Abstract
A high incidence of viral persistence and progression to chronic hepatitis are characteristic features of HCV infection. The aim of this case control study was to investigate the expression of the apoptosis related proteins Fas (CD95) and Bcl-2 in peripheral blood mononuclear cells (PBMCs) from patients with chronic HCV and schistosomiasis, and to evaluate their contribution in the development and maintenance of the different clinical forms of these diseases. The level of Fas and Bcl-2 expression in PBMCs was detected using flow cytometry in 85 cases in this study, including chronic HCV (40 patients) and schistosomiasis (25 patients) in addition to 20 healthy controls. Results showed that the increased apoptotic level correlated with increased Fas expression on the surface of PBMCs in chronic HCV patients (p = 0.001) which did not show increased expression of the anti-apoptotic factor Bcl-2 (p = 0.19). Apoptotic PBMCs in intestinal schistosomiasis patients showed increased expression of Fas (p = 0.001) but again did not exhibit a difference in Bcl-2 expression (p = 0.61) when compared to either hepatoplenic schistosomiasis or control patients. The Fas expression was highly significant in HCV > schistosomiasis > control (p < 0.002) while non-significant difference was found between the 3 groups as regards to Bcl-2 expression (p > 0.12). It was concluded that the detection of apoptosis of PBMCs in patients with chronic HCV was associated with increased expression of apoptotic markers in comparison with schistosomiasis patients, which may be an indicator of disease progression and severity. Modulation of this process may offer valuable methods of HCV therapy.
Keywords:
1 Introduction
In the immune system, apoptosis appears to play a crucial role in selection of the T cell repertoire, deletion of self-reactive T and B lymphocytes, removal of peripheral effector T cells following termination of an immune response, regulation of immunological memory and in the cytotoxicity of target cells by cytotoxic lymphocytes (CTLs) and natural killer (NK) cells [Citation1].
The cell's death is choreographed by a set of previously dormant proteases, the caspases, which cleave several hundred cellular substrates [Citation2]. Two principal pathways to caspase activation have been recognized [Citation3]. One is the death receptor pathway (extrinsic pathway), which is triggered by binding of FAS ligand (FASLG) to FAS (CD95) [Citation4]. The second apoptosis pathway (intrinsic pathway) is induced by mitochondria in response to DNA damage, oxidative stress, and viral proteins [Citation5]. Mitochondrion-dependent apoptosis is mediated by pro-apoptotic genes (BAX, BAD, BAK, and others), whereas proteins like BCL-2 and BCL-XL are antiapoptotic [Citation6].
Hepatitis C virus (HCV) is a major worldwide causative pathogen of chronic hepatitis (CHC), liver cirrhosis, and hepatocellular carcinoma [Citation5]. The fact that HCV exists as an evolving quasispecies plays an important role in the selection of escape mutants. Furthermore, several viral proteins interfere with cellular functions, in particular, those involved in the immune response of the host. Several HCV proteins also modulate cells through interaction with different effectors involved in cell proliferation and apoptosis, or in the interferon-signaling pathway [Citation7]. An accumulating body of evidence suggests that apoptosis of hepatocytes and peripheral blood mononuclear cells (PBMCs) are significantly involved [Citation8].
In chronic hepatitis C virus (HCV) lymphocytes infiltrating the liver recognize viral antigens on hepatocytes, become activated and over-express Fas ligand (FasL) on their surface [Citation9]. Spontaneous apoptosis of peripheral T cells from HCV-infected patients was significantly increased compared with that of normal individuals [Citation9,Citation10].
Apoptosis is an important regulator of host responses during infection with a variety of parasites [Citation11]. Schistosomiasis is a leading tropical disease caused by flatworms of the genus Schistosoma. It is estimated to affect over 200 million people globally and cause nearly 300,000 deaths annually [Citation12]. The host granulomatous inflammatory response to deposited worm eggs leads to hepatic and intestinal fibrosis, the major pathological consequences of infection with the parasitic helminth Schistosoma mansoni. Schistosomes appear to have evolved several strategies to down-regulate the host's immune response in order to promote their own survival [Citation13]. It is well-established that cells obtained from patients with chronic infection fail to respond to antigens derived from S. mansoni [Citation14]. Several lines of evidence suggest that antigens of S. mansoni can induce apoptosis of host T cells [Citation15].
