?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A series of azosalicylic acid analogs were newly synthesized by coupling various aryl and heteroarylamine functionalities with salicylic acid nucleus. All the synthesized compounds were structurally confirmed by various modern analytical methods. The said synthesized compounds were screened to investigate their antimicrobial, analgesic and antioxidant activities. The compounds 4e and 4h showed excellent significant antibacterial activity against most of the bacterial strains as no compounds showed significant antifungal activity against Cryptococcus neoformans. The bromine substituted molecule 4e (4-bromo-3-methyl phenyl azosalicylic acid analog) showed the highest significant analgesic activity with 46.10% of inhibition. The results of in vitro antibacterial and analgesic activity were justified with the outcome of in-silico investigation. The results of biological activities were statistically interpreted. The compounds substituted with antipyrinylazo and 4-carboxy phenylazo moiety exhibited potential antioxidant activity.
1 Introduction
The writings of Greek physician Hippocrates revealed that the leaves and barks of willow tree were used as analgesic and antipyretic in early days. The active constituent responsible in this natural source, later identified as salicin, contains both sugar and aromatic component, initially called as spirasure and later salicylic acid. The de-novo synthesis of salicylic acid was first performed in 1852 and its structure was deduced as 2-hydroxy benzoic acid [Citation1]. The salicylic acid derivatives exhibited antioxidant, antiproliferative [Citation2] and cytotoxic activities [Citation3]. The azo salicylic acid derivative sulfasalazine is a proven drug for the last 40 years which is effective against ulcerative colitis (inflammatory bowel disease) [Citation4]. There has been an increase in the side-effects due to the sulfapyridine portion which acts as a carrier. The azo bond breaks due to the bacterial enzyme azo-reductase present at the site of lumen of the colon leaving the 5-aminosalicylic acid. The azobis-salicylic acid derivative olsalazine could be a better alternative for sulfasalazine. Literature survey supports that azo-salicylic acids have biological activity and also are useful precursors for the synthesis of anticarcinogenic, antiviral, antimicrobial and antimalarial agents [Citation3]. Salicylates have analgesic effects similar to that of other NSAIDs to inhibit the enzyme cyclooxygenase (COX) [Citation5]. NSAIDs inhibit both the activity of COX-1 and COX-2 and thereby synthesis of prostaglandin and thromboxane [Citation6]. Literature support also suggests that bromine substituted molecules can show potential analgesic activity [Citation7]. Further, literature survey indicates that pyrazolone nucleus is the key pharmacophore and is responsible for various pharmacological activities such as analgesic [Citation8] and antimicrobial activity [Citation9]. The N-phenyl substituted anthranyl congeners also have analgesic, antirheumatic and antiinflammatory activities [Citation10]. The above information encouraged us to synthesize a new range of azo-salicylic acid congeners with different aryl and heteroaryl functionalities and to investigate the antibacterial, analgesic and antioxidant activities. The structures were confirmed by spectral characterization. The synthesized azosalicylic acid congeners act as ligands individually against the targeted proteins (PDB ID: 3SPU of NDM-1 and 1CX2 of COX-2) by computational docking method for the evaluation of antibacterial and analgesic activities respectively.
2 Materials and methods
All the chemicals used in the present study were of synthetic grade and sourced from Merck Specialties Ltd. (Mumbai, India). The structural conformation of the synthesized compounds from salicylic acid is conducted by various modern analytical techniques viz. FT/IR (JASCO FT/IR 4100 Spectrophotometer using KBr disc), 1H NMR (Bruker 1H NMR 400 MHz) using TMS as an internal standard, LC–MS (Shimadzu-mass spectrometer) and Differential Scanning calorimetric analysis (METTLER TOLEDO STARe system at a heating rate of 10 °C min−1, temperature range 30–350 °C using aluminum cans calibrated with indium) and elemental analysis (Perkin Elmer-2400 CHNO/S analyzer system). Solvent behavior of the compounds was studied by UV–Visible spectrophotometer (JASCO V-630 Spectrophotometer). The melting points were determined by open capillary method (Elico) and were uncorrected. The synthesized ligands were evaluated for their in vitro antimicrobial activity against different pathogens by Agar Well Diffusion method. The results of the potential antibacterial and analgesic activity of the selected ligands were rationalized by molecular docking.
