Abstract
Adipose tissue regulates insulin sensitivity via the circulating adipocytokines, leptin, resistin and adiponectin. Hypoadiponectinemia contributes to the development of obesity and related disorders such as diabetes, hyperlipidemia, and cardiovascular diseases. In this study, we investigated the effects of Brazilian propolis on adiponectin levels in type 2 diabetes mellitus (T2DM), the mechanism of signaling pathway was explored as well. T2DM was induced in male Wistar rats using high fat diet and low dose of streptozotocin (STZ, 35 mg/kg, i.p.). Propolis was administered by oral tubes. Peroxisome proliferator activated receptor gamma (PPARγ) levels in sub abdominal adipose tissue, serum levels of adiponectin, tumor necrosis factor-α (TNF-α) and insulin were detected by Enzyme Linked Immunosorbent Assay (ELISA). Malondialdehyde (MDA) and reduced glutathione (GSH) in sub abdominal adipose tissue, fasting plasma glucose, plasma triglycerides and total cholesterol levels were measured by colorimetric method. Results showed that Brazilian propolis ameliorated hypoadiponectinemia in T2DM rats and relieved high glucose-induced adiponectin decrease. The signaling pathway analysis indicated that PPARγ regulation was involved. In conclusion, Brazilian propolis could have beneficial effect in T2DM by increasing tissue PPARγ levels, restoring serum adiponectin levels, enhancing insulin sensitivity and subsequently, attenuating elevated glucose level.
1 Introduction
It is undeniable to say that there are more than 194 million people with diabetes worldwide [Citation1,Citation2]. Diabetes mellitus is characterized by high blood glucose levels and is associated with devastating and life-threatening complications that affect various body organs, such as blood vessels, eyes, kidneys and nerves [Citation3,Citation4]. Among different types of diabetes mellitus, Type 2 account for 90% of diagnosed patients. Type 2 diabetes mellitus (T2DM) is a metabolic syndrome, which is characterized by both fat accumulation and impairment in insulin action, insulin production, or both; a condition called insulin resistance. Insulin resistance leads to the development of hyperglycemia. Such harmful hyperglycemia produced tissue damaging glucotoxicity, which is the major cause of diabetic complications [Citation5]. In addition, abnormal metabolism of accumulated fat in adipose tissues can cause lipotoxicity, which can further exacerbate diabetic complications [Citation6].
The management of T2DM entails lifestyle modification and/or pharmaceutical treatment such as insulin, biguanides, sulfonylureas, and alpha glucosidase inhibitors. However, these anti-diabetic medications are far from being satisfactory because of limited efficacy and many undesirable side effects [Citation7]. As a consequence, T2DM is still an incurable disease with poor quality of life, high morbidity and mortality. Thereby, the social and economic burdens of this disease pose an urgent need for development of novel therapeutic strategies for treatment with satisfactory efficacy and no adverse effects [Citation8].
Adipose tissue secretes many proteins and hormones such as adipocytokines, resistin, leptin, and adiponectin to control insulin sensitivity [Citation9]. Adiponectin is a protein hormone secreted into the blood stream in average 0.01% of total plasma proteins. The important role of adiponectin comes from modulation of glucose and lipid metabolism in insulin sensitive tissue [Citation10,Citation11]. Accumulating evidence showed that hypoadiponectinemia played a key role in the pathogenesis of obesity and related diseases [Citation12,Citation13]. Furthermore, adiponectin administration to obese or diabetic mice can reduce body weight and blood glucose levels while enhancing insulin sensitivity [Citation14,Citation15]. Based on these data, adiponectin was conceived to be a novel therapy target for obesity and insulin resistance [Citation16].
Various factors are involved in regulation of adiponectin expression. These factors include, peroxisome proliferator-activated receptor (PPAR-γ), CCAAT-enhancer-binding protein (C/EBP) α, Kruppel-like factor 7 (KLF7), and sterol regulatory element binding protein-1c (SREBP-1c). Among these factors, PPARγ is recognized as the master regulator of gene transcription and plasma concentrations of adiponectin [Citation9]. PPARγ binds directly to a functional PPAR-responsive element (PPRE) in adiponectin promoter, leading to enhancement of adiponectin gene transcription [Citation17]. Indeed, adiponectin was believed to be a marker for activity of PPARγ [Citation18].
