Abstract
Patients with either diabetes or hypercholesterolemia develop atherosclerosis and impair healing of bone. In the present work we illustrated their role in limb ischemia during bone cells differentiation of Wistar rat fetuses. Pregnant Wistar rats (n = 20 each) were arranged in three groups: control, diabetic or hypercholesterolemic. Diabetes was induced at the fifth day of gestation using streptozotocin, and hypercholesterolemia was carried by feeding virgin rats a diet containing 3% cholesterol for 6 wks prior to the onset of conception. At 13, 15, 17, and 19 d prenatal, pregnant rats were sacrificed, dissected, and fetuses were removed. Hind limbs were separated and subjected to histological and transmission electron microscopic examination, ossification, total calcium content of fetuses, isoenzymes alkaline and acid phosphatase and lactic dehydrogenase electrophoresis and DNA damage. Fetuses of diabetic or hypercholesterolemic mothers exhibited delayed histo-cytological differentiation of chondrocytes, and decreased periosteal ossification. Alkaline and acid phosphatase as well as lactic dehydrogenase isoenzymes showed altered diffusion rate and intensities of their bands reflecting their activities in both diseases comparing with the control. Assessments of bone calcium contents revealed marked reduction. Genomic expression of the degree of laddering (total DNA fragmented) or single-cell gel electrophoresis was found to be increased in cartilage and bone cells of fetuses of diabetic or hypercholesterolemic mothers. The authors concluded that both diseases had a selective, dramatic effect during fetus development in this model by retarding the histo- and cytological differentiation during limb bone growth. Both diseases increased the average cell death in skeletal elements and blood vessels as a consequence of altered alkaline and acid phosphatases and lactic dehydrogenase isoenzymes in accordance with DNA damage.
1 Introduction
Hypercholsterolemia represents the common pathogenetic factor for bone disease [Citation1,Citation2]. Researchers are still figuring out exactly how diabetes changes cholesterol levels and contributes to vascular complications such as atherosclerosis [Citation3]. Diabetes [Citation4] or hypercholesterolemia [Citation5] was interrelated with each other and increased the oxidative stress and cell damage.
Recently, we reported that maternal diabetic or hypercholesterolemic mothers exhibited massive damage of both blood vessels and myocardial fibers of their fetuses [Citation6] as well as induce apparent hepatocyte damage [Citation7] which may co-operate in bone defects.
A very few number of studies have reported that type 1 diabetes altered bone remodeling by reducing the formation of new bone, leading to osteopenia in humans and animals [Citation8,Citation9]. Type 1 diabetes was found to cause a significant delay in fracture healing [Citation10](Lu et al., 2003) through a reduction of bone mineral density as assessed in the lumbar spine and proximal femur [Citation11] and a decrease of bone formation compared with normal individuals [Citation8].
Experimental studies on diabetic mice and rats revealed that the disease was concerned with reduction of bone formation, expression of osteocalcin, collagen types and decrease in bone healing [Citation10], as well as increasing apoptosis of bone-lining cells [Citation11]. Kayal et al. [Citation12] observed a decrease of cartilage tissue and new bone area post 16 days of onset of diabetes in CD-1 mice. Won et al. [Citation13] examined the inter-vertebral disk of OLETF (diabetic) rats at 6 and 12 months of age and detected increased incidence of apoptotic index of notochordal cells, which led to early intervertebral disc degeneration.
Atherosclerosis, like osteoporosis, is closely associated with each other [Citation1]. The enhanced risk of bone fracture was the end result of accumulated damage [Citation14]. Osteoclast formation and function were influenced by numerous inflammatory factors mediated in atherogenesis such as tumor necrosis factor -α which activated osteoclasts formation [Citation15]. In experimental rats, Funaba et al. [Citation16] reported a reduction of bone formation in 10-week-old hypercholesterolemic rats, suggesting inhibiting factor(s) for bone growth.
From the literature, there are no reports illustrating the direct effects on the differentiation of skeletal cells. The present study aims to outline the exact role of diabetes or hypercholesterolemia in histogenesis and cytological structures of bone elements, bone isoenzyme lactic dehydrogenase electrophoresis, and pattern of DNA damage of hind limb of Wistar rat during prenatal growth.
2 Materials and methods
2.1 Induction of diabetes
Experimental diabetes mellitus was induced by a single intraperitoneal injection of streptozotocin (60 mg/kg) in the citrate buffer (pH 4.5) on the fifth day of gestation [Citation17]. Control animals received physiologic saline as a vehicle. Hyperglycemia was verified by measuring the blood glucose at approximately 350 mg/dL.
2.2 Induction of hypercholesterolemia
The experimental group was fed a hypercholesterolemic diet according to Enkhmaa et al. [Citation18]. The component of the diet was illustrated in . The hypercholesterolemic diet was composed of 3% cholesterol and 15% cocoa butter in accordance with the standard diet formula. The rats were fed for 6 wks before the onset of gestation. The control group was supplied a standard diet free from atherogenic components.
Table 1 Description of the percentages of diets constituents in control, diabetic and hypercholesterolemic groups.