The aim of this case control study was to assess the expression of the apoptosis and its related proteins Fas (CD95) and Bcl-2 in peripheral blood mononuclear cells from patients with chronic HCV and schistosomiasis, and to evaluate the contribution of peripheral blood T cell apoptosis to the development and maintenance of the different clinical forms of these diseases.
2 Patients and control
This case control study which carried out on 85 cases attending the clinics of the Tropical Medicine Department at Mansoura University Hospital. Group I of these cases comprised 40 chronic HCV patients, which were further sub-grouped as compensated (n = 22) and decompensated (n = 18), while group II included 25 patients with schistosomiasis, either intestinal (n = 9) or hepatosplenic (n = 16). In addition, 20 age and sex matched healthy individuals were also included as control subjects.
Patients infected with other hepatic viruses and/or parasites, autoimmune diseases, chronic heart diseases, chronic renal diseases, malignancy, diabetes mellitus, pregnancy, and cases with anti-schistosomal therapy (within the last 3 years) were excluded. All participants were subjected to complete history taking and thorough clinical examination, and the required laboratory and radiological investigations were performed.
Micro-enzyme immunosorbent assay (Axsym; Abbott Diagnostics-Abbott Max-Planck-Ring2, Wiesbaden, Germany) was used and confirmed by the detection of circulating HCV RNA by RT-PCR (Perkin–Elmer Thermal cycler). Confirmation of S. mansoni infection was carried out through detection of S. mansoni ova in stool as determined by formol-ether-concentration technique or rectal snip [Citation16] and indirect hemagglutination assay (Fumouze Diagnostics-Levallois-Perret, France). The research protocol was reviewed and approved by Medical Mansoura University Ethics Committee for Human Subject Research. The study protocol respected the most recent declaration of Helsinki, and all patients gave informed consent before sharing in the study [Citation17].
3 Methods
3.1 Lymphocyte separation
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by a standard density gradient centrifugation procedure using Ficoll-Hypaque [Citation18]. Five ml of heparinized blood were collected in a 15 ml sterile falcon tube and allowed to stand with an equal volume of dextran/saline solution for 45 min at 20–25 °C. The leukocyte-rich plasma (buffy coat) was aspirated and centrifuged at 170 × g for 10 min. Pellets were then suspended in a volume of PBS (phosphate-buffered saline) to the starting volume of blood, placed on top of Ficoll solution and centrifuged at 400 × g at 20 °C for 40 min. The supernatant was discarded and the pellets were washed with 0.34 M sucrose to remove platelets. A few remaining erythrocytes were disrupted by hypotonic lysis with 10% ammonium chloride (cold 0.2% NaCl for 30 s). Isolation was restored by 1.6% NaCl. Lymphocytes were finally washed and suspended in PBS.
3.2 Culture of lymphocytes
Cells were counted and adjusted to give a final concentration of 2 × 106 cells/ml in complete RPMI-1640 medium containing 25 mM HEPES buffer (pH 7.3) supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin, and 100 mcg/ml streptomycin. The cell suspension was divided into 4 aliquots to measure apoptosis at zero time (baseline), then at 24 h, 48 h, and 72 h. Cells were incubated at 37 °C in 5% carbon dioxide. After incubation, the cells were centrifuged (200 × g for 5 min at 5 °C), then resuspended in ice-cold PBS [Citation18].
3.3 Assessment of cell viability
At time zero and then subsequent times, cells were removed from culture and counted on a hemocytometer. Cell viability was determined by trypan blue dye which stains only damaged cells, so the viable cells which exclude the dye were counted. This method is used to determine the cell concentration (cell number/ml) in batch cell culture to ensure that optimal level of growth was reached before routine subculture [Citation18].
3.4 Morphological detection of apoptotic cells
For reliable detection of apoptosis, it is generally recommended to employ at least two different methods together with microscopic verification of the distinctive morphological alterations. This was done using Grunwald–Giemsa (A) and acridine orange stain (B) as well as DNA ladder for determination of DNA fragmentation by extraction of DNA followed by gel electrophoresis.
3.4.1 Morphological assessment using Giemsa stain
Cells were removed from culture, fixed in methanol, harvested on slides, and stained with May Grunwald–Geimsa, then examined by oil immersion light microscope. Five hundred cells were counted per sample and data were reported as percentage of cells with apoptotic morphology, as assessed according to the following criteria: cellular condensation eventually resulting in apoptotic bodies decreases in cell size, and cytoplasmic vacuolation [Citation19].