Propolis (Brazilian) is a sticky resinous mixture that honey bees collect from tree buds, sap flows, or other botanical sources. Its color varies depending on its botanical source, the most common being dark brown [Citation19]. The chemical compositions of propolis are mainly flavonoids, aromatic acids and esters, aldehydes and ketones, fatty acids and esters, terpenes, steroids, amino acids, polysaccharides, hydrocarbon, alcohol, hydroxybenzene and other compounds [Citation20]. Brazilian propolis composed mainly of phenolic compounds artepillin C. Besides, it was reported to contain 3-prenyl-4-hydroxycinnamic, p-coumaric, caffeic acid, and caffeoylquinic acids, cinnamic acids and the flavonoids pinobanksin and kaempferol [Citation21]. Brazilian propolis has been reported to possess various biological activities including antioxidant, anti-microbial, liver protective, immunoregulatory, anti-inflammatory, and anticancer effects [Citation22]. In addition, it was reported to have hypoglycemic and hypolipidemic effects. Further, propolis was also demonstrated to control metabolic disorders in diabetic rats and to accelerate the tissue regeneration and repair of damaged pancreatic cell [Citation23].
A recent study demonstrated that Brazilian propolis restored obesity-induced down regulation of adiponectin expression. In view of this recent claim, we investigated the effect of Brazilian propolis on adiponectin levels in T2DM induced experimentally in rats [Citation24]. The signaling pathway mechanism was explored along with the regulatory roles of PPARγ.
2 Materials and methods
2.1 Ethics statement
Experimental design and animal handling were according to the guidelines of the Ethical Committee of the Faculty of Pharmacy, Mansoura University, for Animal Use.
2.2 Animals
Male Sprague Dawley rats (160–180 mg) were housed in a certified animal care at a constant temperature (22 °C) under a 12-hour light–dark cycle, and were provided with standard rat food and water.
2.3 Experimental design
The rats were randomly divided into 3 groups with two dietary regimens. Group1 (control group) was fed certified standard chow; Group2 (diabetic untreated group) was fed high fat (HF) diet. Group3 (diabetic group treated by Brazilian propolis) was fed HF diet for an initial period of 2 weeks without treatment. HF diet consists of (58% fat, 25% protein and 17% carbohydrate, as a percentage of total kcal) [Citation25]. After the 2 weeks of dietary manipulation, the rats from group 2 and 3 were injected intraperitoneally (i.p.) with low dose of STZ (Sigma-Aldrich Co, St Louis, MO) (35 mg kg−1) after overnight fasting [Citation26]. The rats with blood glucose levels ≥250 mg/dl were considered diabetic and selected for further studies. The rats were allowed to continue to feed on their respective diets until the end of the study [Citation27]. Type 2 diabetic rats in group 3 were treated with propolis (in aqueous solution, 0.6 g/kg), by oral tube for 21 days. The dose used for propolis in this study was in the range used in other studies applied for the same animal species [Citation28].
At the end of the study, the rats were fasted overnight, and then sacrificed. Blood samples were collected via puncture of retro-orbital venous plexus using heparinized capillary hematocrit tubes. Blood was centrifuged at 3000 rpm for 5 minutes, and then plasma and serum samples were separated for determination of the biochemical parameters. Rats' sub abdominal adipose tissues were isolated, weighed and then homogenized in a 10-fold volume of ice-cold sodium potassium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl. The homogenates were centrifuged at 3000 rpm at 4 °C for 10 minutes and immediately used for determination of oxidative stress or stored at −80 °C until used.
2.4 Assessment of biochemical parameters
Fasting plasma glucose concentrations were determined using the glucose oxidase method, and Triglyceride (TG) and total cholesterol (TC) were assayed using calorimetric kits purchased from Biodiagnostic Company (Egypt, Cairo), according to manufacturer's instructions.
2.5 Assessment of oxidative stress
Malondialdehyde (MDA) and reduced glutathione (GSH) were estimated in sub abdominal adipose tissue using commercial kits from Biodiagnostic Company (Egypt, Cairo), according to manufacturer's instructions.