2.3 Experimental animal work
This study and all procedures were approved by the Animal Care and Bioethics of the Egyptian Committee, and the animal work was done at Faculty of Science, Mansoura University. Eighty fertile male and virgin female rats of Wistar strain (Rattus norvegicus) (at a ratio of 1 male to 3 females) weighing approximately 125 g body weight, were obtained from Hellwan Breeding Farm, Ministry of Health, Egypt and used for experimentation. They were housed in cages with good ventilation on a 12-h light and dark cycle. Females were mated (1 male/3 females) overnight, and zero dates of gestation were determined the next morning by the presence of sperm in the vaginal smear. The pregnant rats were arranged into three groups (n = 20 per each) as follows: C, control; D, diabetic; H, hypercholesterolemic. Animals were maintained in free excess of diet as mentioned in and water ad Libitum. At the end of treatment, they were sacrificed by light diethyl ether anesthesia and dissected at 13, 15, 17, and 19 d prenatal. The tibia regions from hind limbs were separated immediately from 13, 15, 17, and 19 d-old embryos and investigated as follows:
2.4 Light microscopic investigations
Hind limb of fetuses of both control and experimentally-treated fetuses were incised at 13-&15d-old and fixed in 10% phosphate buffered formalin (pH 7.4), dehydrated in ascending grades of ethyl alcohol, cleared in xylene and mounted in molten paraplast at 58–62 °C. Serial 5-μm histological sections were cut and stained with hematoxylin and eosin and examined under bright-field light microscopy.
2.5 Transmission electron microscopic investigations
Tibia specimens of 15d-old fetuses of both control and experimental groups were separated and fixed immediately in 2% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 12 hours. After washing, the specimens were postfixed in a buffered solution of 1% osmium tetra oxide at 4 °C for 1.5 h, dehydrated in ascending grades of ethyl alcohol, and embedded in epoxy–resin. Ultrathin sections were cut with a diamond knife on an LKB Ultratome IV (LKB Instruments, Bromma, Sweden) and mounted on grids, stained with uranyl acetate and lead citrate, and examined under a Joel 100CX transmission electron microscope (Musashino 3-chome Akishima Tokyo 196- 8558, Japan).
2.6 Ossified length of femur, tibia and fibula
The ossification centers of hind limb of examined fetuses were careful staining with the alizarin red “S” method. The lengths of ossified femur, tibia and fibula were measured for ten fetuses per each developmental stage in all groups.
2.7 Determination of calcium in fetuses
Calcium content was assayed in fresh fetuses (n = 10) from both the control and experimental groups at 15,17 and 19-days prenatal. The fetuses were dissected and remove all the internal organs and dried in aven at 60 °C for 6 hours. Lipid extraction was carried out by using chloroform methyl mixture 2:1. The fetuses' skeleton were weighed and digested in 1 mL of nitric acid at highest purity and diluted with 4 mL bi-distilled water and measure calcium by atomic absorption spectrometry [Citation19].
2.8 Lactate dehydrogenase and alkaline and acid phosphatases isoenzymes electrophoresis
For determination of lactate dehydrogenase (LDH; 3.1.1.27), tissues were homogenized and centrifuged at 3000 × g for 5 min, 4 °C. One mL protein dye (0.001% bromophenol blue) plus 20 mL 2% sucrose were added and thirty μL of the mixture per gel slot was applied for each sample in enzyme electrophoresis. Polyacrylamide gel electrophoretic profiles of LDH isoenzymes were utilized and software analysis of gel scans done. Electrophoresis was essentially carried out according to Lehnert and Berlet [Citation20]. LDH enzyme bands were stained and visualized in the presence of l-lactate as the substrate according to the procedure of Shaw and Prasad [Citation21]. Then, the stained gels were fixed in 7% acetic acid (v/v) and documented via scanning on HP Deskjet F370 All-in-One computer assembly. Limb ALP is separated by electrophoresis through alkaline buffered (pH 9.1) according to [Citation22]. Electrophoresis was performed at a constant current at 2.5 mA per gel column at 18–20°C. Incubation for alkaline phosphatase (E.C.3.1.3.1) was carried out using Naphthol-AS-MX-phosphateas substrate and fast red violet LB salt as the coupler. Incubations of acid phosphatase (E.C.3.1.3.2) was undertaken at pH 5.0 using alpha-naphthyl phosphate as substrate and hexazotized pararosanilin as coupled agent, run in electrophoresis and processed for visualization and photographed [Citation23].
2.9 DNA fragmentation assay
DNA fragmentation was assayed by a modification of Duke and Sellins [Citation24]. Freshly isolated specimens were washed twice with ice-cold phosphate-buffered saline and suspended in 100 mL lysis buffer (10 mM Tris HCl/10 mM EDTA/0.5% Triton X-100, pH 8.0), vortex-mixed, sonicated, and incubated on ice for 20 min. After centrifugation at 4 °C (14,000 × g), the supernatant containing fragmented (soluble) DNA was mixed with lysis buffer (1 mL). Both samples were treated with RNAse A (0.5 mg/mL) for 1 h at 37 °C and then with proteinase-K (0.4 mg/mL; Sigma, St. Louis, MO, USA) for 1 h at 37 °C. After adding 20 mL of 5 M NaCl and 120 mL isopropanol, the samples were incubated overnight at 220 °C, and the DNA concentrations were determined and run on agarose gel electrophoresis and visualized.