The synthesis of the aryl/heteroaryl azo salicylic acid analogs was carried out on the basis of our earlier reported work [Citation11] (Scheme 1).
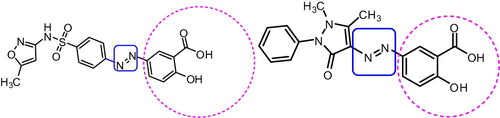
Structures of some newly synthesized azosalicylic acid congeners.
2.1 2-hydroxy-5-(4-sulfamoylphenylazo)-benzoic acid (4a)
Dark red color powder; yield 75%; Rf 0.8; mp (°C); 297–300; UV–vis (λmax, ethanol): 366 nm; IR (KBr, γ, cm−1): 3374 (O–H str.), 1676 (C=O str.), 1587 (C=C str.), 1482 (–N=N–), 1331, 1160 (SO2 str.SO2NH2), 910 (S-N str.), 1096 (C–O str.); 1H NMR (CDCl3, δppm, 400 MHz): 7.46 (s, 2H, SO2NH2), 8.01–8.10 (m, 4H, Ar H), 12.10 (sb. 1H, COOH), 11.69 (sb, 1H, OH), 7.36 (d, 1H, salicylic H-3), 8.11 (d, 1H, salicylic H-4), 8.34 (s, 1H, salicylic H-6); LC–MS (% area); 77.65; m/z; 320.13 (M-1); Analysis for C13H11N3O5S: Calcd % C, 48.59; H, 3.45; N, 13.08; S, 9.98; Found %: C, 48.19; H, 3.48; N, 13.11; S, 9.95.
2.2 2-hydroxy-5-(4-sulfamoylphenylazo)-benzoic acid (4b)
Yellow color powder; yield 72%; Rf 0.8; mp (°C); 328–330; UV–vis (λmax, ethanol): 361 nm; IR (KBr, γ, cm−1): 3431 (O–H str.), 1671 (C=O str.), 1628 (C=C str.), 1448 (–N=N–), 1389, 1206 (SO2 str.SO3H), 1127 (C–O str.); 1H NMR (DMSO-d6, δppm, 400 MHz): 7.83–8.34 (m, 4H, Ar H), 11.69 (sb. 1H, OH), 12.10 (sb, 1H, COOH), 7.28 (d, 1H, salicylic H-3), 8.08 (d, 1H, salicylic H-4), 8.34 (s, 1H, salicylic H-6); LC–MS (% area); 52.33; m/z; 321.08 (M-1); Analysis for C13H10N2O6S: Calcd % C, 48.45; H, 3.13; N, 8.69; S, 9.95; Found %: C, 48.42; H, 3.09; N, 8.62; S, 9.91.
2.3 2-hydroxy-5-(4-nitrophenylazo)-benzoic acid (4c)
Dark red color powder; yield 92%; Rf 0.7; mp (°C); 243–245; UV–vis (λmax, ethanol): 388 nm; IR (KBr, γ, cm−1): 3456, 3210 (O–H str.), 1672 (C=O str.), 1610 (C=C str.), 1482 (–N=N–), 1530, 1344 (NO2 str.), 1106 (C–O str.); 1H NMR (DMSO-d6, δppm, 400 MHz): 7.75–8.25 (m, 4H, Ar H), 11.75 (sb. 1H, OH), 12.09 (sb, 1H, COOH), 7.31 (d, 1H, salicylic H-3), 8.13 (d, 1H, salicylic H-4), 8.35 (s, 1H, salicylic H-6); LC–MS (% area); 91.62; m/z; 286.12 (M-1); Analysis for C13H9N3O5: Calcd % C, 54.36; H, 3.16; N, 14.63; Found % C, 54.26; H, 3.11; N, 14.60.