2.6 Enzyme-linked immunosorbent assay (ELISA)
ELISA technique was used to assess serum adiponectin concentration, serum tumor necrosis factor-α (TNF-α), circulating insulin and PPARγ concentration in sub abdominal adipose tissue according to manufacturer's instructions. The kits were purchased from MyBioSource Company (5520 Hubner Rd, San Diego, CA 92105, United States).
2.7 Statistical analysis
Results are expressed as means ± SEM of 6 animals, and differences between groups were tested for significance using analysis of variance (ANOVA), followed by Tukey's post hoc test. The level of statistical significance was taken at P ≤ 0.05. Statistical analysis of the experimental data was performed using the statistical package SPSS as the definitive analyzer of drug effects.
3 Results
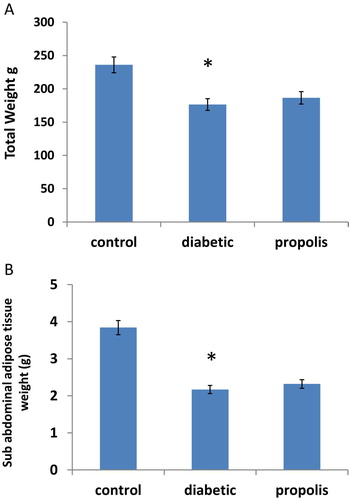
3.1 Effect of Brazilian propolis on body Weight and Sub abdominal adipose tissue weight
As shown in Fig. 1A, B, diabetes induction resulted in significant decrease in body weight by 25.25% and marked reduction in sub abdominal adipose tissue weight by 43.49% without affecting the food intake compared to control group. However, propolis treatment increased body weight by 1.05 folds and sub abdominal adipose tissue weight by 1.07 folds without affecting the food intake compared to diabetic group.
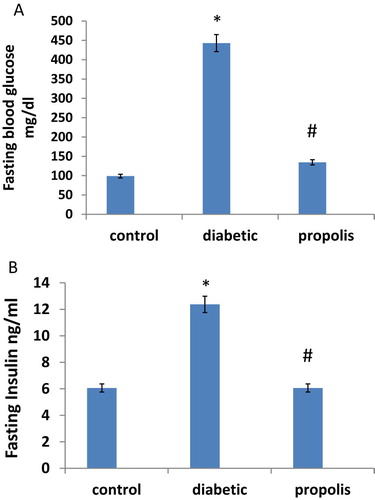
3.2 Effect of Brazilian propolis on fasting blood glucose and fasting insulin levels
Fasting blood glucose and fasting serum insulin levels (M ± SE) in diabetic and control groups are illustrated in Fig. 2A, B. Fasting blood glucose increased 4.48 fold in diabetic group when compared to control group. Besides, fasting serum insulin increased 2.05 folds in diabetic group when compared to control group. Propolis treatment reduced fasting blood glucose level by 69.52% and reduced fasting insulin level by 50% compared to diabetic group.
3.3 Effect of Brazilian propolis on lipid profile
The effects of propolis on lipid profile in rats are given in . The results showed that propolis treatment significantly increased the serum levels of high density lipoprotein 3.99 fold compared to diabetic group. In parallel, propolis treatment markedly decreased low density lipoprotein, total cholesterol, triglyceride, total lipid and very low density lipoprotein compared to diabetic group (P < 0.05).
Table 1 Effect of Brazilian propolis treatment on the level of total cholesterol, total lipids, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, Triglyceride (TG) in rats with type 2 induced diabetes mellitus (Mean ± SE).
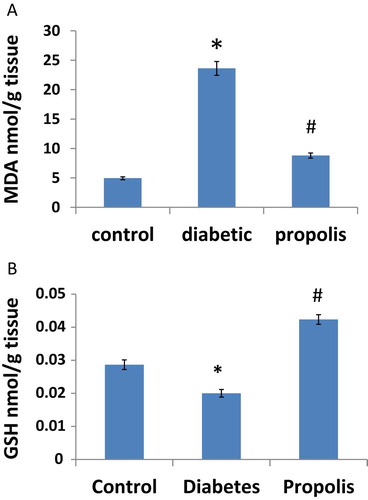
3.4 Effects of Brazilian propolis on oxidative stress
Lipid peroxides in sub abdominal adipose tissue were measured as MDA. Results showed that MDA increased 4.78 fold in diabetic group when compared to control group. However, Propolis treatment reduced MDA by 58.5% compared to diabetic group, Fig. 3A. On the other hand, diabetes significantly reduced GSH levels in sub abdominal adipose tissue of diabetic rats by 31.3% compared to control group. Propolis treatment restored GSH levels in sub abdominal adipose tissue of treated group compared to diabetic group (P < 0.05), Fig. 3B.