3.4.2 Morphological assessment by acridine orange (AO) stain
One drop of cell suspension was added to one drop of AO solution, mixed gently on a slide, and immediately examined with Olympus microscope with fluorescence attachment. Green fluorescence was detected between 500 and 250 nm. Cells exhibiting bright fluorescent intact or fragmented nuclei were interpreted as apoptotic cells, whereas viable cells were interpreted as cells that exhibited a green and diffusely stained nucleus [Citation20].
3.4.3 Morphological assessment by determination of DNA fragmentation using gel electrophoresis
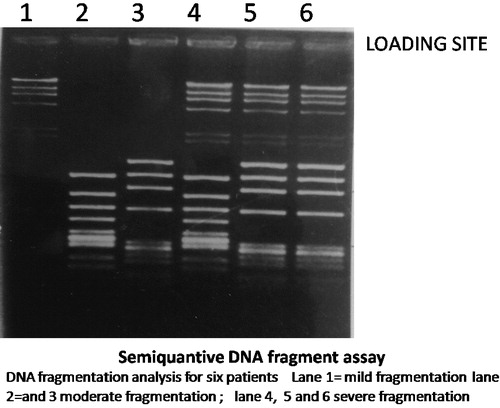
For extraction of DNA, cells were lysed in lysis buffer, and incubated with proteinase K for 2 h at 55 °C and with RNase for 2 h at 55 °C. DNA was precipitated with 2 vol of isopropanol overnight at 20 °C and dissolved in TE buffer. After resuspension of pellets in TE buffer, equal amounts were subjected to electrophoresis, where 2% agarose solution was prepared in TAE buffer. Ethidium bromide was added in a concentration of 0.5 μg/ml. DNA was visualized by fluorescence under UV light [Citation21].
3.4.4 Degree of DNA fragmentation
The grading of DNA fragmentation as mild (+), moderate (++), or severe (+++) can be explained by the location of the bands corresponding to the fragmented DNA (). In the mild (+) degree the location of the bands was nearer to the wells where the DNA sample was loaded. In the severe (+++) degree the location of the bands was away from the wells where the DNA sample was loaded and nearer to the end of the gel. Whereas, in the in the moderate (++) degree the location of the bands was midway between the wells where the DNA sample was loaded and the end of the gel. In other wards if the gel was divided into 3 equal thirds; upper, middle and lower. The (+) DNA fragmentation bands were located in the upper third (the third which contain the wells where DNA samples were loaded). The (++) DNA fragmentation bands were located in the middle third, and the (+++) DNA fragmentation bands were located in the lower third [Citation22].
3.5 Flow cytometry
3.5.1 Flow cytometric measurement of apoptosis via detection of CD95
Viable lymphocytes were incubated with the unlabeled monoclonal antibody (MoAb) and a second layer of fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin with heavy chain specificity (Diaclone, UKAS, Cat. no. 853.001.010). All incubations were performed at room temperature, and 2% pooled human AB serum was added to the buffer throughout all the steps to avoid non-specific binging of MoAbs to Fc receptors.
The following procedure was done: the lymphocytes were separated and the cell pellet was suspended in RPMI-1640, and the cell count was adjusted between 10 × 106 and 50 × 106/ml. The lymphocyte suspension was centrifuged and the supernatant was discarded, cell pellet resuspended in PBS containing 2% (vol/vol) human AB serum, and the cell count adjusted between 10 × 106 and 20 × 106/ml. Fifty ml of the lymphocyte suspension (containing from 0.5 × 106 to 1 × 106 cells) were added to each Falcon tube. Ten ml of the MoAb (mouse monoclonal antihuman CD95 antigen FITC-conjugated) was added, mixed well and incubated for 10 min at RT. After incubation, the cells were washed 3 times with PBS containing 2% human antibody (AB) serum and centrifuged in between at 200 × g for 2 min at RT. After the last wash the supernatant was decanted and the cell pellet resuspended in PBS and analyzed on the flow cytometer (FAC Scan, Becton Dickinson). Negative controls were included in the form of replacement of the AB by an isotopic antibody (IgG2a) or omitting the use of the AB. The cutoff point of positivity was considered when more than 20% of the cells stained with a particular antibody in excess of the background fluorescence in the negative controls [Citation23].
