as a source of natural fibers, the family providing perhaps the worlds three most important fiber crops plants of the family are also used for food, beverages, timber, in traditional medicine and in horticulture [Citation1].
Many researches have been published on the ecology, taxonomy, genetic, cytology, chemotaxonomy, physiology, seed germination and economic uses of family Malvaceae such as [Citation2] in ecology; in taxonomy [Citation3], in chemotaxonomy [Citation4] and in genetic researches [Citation5] studied the pollen.
Aerial parts of many species belong to family Malvaceae have Betaines, Glycine betaines were obtained in high yield (0.5–4.6% dry weight). Also, trigonelline was recorded, but the yield was low (0.005–0.07% dry weight) [Citation4].
Isozymes have been widely used as a molecular markers for the identification the genetic relationships among genera, species and varieties. The phylogenetic relationships in many genera have been studied by isozymes electrophoresis [Citation6] and [Citation7].
Seed protein electrophoresis has been successfully used in define species relationships in various groups of plants [Citation8].
The technology of the molecular biology has been developed over the 20 years and provided new methods for observing the genetic differences among species. These techniques offer and give many advantages over the conventional methods [Citation9].
Therefore, the present study was designed to clarify the genetic relationships among six taxa belong to family Malvaceae collected from ten different accessions from Egypt. This work is very important to document in gene banks for sustainable conservation of plant genetic resources.
2 Materials and methods
2.1 Accessions selection
Ten accessions of the six studied taxa subjected to analysis using available characterization methods. Viable seeds of the studied taxa were collected from 50 mature individuals. Identification and nomenclature of studied species were according to [Citation10] and [Citation11].
Table 1 Names and localities of ten accessions of the six studied taxa collected from Egypt.
2.2 Protein analysis
Electrophoresis analysis of seed proteins followed the method for discontinuous SDS-PAGE technique of [Citation12].
2.3 Native PAGE for isozymes
Isozymes variations identified using native polyacrylamide gel electrophoresis. Three isozymes (esterase, peroxidase and acid phosphatase) studied. These isozymes were separated on polyacrylamide gel according to [Citation13].
2.4 DNA extraction
Genomic DNA of the ten accessions of six taxa was extracted from fresh young leaves according to [Citation14].
2.5 Random amplified polymorphic DNA (RAPD-DNA)
Ten primers were used to generate RAPD markers according to [Citation15] with some modifications. The sequence of these primers is given in . The percentage of polymorphism can be calculated according to this equation.
Table 2 Primers and their composition used in RAPD analysis.
2.6 Data analysis
All gels were photographed and analyzed using Bio-Rad video Documentation system Model Gel Doc 2000. The presence or absence of each band was treated as a binary character in a data matrix (coded 1 and 0 respectively). Data analyses were performed using SYSTAT version 7.0 program [Citation16].
3 Results
3.1 Protein analysis
The electrophoretic banding patterns of extracted proteins have been studied in the ten accessions of the six taxa. These patterns were shown in . The distribution of protein bands in the different accessions of the six taxa based on their molecular weight range was shown in . The following is a brief description of the banding profiles for all accessions.
Fig. 1 Polyacrylasmide gel illustrating seed proteins bands of some taxa belonging to family Malvaceae. (M) marker, 1 (At), 2 (Hs1), 3 (Hs2), 4 (Ht1), 5 (Ht2), 6 (Lc), 7 (MP1), 8 (Mp2), 9 (Sa1) and 10 (Sa2).
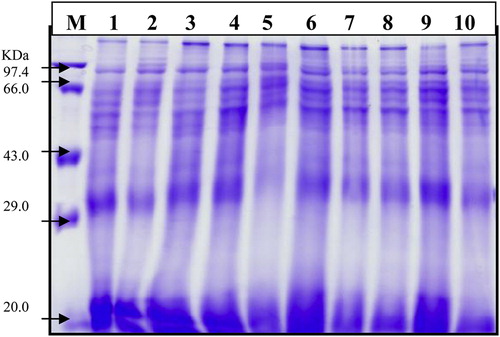
Table 3 Seed protein attributes of some species of family Malvaceae collected from different accessions, for accessions names see .