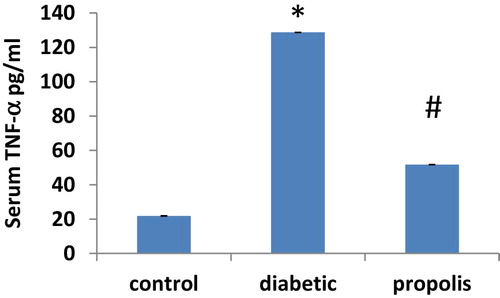
3.5 Effect of Brazilian propolis treatment on TNF-α
As illustrated in Fig. 4, serum TNF-α level increased 5.89 fold in diabetic group when compared to control group. However, propolis treatment reduced TNF-α by 59.78% compared to diabetic group.
3.6 Effect of Brazilian propolis treatment on adiponectin and PPARγ concentration
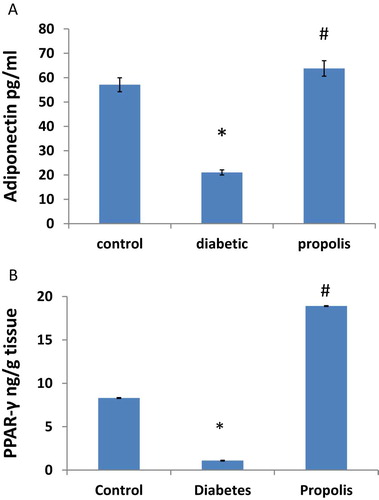
As illustrated in Fig. 5A, B, serum adiponectin decreased by 63.01% in the diabetic group when compared to control group. In addition, sub abdominal adipose tissue (PPAR-γ) levels decreased by 87.71% in diabetic group when compared to control group. On the other hand, propolis treatment markedly increased the serum levels of adiponectin 3 folds and increased the concentration of PPARγ 1.9 fold compared to diabetic group.
3.7 Correlation analysis of studied parameters
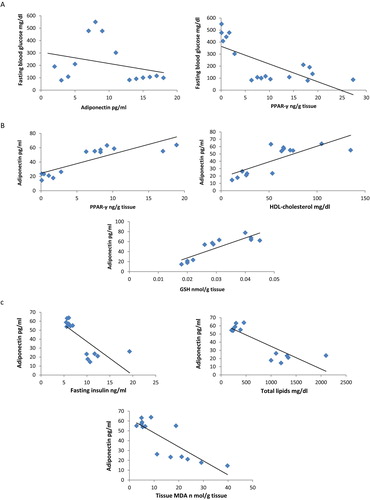
Results illustrated in Fig. 6 showed that fasting blood glucose level negatively correlated with serum adiponectin (r = −0.3, p < 0.05) and PPAR-γ levels (r = −0.7, p < 0.05). In addition, serum adiponectin positively correlated with PPAR-γ (r = 0.677, p < 0.05) and HDL-cholesterol (r = 0.77, p < 0.05) and GSH (r = 0.869, p < 0.05). Moreover, serum adiponectin correlated negatively with insulin (r = −0.765, p < 0.05), total lipids (r = −0.86, p < 0.05), MDA (r = −0.82, p < 0.05).
4 Discussion
The results of the present investigation confirmed earlier reports that propolis treatment could almost control the hyperglycemia in the STZ-induced diabetic rat model [Citation29]. The glycemic control achieved by Brazilian propolis treatment could be due to increasing adiponectin levels, up-regulation of PPARγ levels and enhancing insulin sensitivity.
At the first steps, diabetes induction by STZ caused rapid reduction in body weight. This was in agreement with previous reports [Citation30]. The body weight loss in diabetic rats could be explained by many reasons, including dehydration as well as excessive fats and proteins catabolism [Citation31], which ultimately leads to muscle wasting [Citation32]. On the contrary, propolis treated rats showed non significant increase in body weight compared to control group, which could be attributed to better control of hyperglycemic state compared to the untreated diabetic group.