2.10 Single cell gel electrophoresis (Comet assay)
Specimens of both control and experimental diseased groups were homogenized in chilled homogenizer buffer (pH 7.5) containing 75 mM NaCl and 24 mM Na2EDTA (pH 13), to obtain a 10% tissue solution. Six μL of the homogenate was suspended on 0.5% low melting agarose and sandwiched between a layer of 0.6% normal-melting agarose and a top layer of 0.5% agarose on fully frosted slides. The slides were kept on ice during the polymerization of each gel layer. After solidification, the slides were immersed in a lysis solution (1% sodium surcosinate, 2.5 m NaCl, 100 mM Na2EDTA, 10 mm Tris–HCl, 1% Triton X-100, and 10% DMSO) at 4 °C for 1 h, then placed in electrophoresis buffer to allow DNA to unwind. Electrophoresis was performed for 10 min at 300 mA and 1 V/cm. The slides were neutralized with Tris HCL buffer, pH 7.5, and stained with 20 mg/mL ethidium bromide. Each slide was analysed using a Leitz Orthoplan (Wetzlar, Germany) epifluorescence microscope. One hundred cells were analysed on each slide using the Comet assay II automatic digital analysis system [Citation25].
2.10.1 Statistical analysis
Statistical analysis was carried out between the control and each experimental group and for each developmental stage. Means and standard deviations were calculated for each result. Student's t test was applied and the differences were considered statistically significant from the control at P < 0.05.
3 Results
3.1 Light and ultrastructural observations
At light microscopic level, control 13-d old embryos exhibited the presence of ossification centers in the center of the diaphysis of both femur and tibia. The extra parts of the mentioned regions as well as tarsal, metatarsal and phalanges showed marked chondrification. The femur and tibial shafts were ensheathed by perichondrium sheath, several cell layers thick except the ossification centers, where sprouts of osteoblast progenitor cells become invade. The mid tarsal, metatarsal and phalanges possessed the presence of hypertrophied cartilage cells (Fig. 1 1A & B).
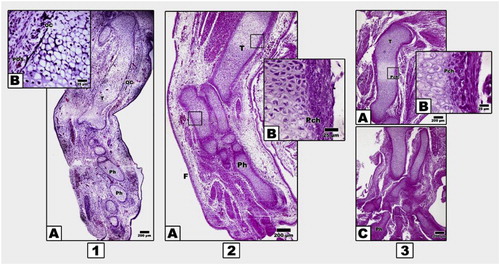
In contrast, 13 d-old embryos of both diabetic or hypercholesterolemic mothers exhibited delayed chondrification of both femur, tibia and distal tarsal, metatarsal and phalanges compared with the control. There is a marked comparatively reduced periosteal ossification centers in both femur and tibia (Fig. 2 2A–C).
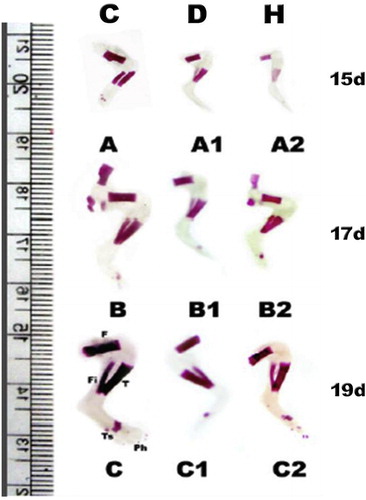
In control 15 d-old embryos, ossification proceeded in different parts of hind limb regions, with characteristic regular arrangement of cartilages zones within the limb regions. The ossification centers proceeded with marked progress of dark-brown deposits of mineralized calcium salts (Fig. 2, 1A–C). In contrast, 15 d-old embryos of either diabetic or hypercholsterolemic mothers exhibited comparatively reduction of ossification centers in comparison with the control. Chondrification of the distal phalanges was detected but not matched with the control (Fig. 2, 2A-D and 2, 3A–C).