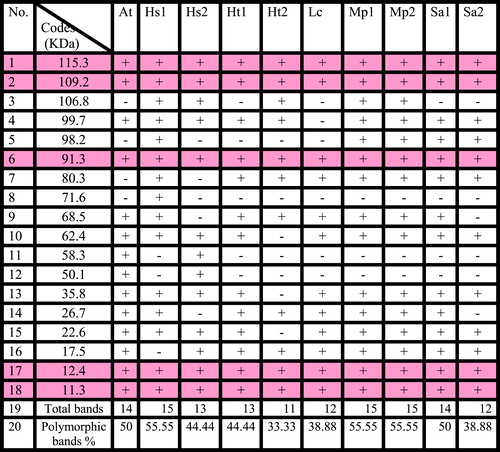
The electrophoretic protein profile of Mp1, Mp2 and Hs1 accessions consist 15 bands, 14 bands in At and Sa1, 13 bands in Hs2 and Ht1, 12 bands in Lc and Sa2, 11 bands in Ht2. the molecular weight bands for all accessions of the six studied taxa ranging from 11.3 to 115.3 KDa . The percentage of polymorphism was given in .
3.2 Isozymes
The electrophoretic analysis of peroxidase, acid phosphatase and esterase isozymes using native PAGE gel for the ten accessions of the six studied taxa recorded in – and .
Table 4 Bands of Acid phosphatase isozyme (ACPH.) for some species of family Malvaceae collected from Egypt.
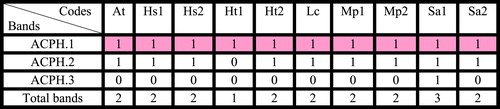
Table 5 Bands of peroxidase isozyme (per.) for some species of family Malvaceae collected from Egypt.
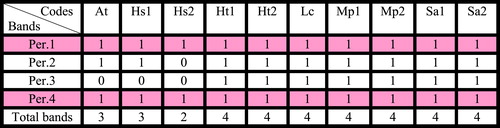
Table 6 Bands of Esterase isozyme (ACPH.) for some species of family Malvaceae collected from Egypt.
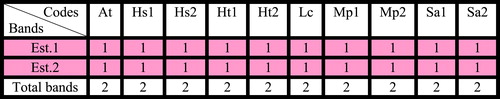
Plate 1 The electrophoresis patterns of three isozymes of some taxa belonging to family Malvaceae. 1 (At), 2 (Hs1), 3 (Hs2), 4 (Ht1), 5 (Ht2), 6 (Lc), 7 (MP1), 8 (Mp2), 9 (Sa1) and 10 (Sa2).
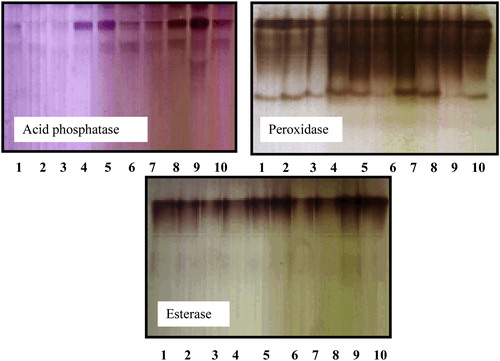
Four loci of peroxidase isozyme distinguished which differ in their amount and relative migration distance. Locus 1 and locus 4 recorded in all taxa under study. Locus 2 recorded in all taxa under study except H. sabdariffa collected from Talka, El-Dakahlyia Governorate. Locus 3 recorded in all taxa under study except Abutilon theophartis and the two accessions of Hibiscus sabdariffa. A total of three loci of acid phosphatase isozyme distinguished which differ in their amount and relative migration distance. Locus 1 found in all taxa under study, locus 2 recorded in all taxa except Hibiscus trionum collected from El- Riyad- Kafr El-Sheikh. Locus 3 found only in Sida alba collected from Menia El-Kamh El-Sharkyia . Two loci of esterase isozyme were found in all studied taxa . Concerning all accessions of the six studied taxa, the highest percentage of polymorphism 66.6% recorded in acid phosphatase isozyme, 50% in peroxidase isozyme and 0% in esterase .