Several studies have documented the association between diabetes mellitus and abnormalities in lipid metabolism [Citation33]. Dyslipidemia is believed to be a major risk factor for development of various diabetic complications. Diabetes-associated dyslipidemia resulted from excessive production of free fatty acids along with abnormal lipoprotein metabolism. Hence, diabetes mellitus is associated with an increase in TG and LDL, and decrease in HDL [Citation34]. Similarly, the results of our investigation revealed disturbance in lipid metabolism in diabetic untreated rats. These effects were attenuated by propolis treatment. Of note, our findings provide ample support to the notion that propolis preparations could modulate lipid metabolism [Citation35].
Insulin resistance is considered as a hallmark of T2DM. Accumulated fat in different body cells disturb their response to insulin, leading to insulin resistance and elevated blood glucose levels [Citation36]. Previous studies have convincingly showed that propolis treatment decreased insulin resistance in obese diabetic rats [Citation37]. In addition, propolis was found to enhance translocation of glucose transporter 4 and glucose uptake in mouse myocyte cell lines, as well as in the ICR mouse strain [Citation38]. In confirmation with these reports, our results demonstrated that propolis markedly reduced fasting plasma glucose level in treated rats compared to untreated diabetic groups. This suggested that propolis could be a beneficial anti-hyperglycemic agent in T2DM.
As already noted, lipid peroxidation plays a significant role among oxidative defects that damages β cells in T2DM [Citation39]. Convincing evidence has established a link between oxidative stress and insulin resistance. Increased free radical levels have deleterious effects on β cells, including decreased insulin secretion in response to glucose, impaired gene expression and cell death, leading ultimately to hyperglycemia and diabetes [Citation40]. Moreover, Elevated free radical concentrations stimulate various signaling pathways that lead eventually to degradation of insulin receptors [Citation41]. Therefore, targeting oxidative stress could be a potential therapeutic approach in T2DM. In the present study, diabetic treated rats showed significantly lower MDA levels and restored GSH levels nearing normal control values. This finding is consistent with the earlier report that propolis caused the partial restoration of β-cell function, possibly by an antioxidant defense mechanism [Citation42]. Therefore, the protective mechanism of propolis against HF-induced diabetic changes could be attributed to its potent anti-oxidative properties.
In addition to oxidative stress, inflammation is considered an important pathogenic factor in the development of insulin resistance in T2DM. Oxidative stress and endoplasmic reticulum stress stimulate inflammatory signaling in T2DM. Inflammatory stimuli, in turn activate multiple serine/threonine kinases that inhibit insulin signaling [Citation43]. Specifically, TNF-α was strongly linked to insulin resistance and diabetes. TNF-α increases free fatty acids production, interferes with insulin receptor signaling, decreased insulin sensitivity and inhibit adiponectin synthesis [Citation44]. Our results showed that diabetes markedly increased serum TNF-α compared to control group. Propolis treatment reduced TNF-α level in treated diabetic rat. These results are in agreement with other studies that reported anti-inflammatory properties of propolis [Citation45].
Adipose tissue is an endocrine organ that plays a crucial role in pathophysiology of T2DM [Citation46]. Adiponectin is defined as anti-diabetic hormone secreted by adipose tissue. Adiponectin was shown to be associated with various metabolic disorders, including obesity, insulin resistance, and obesity related cardiovascular and fatty liver diseases [Citation47]. Moreover, adiponectin production was reported to be negatively correlated with accumulated visceral fat [Citation48]. Further, reduced adiponectin levels were observed in obesity [Citation49] and knocking out adiponectin resulted in severe insulin resistance and diabetes [Citation50]. Similarly, our results showed reduced levels of adiponectin in diabetic rats. Interestingly, adiponectin levels in our study were inversely correlated to the levels of blood glucose, insulin, total lipids and MDA. On the other hand, a high adiponectin level is found to be a consistent indicator of lower risk of T2DM because of its anti-diabetic and anti-atherogenic effects [Citation51]. In line with this study, our results revealed that propolis restored reduced adiponectin levels in treated diabetic rats compared to untreated diabetic rats.