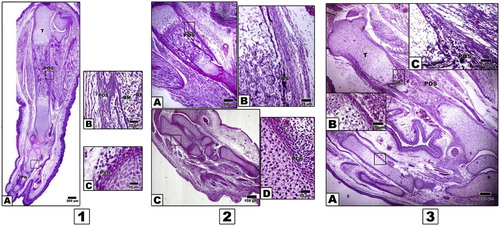
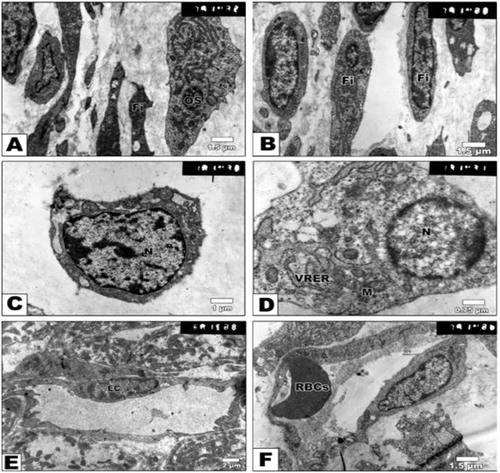
At the ultra-structural level, the control tibia of 15d-old fetuses possessed electron-dense mineralization calcium salts within the periosteum and in-between the hypertrophied chondrocytes. The periosteal sheath showed densely grouping fibroblast cells and characteristic bundles of collagen fibers. Nests of macrophages and osteoblast cells representing osteoprogenitor cells were detected closely adjacent to the calcified matrix of the breakdown chondrocytes. The osteoblast was characterized by the presence of a well-developed rough endoplasmic reticulum with dilated cisternae and large circular Golgi complex containing multiple stacks (Fig. 3 A–H).
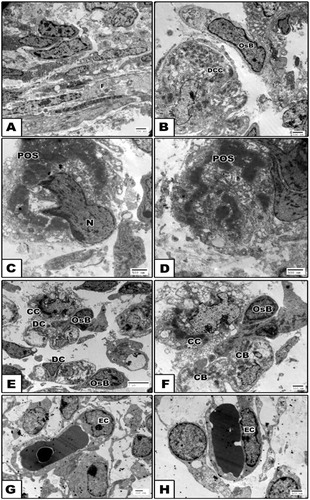
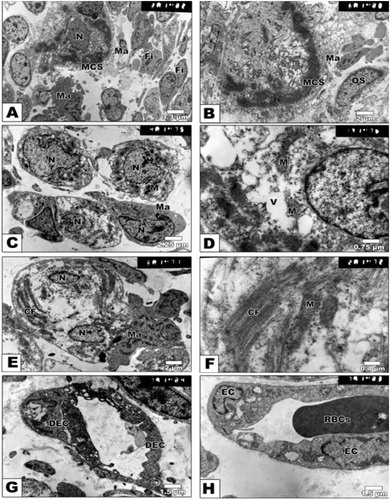
In contrast, embryos of either diabetic or hypercholesterolemic mothers exhibited malformed perichondrial sheath with peculiar presence of fibroblast cells lacking the cytoplasmic dendritic formation. The chondrocytes possessed vacuolated cytoplasm and presence of collagen fibers. Many of the chondrocytes attained considerable hypertrophy with the massive breakdown of their cytoplasmic compartments and nuclei with clumping of their nuclear chromatin (apoptosis) (Figs. 4 A–F and 5 A–H).
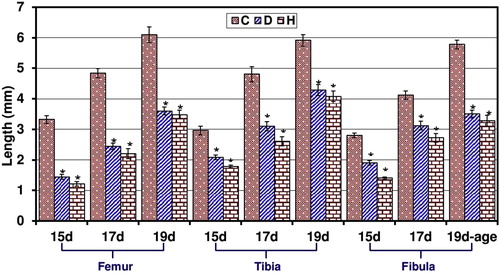
3.2 Ossified length of femur, tibia and fibula
From Figs. 6 and 7, the ossified length of femur, tibia and fibula of 15–19d-old fetuses of diabetic and hypercholesterolemic mothers showed a marked decrease comparing with the control.
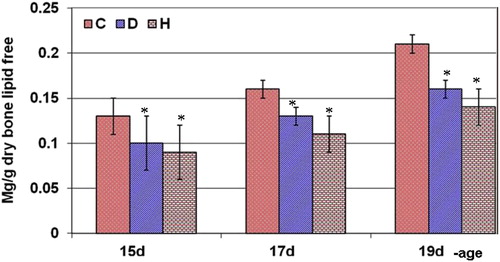
3.3 Fetuses calcium content
From Fig. 8, fetuses of diabetic and hypercholesterolemic mothers showed a marked reduction of calcium content.
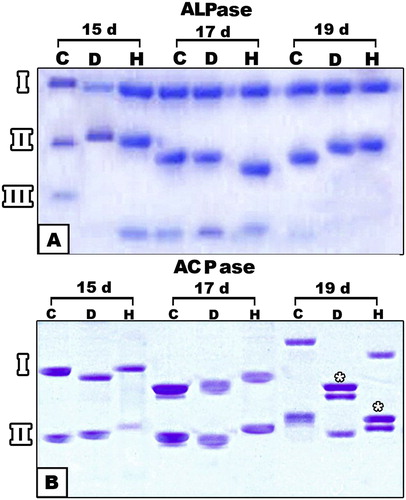
3.4 Differential isoenzyme alkaline and acid phosphatase and lactic dehydrogenase activity
Three bands of alkaline phosphatase isoenzymes are expressed in control bone specimens of 15,17 and 19 d-old fetuses. The rate of diffusion was markedly increased in fetuses of diabetic and hypercholesterolemic mothers, especially at 17 d-old. Isoenzyme fraction III appeared missing in 19 d-old diabetic and hypercholesterolemic fetuses. On the other hand, the intensity of acid phosphatase isoenzymes was markedly reduced. Separation of the isoenzyme and formation of the double band was detected in 19 d-old fetuses of diabetic or hypercholesterolemic groups (Fig. 9).