Table 7 polymorphic bands of some species of family Malvaceae collected from Egypt.
3.3 DNA fingerprint
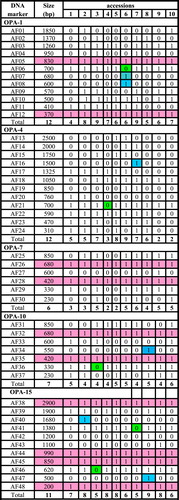
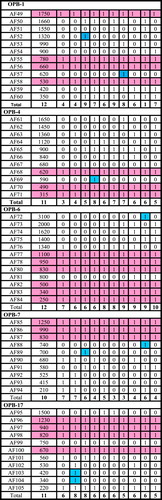
In the present study ten primers used to differentiate among the ten accessions of the six studied taxa as recorded in and . The percentages of polymorphisms were recorded in . The results were reported as follow:
Plate 2 DNA polymorphism based on RAPD- PCR analysis of some species of family Malvaceae, Egypt. (M) marker, 1 (At), 2 (Hs1), 3 (Hs2), 4 (Ht1), 5 (Ht2), 6 (Lc), 7 (MP1), 8 (Mp2), 9 (Sa1) and 10 (Sa2).
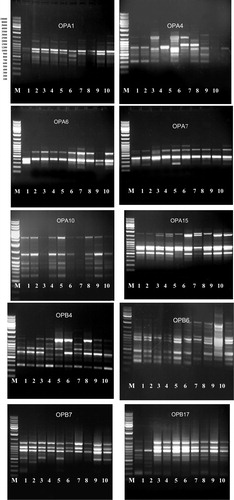
Table 9 Polymorphic bands of some species of family Malvaceae collected from Egypt.
3.4 Primer OPA-1
The results revealed that this primer produced a total number of 12 bands. Bands of molecular size 830 bp and 370 bp were recorded in all taxa under study. Bands of molecular size 600 bp and 680 bp were recorded only in Lavatera cretica so that this band could be used as a positive molecular marker for L. cretica. The band of a molecular size 700 bp recorded in all taxa under study except L. cretica so that this band could be used as a negative molecular marker for this species. The band of a molecular size 1260 bp recorded in H. trionum, H. sabdariffa, Malva parviflora and S. alba.
3.5 Primer OPA-4
The results revealed that this primer produced 12 bands among the ten accessions of the six studied taxa with a molecular size ranging from 310 to 2500 bp. The band of a molecular size 1500 bp recorded only in M. parviflora collected from Menia El-Kamh El-Sharkyia, it could be used as a positive molecular marker for this accession. The band of molecular weight 590 bp recorded in all taxa except two accessions of S. alba.
3.6 Primer OPA-7
The molecular size of the PCR products ranged from 230 bp to 850 bp. Bands of molecular size 680 bp and 420 bp recorded in all accessions of the studied taxa and missed in A. theophartis and the two accessions of H. trionum.
3.7 Primer OPA-10
The primer gave five polymorphic bands and two common bands with molecular size 420 bp and 680 bp. Band of a molecular size 330 bp recorded in all taxa under study except H. sabdariffa collected from Nabrooh –El-Dakahlyia Governorate. Band of a molecular size 550 bp recorded only in M. parviflora collected from Nabrooh, this band could be used as a molecular marker for M. parviflora collected from Nabrooh.
3.8 Primer OPA-15
The molecular sizes of PCR products ranged from 200 to 2900 bp. This primer gave seven polymorphic bands and four common bands with molecular sizes 200, 850, 990 and 2900 bp. Band with a molecular size 500 bp restricted to M. parviflora collected from Nabrooh and the band with molecular size 1680 bp restricted to H. sabdariffa collected from Menia El-Kamh El-Sharkyia Governorate. Band of a molecular size 620 bp recorded in all taxa under study except H. sabdariffa collected from Talkha- El-Dakahlyia Governorate. This band could be used as a negative molecular marker for H. sabdariffa collected from Talkha- El- Dakahlyia. Band of a molecular size 1380 bp recorded in all taxa except M. parviflora collected from Menia El-Kamh. This band could be used as a negative molecular marker for M. parviflora collected from Menia El-Kamh.