3.5.2 Flow cytometric measurement of Bcl-2
The procedure was done as previously described for the first 3 steps after which 50 ml of the lymphocyte suspension (containing 0.5 × 106 to 2 × 106 cells) were added to each Falcon tube. Two ml 4% formaldehyde was added to each of the tubes, which were then incubated for 10 min at RT. The tubes were then centrifuged and supernatant was discarded, and the cells were permeabilized with 0.2% Tween 20 (Sigma Chemicals, St. Louis) for 15 min at 37 °C. The cell pellet was resuspended in PBS and 10 ml FITC-conjugated anti-Bcl-2 MoAb (Dako, Carpentaria, California, Cat. no. F 7053) was added to the tubes which were then incubated for another 25 min and 4 °C. The cells were washed twice with PBS, centrifuged and supernatant discarded, then washed twice again with PBS. After the last wash, the cell pellet was resuspended in 300–500 μl PBS and the sample read on the same day. Flow cytometry was performed on a FAC scan. Negative controls included unstained cells only, FITC-IgG1 (Conjugate control), and FITC-Bcl-2 labeled unstimulated/untreated cells [Citation23].
3.6 Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Science (SPSS) package program. Quantitative data were presented as mean and standard deviation. Least significant difference (LSD) was used to compare quantitative data between each of two groups. Student t test was used to compare quantitative data between the two groups, while one way Anova (F test) was used to compare quantitative data between more than two groups.
Qualitative data was expressed as number and percentage; Chi-square test was used as a test of significance for qualitative data between groups. Significance was considered when p value was less than 0.05 while non-significance when more than 0.05. High significance was considered when p value was less than 0.01 and extreme significance when less than 0.001.
4 Results
Comparison of morphological detection of apoptosis between chronic hepatitis C and schistosomiasis using acridine orange (B) and Giemsa stains (A) (represented as percentage of apoptotic cells) showed that the mean level of detection of apoptotic cells by acridine orange stain was 38.71% vs 16.64% and by Giemsa stain was 37.55% vs 15.36% in the HCV group versus (vs) the schistosomiasis group (), respectively, a difference that was extremely significant (p < 0.001). showed that the degree of DNA fragmentation was (+++) in 57.9% of the HCV group while being only (+) in 52% of schistosomiasis cases, a difference that was also statistically highly significant (p = 0.02) ().
Table 1 Apoptosis detection by acridine orange and Giemsa stains in the chronic hepatitis C group vs the schistosomiasis group.
Table 2 Detection of apoptosis by DNA fragmentation in the chronic hepatitis C group vs the schistosomiasis group.
The increased apoptotic level of peripheral blood mononuclear cells was correlated with increased Fas expression on the surface of PBMCs in chronic HCV patients (59.39 ± 24.33) compared to the control group (9.8 ± 1.31, p = 0.001), although these cells did not show increased expression of the anti-apoptotic factor Bcl-2 (23.51 ± 10.31) when compared with controls (20 ± 17.94, p = 0.19). Intestinal schistosomiasis patients also showed increased expression of Fas (46.5 ± 14.009, p = 0.001), but again did not exhibit a difference in Bcl-2 expression (21.33 ± 34.69, p = 0.61) when compared to either hepatoplenic patients (25.7 ± 5.16 & 40.12 ± 74.41) or to the control group (9.8 ± 1.31 & 20 ± 17.94), respectively. However, Fas expression was highly significant in HCV > schistosomiasis > control (p < 0.002) while non-significant differences were seen between the 3 groups as regard to Bcl2 expression (p > 0.12), as shown in .
Table 3 Detection of Fas and Bcl-2 expression by flow cytometry in the chronic hepatitis C group vs the schistosomiasis group (intestinal and hepatosplenic).
Detection of Fas expression in compensated and decompensated HCV patients showed extremely significant difference (p < 0.001) between the compensated patients (41.4 ± 12.88) vs non decompensated group (64.6 ± 12.19), both of which were significantly increased when compared with the control group (9.86 ± 1.35 – P < 0.001), while Bcl2 expression showed no significant difference between the 3 groups (22.0 ± 3.62, 25.8 ± 10.75 and 20.33 ± 17.84, respectively; p > 0.05) as shown in .
Table 4 Detection of Fas and Bcl-2 expression by flow cytometry in the chronic hepatitis C (compensated vs non compensated cases).