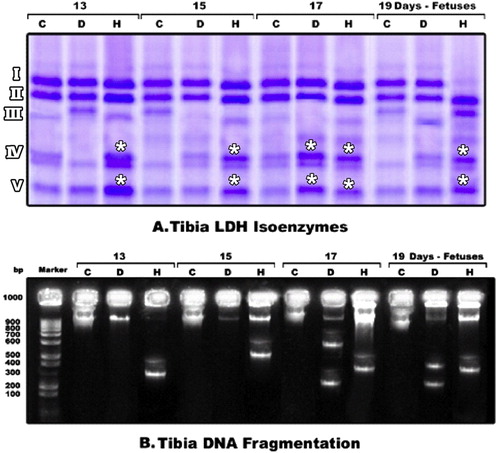
The cartilage and bone elements lactic dehydrogenase express five isoenzymes (LDH1-LDH5) (Fig. 10A). Both diabetes or hypercholesterolemic groups exhibited either no expression of their isoenzyme bands or a decrease in its intensity in 15 d-old embryos. The expression of isoenzymes was markedly detected in 17 and 19 d-old tibia of embryos of diseased groups. However, double bands of isoenzyme IV were clearly detected in hypercholesteromic-groups as well as increased intensities of the expression of fraction V.
3.5 Genomic DNA fragmentation
The genomic expression of the degree of laddering (total DNA fragmented) increased in cartilage and bone cells of fetuses maternally diabetic or hypercholesterolemic. The highest incidence of genomic DNA fragmentation was markedly increased in skeletal cells of diabetic mothers (Fig. 10B).
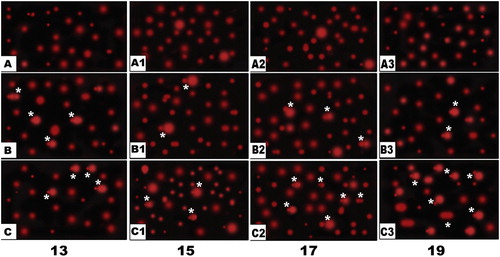
Also, following application of the Comet assay (single-cell gel electrophoresis), the single-strand nucleotide/each skeletal cell of fetuses of both diabetic or hypercholesterolemic mothers were streached with increased tail length and DNA concentration, compared with normal pattern structure manifesting apoptic cell death (Fig. 11).
4 Discussion
Maintenance of skeletal integrity involves a dynamic biological equilibrium between osteoclast-induced bone resorption and osteoblast-mediated bone formation. Ossification of the limb is carried throughout the endochondral period [Citation26]. According to Polanco Ponce et al. [Citation27], maternal diabetes in rats was found to alter fetal development in a very similar manner to that of humans.
The observed findings are the first to reveal that the developing rat fetuses at 13 d-old prenatal of either diabetic or hypercholesterolemic mothers showed a marked delay of histogenesis of perichondrium and periosteal ossification of hind limb. There was a qualitative decrease of both mineralized calcium salts and cartilage-bone formation. Ultra-structurally, both chondroblast and osteoblast cell's differentiation were markedly delayed associated with deformed osteoprogenitor cells within the periosteum sheath, predicting the primitive osteoid bone formation. The observed cytological findings were confirmed by a marked decrease of the ossified length of limb bones as well as decreased mineralized calcium salts in the skeleton of 15, 17 and 19-d old fetuses of diabetic or hypercholesterolemic mothers.
The present findings support the work of Eidem et al. [Citation28] and El-Sayyad et al. [Citation29], who studied the effects of either diabetes or hypercholesterolemia in pregnant mother and their offspring. Our findings declare that decreased ossification resulted from a reduction of calcium contents of embryonic bone tissues. Ossification defects may be attributed either to disruption of either the Ca2 supply or the bone maturation itself [Citation30], or decreased placental transport capacity [Citation31]. The ossification process in the fetus itself was deficient, as indicated by decreased osteocalcin levels [Citation32]. Retarded both bone growth [Citation33,Citation34] as well bone marrow osteogenic progenitor cells [Citation35] were the main consequences of diabetes. Also, the mentioned authors mentioned that diabetes cause a significantly decrease in levels of chondroitin sulphate and heparan sulphate in the vertebrae of ovariectomized diabetic rats. Both chondroitin sulphate and heparan sulphate represent the main sources of cartilage and bone formation [Citation36]. Violation of the oxidant/antioxidant balance by high glucose concentrations can cause massive cell damage, increase in apoptotic events and defective embryonic development [Citation37,Citation38]. Viccica et al. [Citation39] reported that cholesterol was synthesized by the liver and secreted as circulating lipoproteins and plays a role in osteoblast differentiation. Also, bone growth defects may be attributed to the pathogenesis of non-alcoholic steatohepatits induced by hypercholesterolemia [Citation7] as mentioned by Nakano et al. [Citation40]. The liver represents the main target of conversion of cholecalciferol (vitamin D3) to 25-hydroxyvitamin D3 as well as 25-hydroxyvitamin D2 [Citation41] which regulating the concentration of calcium and phosphate in the bloodstream and promoting remodeling of bone [Citation42]. Liver damage may disrupt bone differention through reducing vitamin D synthesis.