3.9 Primer OPB-1
The results revealed that this primer produced a total number of 12 bands. Band of a molecular size 620 bp recorded only in M. parviflora collected from Menia El-Kamh. It could be used as a positive molecular marker for M. parviflora collected from this accession. Band of a molecular size 1320 bp recorded only in H. sabdariffa collected from Talkha- El-Dakahlyia Governorate. This band could be used as a molecular marker for H. sabdariffa collected from this accession. This primer gave four common bands with molecular sizes 530 bp, 660 bp, 780 bp and 1750 bp.
3.10 Primer OPB-4
The molecular size of the PCR products ranged from 315 bp to 1650 bp. This primer gave three common bands with molecular sizes 315 bp, 490 bp, 620 bp and eight polymorphic bands. Band of a molecular size 590 bp recorded only in H. trionum collected from El- Riyad- Kafr El-Sheikh Governorate. It could be used as a positive marker for H. trionum collected from this accession.
3.11 Primer OPB-6
This primer produced a total number of 12 bands. Six bands are common and six polymorphic bands. Band of a molecular size 3100 bp recorded only in S. alba collected from Menia El-Kamh. This band could be used as a positive marker for S. alba collected from Menia El-Kamh.
3.12 Primer OPB-7
This primer produced a total number of ten bands, three of which common bands and seven polymorphic bands. The band of a molecular size 740 bp recorded only in S. alba collected from Menia El-Kamh so that, this band could be used as a molecular marker for S. alba collected from Menia El-Kamh. The band of a molecular size 700 bp recorded only in H. sabdariffa collected from Talkha- El- Dakahlyia. This band could be used as a positive marker for H. sabdariffa collected from Talkha – El-Dakahlyia.
3.13 Primer OPB-17
The molecular size of the PCR products from 220 bp to 1500 bp. This primer OPB-17 gave seven polymorphic bands and four common bands. Bands of molecular sizes 420, 340 bp recorded only in H. sabdariffa collected from Menia El-Kamh El-Sharkyia. These bands could be used as positive molecular markers for H. sabdariffa collected from Menia El-Kamh El-Sharkyia.
Regarding the polymorphism of all accessions of the six studied taxa, the maximum value of polymorphism 100% recorded in primer OPA-4. However, the remaining percentages of polymorphism took specific trends where 83.3% in primer OPA-1, 72.7% in primer OPB-4, 71.4 in primer OPA-10, 70% in primer OPB-7, 66.6% in primers (OPA-7, OPB-1), 63.3% in primers (OPB-17, OPA-15) and 50% in primer OPB-6.
4 Discussion
Biochemical and molecular techniques are provided approaches for evaluating genetic diversity in plants. These methods are favored because they are independent of the developmental stage of the plant [Citation17]. Biochemical evidences such as seed storage protein electrophoresis and isozyme polymorphisms are convenient evidences for assessing genetic relationships [Citation18] and [Citation19].
The variation in SDS-PAGE of seed protein profiles have successfully been used to differentiate between species [Citation20] and provide a valid source of taxonomic evidence for addressing the relationships at the different taxonomic levels [Citation21].
The electro-phenogram of the examined ten accessions of the six studied taxa revealed a total number of eighteen bands. The highest number of bands 15 was observed in H. sabdariffa collected from Menia El-Kamh, El-Sharkyia and the two accessions of M. parviflora. The molecular weight of these bands ranging from 11.3 to 115.3 KDa, where as the lowest number 11 bands were recorded in H. trionum collected from Talkha- El-Dakahlyia Governorate the molecular weight of these eleven bands ranging from 11.3 KDa to 115.3 KDa.
Isozymes polymorphisms are used effectively to assess genetic relationships among individuals, populations and closely related species [Citation22] and [Citation23]. The applications of isozymes polymorphism are still important for population genetic studies and in addressing infra-specific relationships [Citation24].