This finding led us to study how propolis may lead to down regulation of adiponectin expression. Gene expression of adiponectin is mainly regulated by nuclear transcriptor PPARγ. PPARγ is known to regulate adipocyte differentiation and to control the transcription of many adipocyte-specific genes. Research demonstrated that PPARγ agonists increased the circulating adiponectin in high fructose fed rat model [Citation52]. Hence, adiponectin expression was believed to be a pertinent target for PPARγ agonists. In addition, epidemiological study proved that PPARγ gene polymorphism would reduce the serum adiponectin levels [Citation53]. In the present investigation, decreased protein concentration of PPARγ and adiponectin was observed in diabetic rats. These adverse changes were counter regulated by propolis treatment. These results were further confirmed by the observed significant positive correlation between PPARγ and adiponectin levels, suggesting PPARγ activation as a possible pathway involved in propolis protective effect. Moreover, PPARγ activation was found to attenuate insulin resistance by elevating the number of mature adipocytes, increasing glucose disposal rate and decreasing circulating free fatty acids levels [Citation54]. In this context, our study revealed that both tissue PPARγ and serum adiponectin levels negatively correlated with fasting blood glucose level.
5 Conclusion
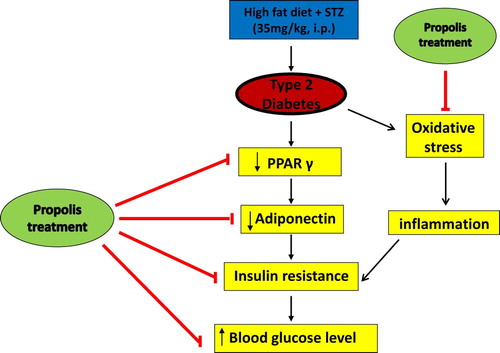
In conclusion, this study demonstrated that Brazilian propolis can reverse changes evoked by T2DM induced experimentally in rats, possibly by combating oxidative stress, activating PPARγ, elevating adiponectin levels and reducing insulin resistance, Fig. 7. This ability of Brazilian propolis to target various pathways involved in T2D makes it a promising therapy for management of T2D.
References
- LiaoZ.ChenX.WuM.Antidiabetic effect of flavones from Cirsium japonicum DC in diabetic ratsArch Pharm Res332010353362
- ZhuC.F.PengH.B.LiuG.Q.ZhangF.LiY.Beneficial effects of oligopeptides from marine salmon skin in a rat model of type 2 diabetesNutrition26201010141020
- Y.M.KimS.NamkoongYunY.G.HongH.D.Y.C.LeeHaK.S.et alWater extract of Korean red ginseng stimulates angiogenesis by activating the PI3K/Akt-dependent ERK1/2 and eNOS pathways in human umbilical vein endothelial cellsBiol Pharm Bull30200716741679
- WangZ.WangJ.P.ChanTreating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbsEvid Based Complement Alternat Med20132013 343594
- S.WildG.RoglicA.GreenR.SicreeH.KingGlobal prevalence of diabetes: estimates for 2000 and projections for 2030Diabetes Care275200410471053
- ChangC.L.LinY.A.P.BartolomeChenY.C.S.C.ChiuYangW.C.Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compoundsEvid Based Complement Alternat Med20132013 378657
- M.KobayashiM.IwataT.HarutaClinical evaluation of pioglitazoneNippon Rinsho582000395400
- LiW.L.ZhengH.C.J.BukuruN.De KimpeNatural medicines used in the traditional Chinese medical system for therapy of diabetes mellitusJ Ethnopharmacol9212004121
- N.MaedaM.TakahashiT.FunahashiS.KiharaH.NishizawaK.Kishidaet alPPARγ ligands increase expression and plasma concentrations of adiponectin, an adipose-derived proteinDiabetes509200120942099
- K.HottaT.FunahashiY.AritaM.TakahashiM.MatudaY.Okamotoet alPlasma concentration of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patientsJ Clin Endocrinol Metab86200119301935
- M.KumadaS.KiharaS.SumitsujiT.KawamotoS.MatsumotoN.Ouchiet alAssociation of hypoadiponectinemia with coronary artery disease in menArterioscler Thromb Vasc Biol23120038589