5 Discussion
Although few studies have compared apoptosis in patients with different clinical forms of S. mansoni infection with patients with chronic hepatitis C virus infection, the present study showed that the level of apoptosis of peripheral T cells as detected morphologically in patients with chronic hepatitis was found to be significantly higher than that detected in the schistosomiasis patients, which may be due to the finding of significant levels of apoptosis in both compensated and decompensated cases of chronic hepatitis C [Citation24] but only in cases of intestinal schistosomiasis, while patients with hepatosplenic schistosomiasis showed non-significant apoptosis of peripheral T cells [Citation14,Citation25].
A high incidence of viral persistence and progression to chronic hepatitis are characteristic features of HCV infection [Citation8]. Lymphocytes infiltrating the liver recognize viral antigens on hepatocytes, become activated and over-express Fas ligand (FasL) on their surface [Citation9]. Possible mechanisms by which HCV increases the sensitivity of T cells to undergo apoptosis include enhancement of T-cell apoptosis as well as increasing Fas sensitivity to FasL binding on T cells, ultimately acting to tip the balance in favor of the virus [Citation10]. This study demonstrated increased apoptosis of peripheral blood T cells which was associated with increased surface expression of Fas in patients with chronic hepatitis C, a finding similar to that reported by another study which showed that the surface expression of Fas was significantly high in T cells derived from patients with chronic hepatitis C [Citation26]. It was found that a close relationship exists between hepatitis C virus infection of PBMCs and cell surface Fas expression in patients with hepatitis C [Citation27]. The expression of Fas on PBMCs was found to be significantly increased in these patients, causing apoptotic cell death. The preferential detection of HCV RNA from Fas-positive cells, but not from Fas-negative cells, suggests that HCV infection of PBMCs might induce Fas expression, thus inducing apoptosis in these Fas-presenting cells, which may explain the decrease in number of PBMCs observed in patients with chronic hepatitis C. A progressive increase in its expression with the severity of liver disease from chronic hepatitis to cirrhosis was observed in several studies [Citation28]. Significantly increased expression of Fas receptor in PBMCs were demonstrated in hepatitis C patients with fibrosis and absent necroinflammation [Citation29], suggesting that Fas receptor expression may represent a self-limiting mechanism of the immune response [Citation30], while in hepatitis C patients with cirrhosis and intense necroinflammation, a decrease in Fas expression in PBMC's was observed [Citation29].
However, other studies showed that Fas was similarly expressed on activated T cells of HCV patients compared with that of healthy individuals [Citation26]. HCV core proteins have been shown to suppress the immune response of peripheral T lymphocytes to HCV infection, which has been shown to be due to increased Fas-mediated apoptosis of cells, contributing to HCV persistence [Citation31]. It has been demonstrated that the core protein binds to the cytoplasmic tail of Fas, which may enhance the downstream signaling event of Fas-mediated apoptosis. On the other hand, core proteins did not alter the cell surface expression of Fas, indicating that the increased sensitivity of core-expressing cells to Fas ligand was not due to up-regulation of Fas.
These results suggest that the core protein may promote the apoptosis of immune cells during HCV infection via the Fas signaling pathway, thus facilitating HCV persistence [Citation32].
Increased T cell apoptosis observed primarily in the intestinal group of schistosomiasis patients was associated with enhanced Fas expression compared with the controls. Increase in apoptosis of lymphocytes isolated from S. mansoni-infected patients in another study supports the hypothesis that activation-induced cell death (AICD) is potentially a contributing factor in T helper (Th) cell regulation during chronic stages of schistosomiasis, which represents a critically determinant factor in the host–parasite interaction and might influence the destiny of parasitic infections either towards establishment of chronic infection or towards host death [Citation33].
The lack of difference in Bcl-2 expression in peripheral blood T cells between HCV-infected patients, either compensated or decompensated, and normal controls in our study is in concordance with several similar findings, including those who found no significant difference in percentage of Bcl-2 in T cells among chronic hepatitis C patients and normal subjects [Citation26], and others who also showed that Bcl-2 expression was similarly expressed in activated T cells of HCV and normal individuals [Citation24,Citation34]. The finding that the expression of Bcl-2 is not increased in the peripheral T cells of patients with chronic HCV infection signifies that Bcl-2 does not exert an anti-apoptotic effect in HCV-infected patients [Citation35]. Similarly, the expression of Bcl-2 on peripheral blood T lymphocytes in patients with intestinal schistosomiasis was not found to be increased in our study.