There are three isozymes (esterase, peroxidase and acid phosphatase) were used to differentiate among the ten accessions of the six studied taxa belonging to family Malvaceae. It was found that acid phosphatase and peroxidase isozymes are more effectively in differentiation among these accessions of the studied taxa, while esterase isozyme was not able to differentiate.
Proteins and isozymes electrophoretic markers have been used in many crops to some extent. The major limitation of these two procedures is the lack of enough polymorphism among closely related cultivars [Citation25]. For this reason, DNA based genetic markers have been integrated into several plant systems and are playing a very important role in molecular genetics and plant breeding [Citation25] and [Citation26].
Randomly amplified polymorphic DNA (RAPD) technique has been used in many different applications involving the detection of DNA sequence polymorphisms [Citation27] to identify varieties [Citation28] and to assess the genetic diversity [Citation29] and [Citation30]. In this study ten primers used to differentiate among the ten accessions of the six studied taxa belonging to family Malvaceae. The primers gave reproducible results but the best primer used to differentiate was primer OPA-4 and gave the highest percentage of polymorphism. Primers OPA-7 and OPB-1 gave the same percentages of polymorphism, primers OPB-17 and OPA-15 also gave the same percentages of polymorphism.
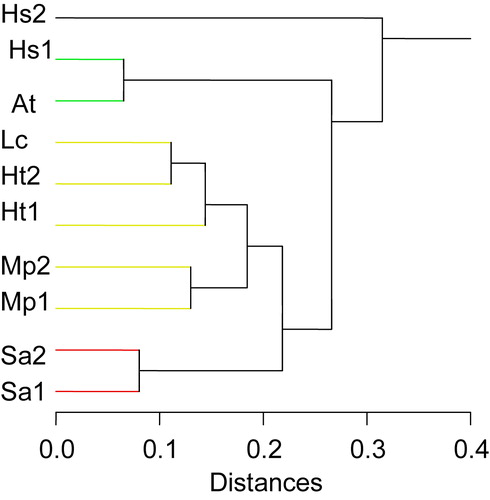
Cluster analysis was conducted to generate a dendrogram clustering possible relationships among the ten studied accessions of six species of family Malvaceae in Egypt based on compiled all matrix data. Investigated accessions were divided into two groups at 0.315, the first group includes Hs2 collected from Talkha district, and the second group is divided into two subgroups at a distance of 0.266. The first subgroup includes Hs1 and At, while the second subgroup includes Lc, Ht1, Ht2, Mp1, Mp2, Sa1 and Sa2. In the second subgroup Sa1 and Sa2 were separated from the rest at a distance 0.218.
Notes
Peer review under responsibility of Mansoura University.
References
- Hinsely RS. Economic uses of Malvaceae- Overview. 2004 and 2008.
- A.O.El-RjoobM.N.OmariHeavy metal contamination in Malva parviflora, (Malvaceae) grown in soils near the Irbid-Amman high wayJ Bot Environ Appl Sci442009433441
- J.A.TateJ.F.AguilarS.J.WagstaffJ.C.La DukeT.A.Bodo SlottaB.B.SimpsonPhylogenetic relationships within the Malveae (Malvaceae, subfamily Malvoideae)Amer J Bot922005584602
- G.BlundenA.V.PatelN.J.ArmstrongJ.GorhamBetaine distribution in the MalvaceaePhytochemistry582001451454
- D.A.BaumS.D.SmithA.YenW.S.AlversonR.NyffelerB.A.Whitlocket alPhylogenetic relationships of Malvatheca ( Bombacoideae and Malvoideae; Malvaceae sensu Lato) as inferred from plastid DNA sequencesAmeri J Bot91200418631871
- VriesADeJ.P.J.BramerZantenBovanA.HofmanR.BijIsmaAllozyme variation in populations of four Racopilum species, including the polyploidy R. tomentosumLindbergia1519904759
- N.CronbergIsozyme evidence of relationships within Sphagnum sect. Acutifolia (Sphagnaceae, Bryophyta)PI Syst Evol20319904146