From our findings, diabetes or hypercholesterolemia exhibited missing expression of lactic dehydrogenase isoenzyme III in 15 d-old embryos. However, double bands of isoenzyme IV were clearly detected in hypercholesteromic-groups as well as increased intensities of the expression of fraction V. These alterations of the isoenzymes reflect alterations of their metabolic activities and may share in bone cell's damage. Lactate dehydrogenase (LDH) has been used extensively as a marker for cell death both in vitro and in vivo [Citation43]. It is more active in osteocytes [Citation44]. Also dehydrogenases except (glutamic dehydrogenase) were detected in osteocytes, osteoblasts and osteoclasts in mandible of 5 day old rat. The bone cells possess fully functional citric acid cycles, pentose cycles, and the capacity to metabolize fatty acids and carbohydrates [Citation45]. Experimental diabetes was found to be associated with alterations of oxidative and glycolytic enzymes, including lactic dehydrogenase [Citation46].
Also, there was a marked alterations of bone alkaline and acid phosphatase isoenzymes. The isoenzyme fraction III of alkaline phosphase showed increased diffusion rates and missing in 19 day-old fetuses. Acid phosphatase isoenzymes showed marked reduction of their intensities and increase diffusion rate. Bone-alkaline phosphatase is a useful parameter for monitoring changes in bone formation [Citation47]. The distribution of acid and alkaline phosphatase activity were identified in osteoclasts and adjacent osteocytes extracellularly lining Howship's lacunae in endosteal resorbing surfaces of rat tibia [Citation48].
Also, feeding on diet high of cholesterol level revealed a reduction of its utilization by fetal tissues, impairing growth and consequently increasing the incidence of developmental defects, including morphological and delayed ossification of bones. The estimated ossified length of hind limb in fetuses of hypercholesterolemic mothers showed apparent reduction. The fetal growth defects may be associated with the damage of myocardium and the development of atherosclerosis in blood vessels, which reduces the transport of nutrients and oxygen to tissues, as well as the cytotoxicity of hypercholesterolemia [Citation6].
There a close association between both diabetes [Citation49] or hypercholesterolemia [Citation50] and disruption of vitamin D synthesis via decrease serum concentrations of 25-hydroxycholecalciferol which intern impair bone cell differentiation.
Also, there was a detected increase of DNA fragmentation in bone tissues of fetuses of either hypercholesterolemic or diabetic mothers. Chronic diabetes is one of the causes of reactive oxygen species formation [Citation51] where the cellular response to reactive oxygen species led to the formation of severe metabolic dysfunction, peroxidation of membrane lipids, oxidative protein damage, alteration of cytoplasmic and nuclear signal transduction, and DNA damage [Citation52].
Takasu et al. [Citation53] stated that DNA fragmentation supposedly resulted from the accumulation of superoxide or hydroxyl radicals.
Also, our findings revealed that defects in blood vessel formation during development of perichondrial and periosteal sheath of the tibia of 15 d-old embryos of diabetic or hypercholesterolemic mothers may reduce nutrient and oxygen demands of skeletal elements, decreasing their differentiation and mineralization.
As we know that the risk of peripheral vascular disease is increased in diabetic and hypercholesterolemic patients. Endothelial dysfunction, vascular smooth muscle cell dysfunction, inflammation and hypercoagubility are the key factors for peripheral vascular disease [Citation6,Citation54]. These complications may cause ischemia during bone differentiation and consequently retarded cell growth and development.
Finally, we conclude that maternal diabetes and hypercholesterolemia have a selective, dramatic effect during fetus development, altering the histo- and cytological differentiation and retarding bone growth. On the other hand, maternal diabetes and hypercholesterolemia increased the rate of cell death in skeletal elements and blood vessels as a result of increased oxidative stress and altered lactic dehydrogenase marker of cell damage in accordance with DNA damage.
Competing interests
The authors declare they have no competing interests.
Funding
There are no funding sources to declare.
Notes
Peer review under responsibility of Mansoura University.