- O.RenaldiB.PramonoH.SinoritaL.B.PurnomoR.H.AsdieA.H.Asdieet alHypoadiponectinemia: a risk factor for metabolic syndromeActa Med Indones41120092024
- J.J.DiezP.IglesiasThe role of the novel adipocyte-derived hormone adiponectin in human diseaseEur J Endocrinol14832003293300
- J.FruebisT.S.TsaoS.JavorschiD.Ebbets-ReedM.R.EricksonF.T.Yenet alProteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in miceProc Natl Acad Sci U S A984200120052010
- T.YamauchiJ.KamonH.WakiY.TerauchiN.KubotaK.Haraet alThe fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesityNat Med782001941946
- V.DeClercqD.StringerR.HuntC.G.TaylorP.ZahradkaAdipokine production by adipose tissue: a novel target for treating metabolic syndrome and its sequelaeWangM.Metabolic syndrome: underlying mechanisms and drug therapies2011John Wiley & Sons, Inc.Hoboken, NJ73131
- M.IwakiM.MatsudaN.MaedaT.FunahashiY.MatsuzawaM.Makishimaet alInduction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptorsDiabetes527200316551663
- K.LakotaWeiJ.M.CarnsM.HinchcliffJ.LeeM.L.Whitfieldet alLevels of adiponectin, a marker for PPAR-gamma activity, correlate with skin fibrosis in systemic sclerosis: potential utility as biomarker?Arthritis Res Ther1432012102
- H.KitamuraY.NaoeS.KimuraT.MiyamotoS.OkamotoC.Todaet alBeneficial effects of Brazilian propolis on type 2 diabetes in ob/ob miceAdipocyte242013227236
- WangL.WangA.N.S.MineshitaI.GaAnti-inflammatory effects of propolisJpn J Pharmacol Therapeut241993223226
- N.PaulinoS.R.AbreuY.UtoD.KoyamaH.NagasawaH.Horiet alAnti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolisEur J Pharmacol5871–32008296301
- A.H.BanskotaN.TakemaY.S.LuciaT.YasuhiroA.SureshK.Midorikawaet alAntiproliferative activity of the Netherlands propolis and its active principles in cancer cell linesJ Ethnopharmacol8020026773
- H.U.FuliangH.R.HepburnXuanH.ChenM.S.DayaS.E.RadloffEffects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitusPharmacol Res512005147152
- R.IkedaM.YanagisawaN.TakahashiT.KawadaS.KumazawaN.Yamaotsuet alBrazilian propolis-derived components inhibit TNF-α-mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytesBiochim Biophys Acta181072011695703
- M.J.ReedK.MeszarosL.J.EntesM.D.ClaypoolJ.G.PinkettT.M.Gadboiset alA new rat model of type 2 diabetes: the fat-fedMetabolism4911200013901394
- K.SrinivasanB.ViswanadL.AsratC.L.KaulP.RamaraoCombination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screeningPharmacol Res522005313320
- ZhangF.YeC.LiG.DingW.ZhouW.ZhuH.et alThe rat model of type 2 diabetes mellitus and its glycometabolism charactersExp Anim522003401407
- K.MatsushigeP.BasnetK.HaseS.KodotaK.TanakaT.NambaPropolis protects pancreatic beta-cells against the toxicity of streptozotocin(STZ)Phytomedicine321996203209
- J.ThulesenC.OrskovJ.J.HolstS.S.PoulsenShort-term insulin treatment prevents the diabetogenic action of streptozotocin in ratsEndocrinology138119976268
- R.M.SalamaM.F.SchaalanA.A.ElkoussiA.E.KhalifaPotential utility of sodium selenate as an adjunct to metformin in treating type II diabetes mellitus in rats: a perspective on protein tyrosine phosphataseBiomed Res Int1020131155
- Z.S.HakimB.K.PatelR.K.GoyalEffects of chronic ramipril treatment in streptozotocin induced diabetic ratsIndian J Physiol Pharmacol411997353360
- L.RajkumarN.SrinivasanK.BalasubramanianP.GovindarajuluIncreased degradation of dermal collagen in diabetic ratsIndian J Exp Biol29199110811083