However, another study demonstrated that the increased susceptibility of PBMCs in patients with chronic hepatitis C to apoptosis was associated with diminished intracellular expression of the anti-apoptotic protein Bcl-2 [Citation36]. This down-regulation of Bcl-2 expression may contribute to viral persistence and progression of liver disease in chronic hepatitis C. It was also shown that signal transducer and activator of Bcl-2 was significantly stimulated in HCV-infected patients [Citation37,Citation38].
6 Conclusion
Apoptosis of PBMCs in patients with chronic HCV showed increased expression of apoptotic markers when compared with schistosomiasis patients, which may be an indicator of disease progression and severity. This was due to an increase in surface receptor expression of pro-apoptotic Fas, associated with lack of expression of the anti-apoptotic Bcl-2, suggesting that this factor does not afford protection against apoptosis. Modulation of apoptotic processes, especially the activation of the Fas surface receptor, may thus offer valuable methods of therapy for chronic HCV patients.
Acknowledgment
We would like to thank Dr. Heba Eldegla (Lecturer of microbiology & immunology – Mansoura Faculty of Medicine) for her technical support during the revision & submission of the manuscript.
Notes
Peer review under responsibility of Mansoura University.
References
- S.GuptaMolecular signaling pathways in death receptor and mitochondrial pathways of apoptosisInt J Oncol22120031520
- N.A.ThornberryY.LazebnikCaspases: enemies withinScience2815381199813121316
- A.AshkenaziTargeting death and decoy receptors of the tumour-necrosis factor superfamilyNat Rev Cancer262002420430
- K.MachidaH.TsukamotoJ.C.LiuY.P.HanS.GovindarajanM.M.Laic-Jun mediates hepatitis C virus hepatocarcinogenesis through signal transducer and activator of transcription 3 and nitric oxide-dependent impairment of oxidative DNA repairHepatol522010480492
- R.FischerT.BaumertH.E.BlumHepatitis C virus infection and apoptosisWorld J Gastroenterol1336200748654879
- C.BrenneS.GrimmThe permeability transition pore complex in cancer cell deathOncogene25200647444756
- N.PavioM.M.LaiThe hepatitis C virus persistence: how to evade the immune system?J Biosci2832003 Apr287304
- J.MankouriM.L.DallasM.E.HughesS.D.GriffinA.MacdonaldC.PeersSuppression of a pro-apoptotic K+ channel as a mechanism for hepatitis C virus persistenceProc Natl Acad Sci U S A106200915903
- M.SarihN.BouchritA.BenslimaneDifferent cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infectionImmunol Lett7422000117120
- G.PiazzollaC.TortorellaO.SchiraldiS.AntonaciRelationship between interferon-gamma, IL-10, and IL-12 production in chronic hepatitis C and in vitro effects of interferon-alphaJ Clin Immunol201020005461
- S.K.LuderU.GrossM.F.LopezIntracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactionsTrends Parasitol102001480486
- P.J.HotezA.FenwickSchistosomiasis in Africa: an emerging tragedy in our new global health decadePLoS Negl Trop Dis32009485
- S.K.LundyS.P.LermanD.L.BorosSoluble egg antigen-stimulated T helper lymphocyte apoptosis and evidence for cell death mediated by FasL+ T and B cells during murine Schistosoma mansoni infectionInfect Immun6912001271280
- P.Carneiro-SantosO.Marins-FilhoL.F.Alves-OliveiroA.M.SilveiroP.Couro-FilhoI.R.ViannaApoptosis: a mechanism of immunoregulation during human schistosomiasis mansoniParasite Immunol2262000267277
- R.S.KasinathanR.M.GreenbergSchistosoma mansoni soluble egg antigens trigger erythrocyte cell deathCell Physiol Biochem262010767774