References
- T.MajimaA.ShimatsuY.KomatsuN.SatohA.FukaoK.Ninomiyaet alIncreased bone turnover in patients with hypercholesterolemiaEndocr J512008143151
- M.L.IsidroB.RuanoBone disease in diabetesCurr Diabetes Rev632010144155
- T.1O'BrienT.T.NguyenB.R.ZimmermanHyperlipidemia and diabetes mellitusMayo Clin Proc73101998969976
- S.M.HwangJ.S.KimY.J.LeeJ.J.YoonS.M.LeeD.G.Kanget alAnti-diabetic atherosclerosis effect of Prunella vulgaris in db/db mice with type 2 diabetesAm J Chin Med402012937951
- K.PrasadE.D.McNairA.M.QureshiG.Casper-BellVitamin E slows the progression of hypercholesterolemia-induced oxidative stress in heart, liver and kidneyMol Cell Biochem3682012181187
- H.I.El-SayyadM.S.Al-HaggarH.A.El-GhawetI.H.BakrCardiomyopathy and angiogenesis defects of Wistar rat fetuses of diabetic and hypercholesterolemic mothersNutrition2820123343
- H.I.H.El-SayyadM.M.S.Al-HaggarH.A.El-GhawetI.H.M.BakrEffect of maternal diabetes and hypercholesterolemia on fetal liver of albino Wistar ratsNutrition302014326336
- J.C.KrakauerM.J.McKennaN.F.BudererD.S.RaoF.W.WhitehouseA.M.ParfittBone loss and bone turnover in diabetesDiabetes441995775782
- M.N.Horcajada-MolteniB.ChanteranneP.LebecqueM.J.DaviccoV.CoxamA.Younget alAmylin and bone metabolism in streptozotocin-induced diabetic ratsJ Bone Min Res162001958965
- H.LuD.KrautL.C.GerstenfeldD.T.GravesDiabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiationEndocrinology1442003346352
- H.HeR.LiuT.DestaC.LeoneL.C.GerstenfeldD.T.GravesDiabetes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone lossEndocrinology1452004447452
- R.A.KayalD.TsatsasM.A.BauerB.AllenM.O.Al-SebaeiS.Kakaret alDiminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activityJ Bone Min Res222007560568
- H.Y.WonJ.B.ParkE.Y.ParkK.D.RiewEffect of hyperglycemia on apoptosis of notochordal cells and intervertebral disc degeneration in diabetic ratsJ Neurosurg Spine112009741748
- K.J.JepsenThe aging cortex: to crack or not to crackOsteoporos Int1452003S57S62
- K.FullerC.MurphyB.KirsteinS.W.FoxT.J.ChambersTNFalpha potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKLEndocrinology143200211081118
- M.FunabaT.MurataE.MurataM.AbeM.TakahashiK.ToriiIncreased cartilage and bone formation in spontaneously hypercholesterolemic ratsLife Sci611997645652
- S.P.PovoskiP.J.McCulloughW.ZhouR.H.BellJr.Induction of diabetes mellitus in Syrian golden hamsters using stored equilibrium solutions of streptozotocinLab Anim Sci431993310314
- B.EnkhmaaK.ShiwakuE.AnuuradA.NogiK.KitajimaM.Yamasakiet alPrevalence of the metabolic syndrome using the third report of the national cholesterol educational program expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (ATP III) and the modified ATP III definitions for Japanese and MongoliansClin Chim Acta3522005105113
- J.ScancarR.MilacicM.BenedikP.BukovecDetermination of trace elements and calcium in bone of the human iliac crest by atomic absorption spectrometryClin Chim Acta2931–22000187192
- T.LehnertH.H.BerletSelective inactivation of lactate dehydrogenase of rat tissues by sodium deoxycholateBiochem J1771979813818
- C.R.ShawR.PrasadStarch gel electrophoresis of enzymes–a compilation of recipesBiochem Genet41970297320
- L.SundbladM.Wallin-NilssonJ.BrohultCharacterization of alkaline phosphatase isoenzymes in serum by agar gel electrophoresisClin Chim Acta4531973219223
- A.FrazenG.HasselgrenElectrophoresis separation of alkaline and acid phosphatase isoenzymes from the pulp of monkey teethActa Odontol Scand3661978371375
- R.C.DukeC.B.SellinsCellular Basis of Immune ModulationJ.G.KaplanD.R.GreenR.C.Bleackley19th International leukocyte culture conference1989AR LissNew York311314
- Y.F.SasakiA.SagaM.AkasakaK.YoshidaE.NishidateY.Q.Suet alIn vivo genotoxicity of ortho-phenylphenol, biphenyl, and thiabendazole detected in multiple mouse organs by the alkaline single cell gel electrophoresis assayMutat Res3951997189198
- R.P.HeaneyThe bone-remodeling transient: implications for the interpretation of clinical studies of bone mass changeJ Bone Min Res9199415151523
- A.C.Polanco PonceM.C.Revilla MonsalveM.A.Palomino GaribayS.Islas AndradeEffect of maternal diabetes on human and rat fetal developmentGinecol Obstet Mex732005544552
- I.EidemL.C.SteneT.HenriksenK.F.HanssenS.VangenS.E.Vollsetet alCongenital anomalies in newborns of women with type 1 diabetes: nationwide population-based study in Norway, 1999–2004Acta Obstet Gynecol Scand89201014031411