2.4 2-hydroxy-5-(4-methoxyphenylazo)-benzoic acid (4d)
Black color powder; yield 95%; Rf 0.8; mp (°C); 238–240; UV–vis (λmax, ethanol): 374 nm; IR (KBr, γ, cm−1): 3464 (O–H str.), 2926 (CH2 str.), 1667 (C=O str.), 1596 (C=C str.), 1491 (–N=N–), 1111 (C–O str.); 1H NMR (DMSO-d6, δppm, 400 MHz): 7.05–7.75 (m, 4H, Ar H), 3.83 (s, 3H, ArOCH3), 11.37 (sb. 1H, OH), 12.13 (sb, 1H, COOH), 7.37 (d, 1H, salicylic H-3), 8.11 (d, 1H, salicylic H-4), 8.27 (s, 1H, salicylic H-6); LC–MS (% area); 71.88; m/z; 273.21 (M+1); Analysis for C14H12N2O4: Calcd % C, 61.76; H, 4.44; N, 10.29; Found %: C, 61.86; H, 4.34; N, 10.19.
2.5 5-(4-bromo-3-methylphenylazo)-2-hydroxybenzoic acid (4e)
Brown color powder; yield 85%; Rf 0.8; mp (°C); 288–290; UV–vis (λmax, ethanol): 361 nm; IR (KBr, γ, cm−1): 3451 (O–H str.), 2937 (CH str.), 1661 (C=O str.), 1587 (C=C str.), 1489 (–N=N–), 1147 (C–O str.), 748 (C–Br str ); 1H NMR (CDCl3, δppm, 400 MHz): 7.54–7.72 (m, 3H, Ar H), 2.44 (s, 3H, ArCH3), 11.49 (sb. 1H, OH), 11.87 (sb, 1H, COOH), 6.86 (d, 1H, salicylic H-3), 7.86 (d, 1H, salicylic H-4), 8.28 (s, 1H, salicylic H-6); LC–MS (% area); 100; m/z; 333.03 (M-1); Analysis for C14H11BrN2O3: Calcd % C, 50.17; H, 3.31; N, 8.36; Found %: C, 50.27; H, 3.41; N, 8.56.
2.6 5-((1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)azo)-2- hydroxybenzoic acid (4f)
Brown color powder, yield 85%; Rf 0.7; mp (°C); 256–260, UV–vis (λmax, ethanol): 368 nm; IR (KBr, γ, cm−1): 3410 (O–H str.), 2926 (CH str.), 1662 (C=O str. of carboxylic group), 1606 (C=C str.), 1486 (–N=N–), 1153 (C–O str); 1H NMR (DMSO, δppm, 400 MHz): 6.85–7.30 (m, 5H, –N–C6H5), 2.66 (s, 3H, =C-CH3), 3.15 (s, 3H, –N–CH3), 11.65 (sb. 1H, OH), 12.17 (sb, 1H, COOH), 7.32 (d, 1H, salicylic H-3), 7.41 (d, 1H, salicylic H-4), 7.87 (s, 1H, salicylic H-6); LC–MS (% area); 39.20; m/z; 353.07 (M+1); Analysis for C18H16N4O4: Calcd % C, 61.36; H, 4.58; N, 15.90; Found % C, 61.46; H, 4.38; N, 15.87.
2.7 5-(4-carboxyphenylazo)-2-hydroxybenzoic acid (4g)
Black color powder; yield 95%; Rf 0.7; mp (°C); 286–290, UV–vis (λmax, ethanol): 360 nm; IR (KBr, γ, cm−1): 3481 (O–H str.), 1692 (C=O str.), 1608 (C=C str.), 1493 (–N=N–), 1180 (C–O str.); 1H NMR (DMSO-d6, δppm, 400 MHz): 8.13–8.41 (m, 4H, Ar H), 11.33 (sb. 1H, OH), 12.54 (sb, 1H, COOH), 7.33 (d, 1H, salicylic H-3), 8.11 (d, 1H, salicylic H-4), 8.29 (s, 1H, salicylic H-6); LC–MS (% area); 100; m/z; 285.00 (M-2); Analysis for C14H10N2O5: Calcd % C, 58.74; H, 3.52; N, 9.79; Found % C, 58.77; H, 3.12; N, 9.49.