- M.S.ArafaH.A.HamadtoA.M.El-RidiA study of the rectal snip transparency and Kato-technique in the diagnosis of intestinal schistosomiasisJ Egypt Soc Parasitol1321983273278
- World Medical Association (WMA)Declaration of Helsinki; ethical principles for medical research involving human subjectsOctober 2008 59th WMA general assembly Seoul
- A.J.McGahonS.J.MartinR.P.BissonetteA.MahbouliY.ShiR.J.MogilThe end of the cell line: methods for the study of apoptosis in vitroMethods Cell Biol461995153185
- J.J.CohenApoptosisImmunol Today1431993126130
- M.KumagaiE.Coustan-SmithL.MurrayO.SilvennoinenK.G.MurtiW.E.EvansLigation of CD38 suppresses human B lymphopoiesisJ Exp Med1813199511011110
- A.HuiJ.KulkarniW.HunterC.A.McCullochT.F.CruzPaclitaxel selectively induces mitotic arrest and apoptosis in proliferating bovine synoviocytesArthritis Rheum406199710731084
- M.HafezH.HawasM.F.M.El-BattotyZ.El-MorsyM.M.AliA.ShaltoutApoptosis and autoantibodies in relation to activity and severity in juvenile rheumatoid arthritisEgypt J Pediatr17200083105
- E.J.QuackenbushJ.LetarteIdentification of several surface proteins of non-T, non-B acute lymphoblastic leukaemia by using monoclonal antibodiesJ Immunol1342199512761285
- E.ToubiA.KesselL.GoldsteinG.SlobodinE.SaboZ.ShmuelEnhanced peripheral T cell apoptosis in chronic hepatitis C virus infection: association with liver disease severityHepatology3562001774780
- J.KountourasC.ZavrosD.ChatzopuolosApoptosis in hepatitis CJVH1052003335342
- S.OgawaK.SakaguchiA.TakakiK.ShiragaT.SawayamaH.MouriIncrease in CD95 (Fas/Apo-1)-positive CD4+ and CD8+ cells in peripheral blood derived from patients with autoimmune hepatitis or chronic hepatitis C with autoimmune phenomenaJ Gastroenterol Hepatol15120006975
- N.TayaY.TorimotoM.ShindoH.HiraiC.HasebeY.KohgoFas-mediated apoptosis of peripheral blood mononuclear cells in patients with hepatitis CBr J Haematol110120008997
- M.BortolamiA.KotsaftiR.CardinF.FarinatiFas/FasL system, IL-1b expression and apoptosis in chronic HBV and HCV liver diseaseJVH152008515522
- G.AlbertoniC.P.ArnoniF.R.LatiniS.S.AndradeP.R.AraujoM.C.J.A.Mendes-CorreaAltered of apoptotic markers of extrinsic and intrinsic pathways induced by hepatitis C virus infection in peripheral blood mononuclear cellsVirol J92012314
- T.PatelG.GoresApoptosis and hepatobiliary diseaseHepatology21199517251741
- K.M.ChangR.ThimmeJ.J.MedpolderD.OldachJ.PembertonMoorhead-LoudisDifferential CD4+ and CD8+ T cell responsiveness in hepatitis C virus infectionHepatology3312001267276
- Y.S.HahnC.SogueroM.CruiseTowards a reliable parameter of liver damage in hepatitis C: tunel versus caspase activationHepatology344 Pt. 12001840841
- H.M.GhoneimS.R.DemianM.G.HeshmatN.S.IsmailL.H.El-SayedActivation-induced apoptosis in peripheral blood mononuclear cells during hepatosplenic Schistosoma mansoni infectionsEgypt J Immunol15220086372
- M.HondaS.KanekoT.ShimazakiE.MatsushitaK.KobayashiL.H.PingHepatitis C core protein induces apoptosis and impairs cell-cycle regulationHepatology316200013511359
- J.Y.N.LauX.XieM.M.C.LaiP.C.WuApoptosis and viral hepatitisSemin Liver Dis1821998169176
- Y.NakamotoS.KanekoK.KobayashiIncreased susceptibility to apoptosis and attenuated Bcl-2 expression in T lymphocytes and monocytes from patients with advanced chronic hepatitis CJ Leuko Biol72120024955
- Y.LiQ.ZhangY.LiuZ.LuoL.KangJ.QuHepatitis C virus activates Bcl-2 and MMP-2 expression through multiple cellular signaling pathwaysJ Virol862320121253112543
- C.A.RumbleyH.SugayaS.A.ZekevatP.J.PerrinS.M.PhillipsElimination of lymphocytes, but not eosinophils, by Fas-mediated apoptosis in murine schistosomiasisAm J Trop Med Hyg6552001442449