- A.KumarV.SinghAtherogenic dyslipidemia and diabetes mellitus: what's new in the management arena?Vasc Health Risk Manag62010665669
- A.D.MooradianDyslipidemia in type 2 diabetes mellitusNat Clin Pract Endocrinol Metab532009150159
- I.IchiH.HoriY.TakashimaN.AdachiR.KataokaK.Okiharaet alThe beneficial effect of propolis on fat accumulation and lipid metabolism in rats fed a high-fat dietJ Food Sci7452009127131
- J.L.LeahyI.B.HirschK.A.PetersonD.SchneiderTargeting beta-cell function early in the course of therapy for type 2 diabetes mellitusJ Clin Endocrinol Metab959201042064216
- W.AoiS.HosogiN.NiisatoN.YokoyamaH.HayataH.Miyazakiet alImprovement of insulin resistance, blood pressure and interstitial pH in early developmental stage of insulin resistance in OLETF rats by intake of propolis extractsBiochem Biophys Res Commun43242013650653
- M.UedaK.HayashibaraH.AshidaPropolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K- and AMPK-dependent pathways in skeletal muscleBiofactors3942013457466
- H.OkutanN.OzcelikR.H.YilmazE.UzEffects of caffeic acid phenethyl ester on lipid peroxidation and antioxidant enzymes in diabetic rat heartClin Biochem3822005191196
- K.ParkM.GrossD.H.LeeP.HolvoetJ.H.HimesJ.M.Shikanyet alOxidative stress and insulin resistance: the coronary artery risk development in young adults studyDiabetes Care327200913021307
- J.L.EvansB.A.MadduxI.D.GoldfineThe molecular basis for oxidative stress-induced insulin resistanceAntioxid Redox Signal77–8200510401052
- A.NoorafshanB.Esmail-ZadehS.BahmanpourA.Poost-PasandEarly stereological changes in liver of Sprague-Dawley rats after streptozotocin injectionIndian J Gastroenterol2432005104107
- K.E.WellenG.S.HotamisligilInflammation, stress, and diabetesJ Clin Invest1155200511111119
- A.Fernández-SánchezE.Madrigal-SantillánM.BautistaJ.Esquivel-SotoA.Morales-GonzálezC.Esquivel-Chirinoet alInflammation, oxidative stress, and obesityInt J Mol Sci125201131173132
- L.F.MartinE.M.RochaS.B.GarciaJ.S.PaulaTopical Brazilian propolis improves corneal wound healing and inflammation in rats following alkali burnsBMC Complement Altern Med132013337
- A.J.ScheenPathophysiology of type 2 diabetesActa Clin Belg5862003335341
- C.BuechlerJ.WanningerM.NeumeierAdiponectin, a key adipokine in obesity related liver diseasesWorld J Gastroenterol1723201128012811
- T.YatagaiaS.NagasakaA.TaniguchibM.FukushimacT.NakamuraaA.Kuroeet alHypoadiponectinemia is associated with visceral fat accumulation and insulin resistance in Japanese men with type 2 diabetes mellitusMetabolism5210200312741278
- C.WeyerT.FunahashiS.TanakaK.HottaY.MatsuzawaR.E.Pratleyet alHypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemiaJ Clin Endocrinol Metab865200119301935
- N.MaedaI.ShimomuraK.KishidaH.NishizawaM.MatsudaH.Nagaretaniet alDiet-induced insulin resistance in mice lacking adiponectin/ACRP30Nat Med82002731737
- N.SattarS.G.WannametheeN.G.ForouhiNovel biochemical risk factors for type 2 diabetes: pathogenic insights or prediction possibilitiesDiabetologia516200892694039
- Y.SharabiM.Oron-HermanY.KamariI.AvniE.PelegZ.Shabtayet alEffect of PPAR-gamma agonist on adiponectin levels in the metabolic syndrome: lessons from the high fructose fed rat modelAm J Hypertens2022007206210
- Y.YamamotoH.HiroseK.MiyashitaK.NishikaiI.SaitoM.Taniyamaet alPPAR gamma 2 gene Pro12Ala polymorphism may influence serum level of an adipocyte-derived protein, Adiponectin, in the Japanese populationMetabolism5111200214071409
- J.M.OlefskyTreatment of insulin resistance with peroxisome proliferator activated receptor gamma agonistsJ Clin Invest1062000467472