2.8 2-hydroxy-5-((4-(N-(5-methylisoxazol-3-yl)-sulfamoyl)-phenyl) azo) benzoic acid (4h)
Black color powder; yield 73%; Rf 0.8; mp (°C); 227–230; UV–vis (λmax, ethanol): 370 nm; IR (KBr, γ, cm−1): 3461 (O–H str.), 3138 (NH str.), 2922 (CH2 str.), 1668 (C=O str.), 1614 (C=C str.), 1473 (–N=N–), 1315, 1170 (SO2 str.SO2NH), 928 (S–N str.); 1H NMR (DMSO-d6, δppm, 400 MHz): 8.02–8.34 (m, 4H, Ar H), 11.69 (sb. 1H, OH), 12.11 (sb, 1H, COOH), 11.12 (s, 1H, SO2NH), 2.30 (s, 3H, CH3), 6.17 (s, 1H, isoxazolyl H-4), 7.00 (d, 1H, salicylic H-3), 7.97 (d, 1H, salicylic H-4), 8.34 (s, 1H, salicylic H-6); LC–MS (% area); 100; m/z; 403.04 (M+1); Analysis for C17H14N4O6S: Calcd % C, 50.74; H, 3.51; N, 13.92; S, 7.97; Found %: C, 50.54; H, 3.55; N, 13.96; S, 7.89.
2.9 Microbiological evaluation
2.9.1 Antimicrobial activity
The above newly synthesized azosalicylic acid congeners were investigated over different microbial strains viz. Escherichia coli (MTCC 614), Salmonella enterica ser. typhi (MTCC 773), Salmonella enterica typhimurium (MTCC 98), Salmonella enterica paratyphi (MTCC 3220), Shigella flexneri (MTCC 1457), Pseudomonas aeruginosa (MTCC 1035), Vibrio cholera (MTCC 3906), Micrococcus luteus (MTCC 1809), Klebsiella pneumoniae (MTCC 109), Bacillus circulans (MTCC 490), Streptococcus mitis (MTCC 2695), Aspergillus niger (MTCC 9933), Candida albicans (MTCC 3017), Candida glabrata, Cryptococcus neoformans and Trichophyton rubrum, sourced from the Institute of Microbial Technology and Gene bank (IMTECH), Chandigarh, India. Staphylococcus aureus and Bacillus subtilis strain hswx88 [Citation12] were isolated in the Pharmaceutical Biotechnology Division of the University Department of Pharmaceutical Sciences, Utkal University. Freshly subcultured microorganisms were used. Ampicillin and Fluconazole were used as reference antibiotics.
The antimicrobial activities of the novel azosalicylic acid congeners (4a–4h) were performed by agar well diffusion method using sterile molten nutrient agar (antibacterial activity) and Sabouraud dextrose agar (antifungal activity) [Citation13]. The solidified mediums were inoculated and punched in to wells of 6 mm diameter. Each well was filled with stock solution of test and reference compounds (1 µg/µL concentration) of definite volume and incubated for 24 h and 72 h for bacterial and fungal strains respectively at 37 °C. The compounds showing significant activity against most of the bacterial strains were subjected to investigation of their activity against different fungal strains. The diameter of zone of inhibition was measured using the Hi-Antibiotic Zone Scale (Hi-Media).
2.9.2 Minimum inhibitory concentration (MIC)
One milligram per milliliter stock solution of synthesized compounds and reference antibiotic was prepared using 10% DMF solution. Further, five different concentrations (500–31.25 µg/mL) were prepared by serial dilution method. The different concentrations for respective test compounds were loaded into the wells made on bacterial inoculated mediums and incubated at 37 °C for 18–24 h. MIC was defined as the lowest concentration of the test compounds that inhibited the visible growth on agar medium. After incubation, minimum inhibitory concentration was determined [Citation14].
2.10 Pharmacological activity
2.10.1 Animals
In this work, female Wistar rats of 180–200 g (for acute toxicity study) and Swiss albino mice 25–30 g (analgesic evaluation) of either sex of appropriate age were used. The experiments were carried out under the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals and approved by the Institutional Animal Ethical Committee with registration number 1171/C/08/CPCSEA and Ref. No. 60/SPS/IAEC/SOAU.