- G.LadizinskySeed protein electrophoresis of the wild and cultivated species of section Faba of ViciaEuphytica241975785788
- S.RajapakseR.E.BallardCultivar identification using molecular methodsR.L.GenevewJ.E.PreeceS.A.MarkleBiotechnology of ornamental plantsvol. 1531997CAB International Publishers
- V.TackholmStudents’ flora of Egypt2nd ed1974Cairo University, Press523610
- L.BoulosFlora of Egypt. Checklist2009Al-Hadara PublishingCairo, Egypt
- U.K.LaemmliCleavage of structural proteins during the assembly of the head of the bacteriophage T4Nature271970680685
- H.StegmannW.BurgermeisterH.E.FrankcksenE.KrogerrecklenfortManual of gel electrophoresis and isoelectric focusing with the apparatus PANTA- PHORInst Biochem Messeweg111985D-3300 Braunsxhweig West- Germany
- S.L.DellaportaJ.WoodJ.B.HicksA plant DNA mini preparation. Version IIIPlant Mol Biol Rep119831921
- J.G.K.WilliamA.R.KubelikK.J.LivakJ.A.RafalskiS.V.TingeyDNA polymorphisms amplified by arbitary primers are useful as genetic markersNucl Acids Res18199065316535
- L.WilkinsonSYSTAT: the system analysis for statistics SYSTATEvaston III1997
- I.SwobodaP.L.BhallaRAPD analysis of genetic variation in the Australian fan flower, ScaevolaGenome401997600606
- NourEl-DinM.NahedF.M.El-SaiedA.M.AhmedEcophysiological responses and genetic variants in some species of the genus ZygophyllumJ Agric Sci Mansoura Univ29200423452362
- L.Simova-StoilovaV.VassilevaT.PterovaN.TsenovK.DemievskaU.FellerProteolytic activity in wheat leaves during draught stress and recoveryGen Appl Plant Physiol200691100 Special Issue
- A.BadrH.H.El-ShazlyM.M.Abou El-EnainSeed protein diversity and its implications on the relationships in the genus LathyrusIst Int. Conf Biol Sci Tanta University May 7-82000
- A.BadrM.M.Abou El-EnainH.H.El- ShazlyVariation in seed protein electrophoretic pattern and species relationships in Sesbania6th Egypt. Bot. Conf. Cairo University, November 24–26III1998493501
- D.E.SoltisP.E.SoltisIsozymes in plant Biology. Advances in plant Sciencevol. 41989Dioscorides PressPortland
- R.W.MurphyJ.W.J.SitesD.J.ButhC.H.HauflerProteins, isozymes electrophoresisD.M.HillisC.MorrtzBkMableMolecular systematics1996Dinauer AssociatesSunderland, MA
- A.M.A.MustafaA.BadrM.A.El-GalalyA.MobarakM.G.HassanGenetic diversity among Mentha populations in Egypt as reflected by isozyme polymorphismIntern J Bot12005188195
- J.BeckmannM.SollerRestriction fragment length polymorphism in genetic improvement methodology. Mapping and CostTheor Appl Genet6719933343
- J.BeckmannM.SollerRestriction fragment length polymorphism in genetic improvementB.J.MiflinOxford survey of plant, molecular biologyvol. 31990196250
- R.S.ReiterJ.G.K.WilliamsK.A.FeldmannJ.A.RafalskiS.V.TingeyP.A.ScolinkGlobal and local genome mapping in Arabidopsis thaliana by using recombinant inbred lines and random amplified polymorphic DNAProc Natl Acad Sci U S A89199214771481
- S.HeH.OhmS.MackenzieDetection of DNA sequence polymorphisms among wheat varietiesTheor Appl Genet845–61992573578
- F.M.Abdel- TawabM.A.RashedR.S.DhindsaA.Bahi El-DinA.Abo-DomaMolecular markers for salt tolerance in sorghum bicolorInter Cong Mol Gent1998195204
- F.M.Abdel- TawabA.Abo-DomaA.AllamStability of microsatellite markers in Sorghum bicolor LEgypt J Genet Cytol302001189200