- H.I.El-SayyadA.M.Abou-El-NagaA.A.GadallahI.H.BakrProtective effects of Allium sativum against defects of hypercholesterolemia on pregnant rats and their offspringInt J Clin Exp Med32010152163
- H.O.GarlandA.G.ForshawC.P.SibleyDietary essential fatty acid supplementation, urinary calcium excretion and reproductive performance in the diabetic pregnant ratJ Endocrinol1531997357363
- S.M.HusainT.J.BirdseyJ.D.GlazierM.Z.MughalH.O.GarlandC.P.SibleyEffect of diabetes mellitus on maternofetal flux of calcium and magnesium and calbindin9K mRNA expression in rat placentaPediatr Res351994376381
- J.VerhaegheE.Van HerckR.BouillonUmbilical cord osteocalcin in normal pregnancies and pregnancies complicated by fetal growth retardation or diabetes mellitusBiol Neonate681995377383
- J.R.FunkJ.E.HaleD.CarminesH.L.GoochS.R.HurwitzBiomechanical evaluation of early fracture healing in normal and diabetic ratsJ Orthop Res182000126132
- A.DostT.RohrerJ.FusseneggerC.VogelB.SchenkM.Wabitschet alBone maturation in 1788 children and adolescents with diabetes mellitus type 1J Pediatr Endocrinol Metab232010891898
- M.J.TolosaS.R.ChuguranskyC.SedlinskyL.SchurmanA.D.McCarthyM.S.Molinuevoet alInsulin-deficient diabetes-induced bone microarchitecture alterations are associated with a decrease in the osteogenic potential of bone marrow progenitor cells: preventive effects of metforminDib Res Clin Prac2013177186
- V.GopalakrishnanJ.ArunakaranM.M.AruldhasN.SrinivasanEffects of streptozotocin-induced diabetes mellitus on some bone turnover markers in the vertebrae of ovary-intact and ovariectomized adult ratsBiochem Cell Biol8452006728736
- S.W.ZangenP.YaffeS.ShechtmanD.H.ZangenA.OrnoyThe role of reactive oxygen species in diabetes-induced anomalies in embryos of Cohen diabetic ratsInt J Exp Diabetes Res32002247255
- J.G.ErikssonH.YliharsilaT.ForsenC.OsmondD.J.BarkerExercise protects against glucose intolerance in individuals with a small body size at birthPrev Med392004164167
- G.ViccicaE.VignaliC.MarcocciRole of the cholesterol biosynthetic pathway in osteoblastic differentiationJ Endocrinol Invest302007812
- A.NakanoT.KandaH.AbeBone changes and mineral metabolism disorders in rats with experimental liver cirrhosisJ Gastroenterol Hepatol1112199611431154
- B.W.HollisAssessment of vitamin D nutritional and hormonal status: what to measure and how to do itCalcif Tissue Int581199645
- S.NairVitamin D deficiency and liver diseaseGastroenterology Hepatology682010491493
- M.AllenP.MillettE.DawesN.RushtonLactate dehydrogenase activity as a rapid and sensitive test for the quantification of cell numbers in vitroClin Mater1641994189194
- S.Y.P.WongC.R.DunstanR.A.EvansE.HillsThe determination bone viability: a histochemical method for identification of lactate dehydrogenase activity in osteocytes in fresh calcified and decalcified sections of human bonePathology1441982430442
- H.M.FullmerDehydrogenases in developing bone in the ratJ Histochem Cytochem1231964210214
- G.PyK.LambertO.MilhavetN.EydouxC.PrefautJ.MercierEffects of streptozotocin-induced diabetes on markers of skeletal muscle metabolism and monocarboxylate transporter 1 to monocarboxylate transporter 4 transportersMetabolism512002807813
- C.OhlssonJ.IsgaardT.TönellA.NilssonO.G.P.IsakssonA.LindahlEndocrine regulation of longitudinal bone growthActa PaediatrSuppl. 39119933340
- J.E.WergedalD.J.BaylinkDistribution of acid and alkaline phosphatase activity in undemineralized sections of the rat tibia diaphysisJ Histochem Cytochem17121969799806
- A.TsurB.S.FeldmanI.FeldhammerM.B.HoshenG.LeibowitzR.D.BalicerDecreased serum concentrations of 25-hydroxycholecalciferol are associated with increased risk of progression to impaired fasting glucose and diabetesDiabetes Care365201313611367
- G.K.GuptaT.AgrawalM.G.DelCoreS.M.MohiuddinD.K.AgrawalVitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swineExp Mol Pathol93120128290
- M.LodoviciL.GiovannelliV.PitozziE.BigagliG.BardiniC.M.RotellaOxidative DNA damage and plasma antioxidant capacity in type 2 diabetic patients with good and poor glycaemic controlMutat Res638200898102
- N.IshiiZ.OgawaK.SuzukiK.NumakamiT.SarutaH.ItohGlucose loading induces DNA fragmentation in rat proximal tubular cellsMetabolism45199613481353
- N.TakasuI.KomiyaT.AsawaY.NagasawaT.YamadaStreptozocin- and alloxan-induced H2O2 generation and DNA fragmentation in pancreatic islets. H2O2 as mediator for DNA fragmentationDiabetes40199111411145
- E.HuysmanC.MathieuDiabetes and peripheral vascular diseaseActa Chir Belg10952009587594