2.10.2 Acute toxicity study
Acute Oral Toxicity study was performed on female Wistar rats to establish the safety dose of the synthesized compounds. OECD guideline No.420 (2000) for Acute Oral Toxicity-Fixed Dose Procedure was followed (sighting study and main study) for a period of 14 days to study the acute toxic symptoms and the behavioral changes within the animals.
2.10.3 Writhing model induced by acetic acid
Albino mice of appropriate weight of either sex were kept under controlled conditions of light and temperature. The animals were divided into 10 groups, each carrying 6 animals. Group-1 was treated as control, group-2 served as positive control where they were administered with standard acetyl salicylic acid at a dose of 50 mg/kg body weight, (intra-peritoneal). Animals from groups 3 to 10 were provided with test (4e, 4f, 4g and 4h) compounds respectively at a dose level of 50 and 100 mg/kg body weight orally. Thirty minutes after the administration of acetyl salicylic acid in group 2 and 1 h after administration of test drugs in groups 3–10, all the groups were administered with 0.6% v/v acetic acid solution at a dose level of 1 mL/100 g of body weight (intra-peritoneal) [Citation15]. The onset of writhing was noted. Finally, the percentage of analgesic activity was calculated.
The reaction time was expressed as mean ± SEM. The statistical analysis was done by one way-ANOVA followed by Dunnett's t-test.
2.10.4 Antioxidant activity assay by DPPH model
The free radical scavenging activity of novel azosalicylic acid analogs (4e–4h) was measured by DPPH method with some modification [Citation13]. The reaction mixture of synthesized compounds at different concentration aliquots was taken and the volume was adjusted up to 3 mL with methanol. To this mixture 1 mL of 0.1 mM solution of DPPH in methanol was added. The mixture was kept in the dark for 30 min. The free radical scavenging activity of synthesized compounds was compared with standard Butylated Hydroxytoluene (BHT). Optical density was measured at 517 nm and the inhibition of concentration was calculated. One milliliter of 0.1 mM of methanolic solution of DPPH and 3 mL of methanol was considered as control.
where Acont is the absorbance of control and Atest is the absorbance of the test sample. The sample concentration providing 50% inhibition (IC50) was determined. All the experiments were carried out in triplicate and IC50 values were expressed as mean ± SD.
2.11 In silico studies
For the computational approach by tools of bioinformatics, docking is employed for locating a suitable or leading synthetic compound against a particular target retrieved from Protein Data Bank (PDB), New Delhi Metallo-β-lactamase-1 (NDM-1) of K. pneumoniae with PDB ID: 3SPU as a bacterial target protein [Citation16] and cyclooxygenase-2 of Mos musculus with PDB ID: 1CX2 as an analgesic target protein [Citation7] for docking study. The structures of the synthesized compounds (4e, 4f, 4g, and 4h) are prepared by using Chem Draw ultra 10.0 and converted from .mol file format to pdb format for docking. In silico protein–ligand interaction of the newly synthesized compounds (4e, 4f, 4g, and 4h) was investigated individually using Arugus Lab 4.0 docking software. The protein–ligand interaction was carried out by Discovery studio Visualizer 3.1 software. The resulting score obtained by molecular docking predicts the strongest binders.
2.12 Statistical analysis
The observed data on zone of inhibitions were subjected to one way-analysis of variance. The mean zone of inhibition for each compound on each strain was compared with the reference antibiotic through Dunnett's Post Hoc test (https://www.statsdodo.com/SSizAOV_Pgm.php). The test of significance was done at 5% level of type one error. The research hypothesis was ‘the zone of inhibition for test compound was higher than the reference antibiotic against the hypothesis of no difference (null hypotheses)’, which states that there is no significant difference between the zone of inhibition of the test compound and the reference antibiotics.
2.13 Sample size determination
A minimum sample size of five was calculated taking probability of type 1 error (d) = 0.05, Power (1-β) = 0.8, Number of groups 13 within group SD = 2. However a sample size of six has been taken in the study for each compound against each strain.
3 Results and discussion
3.1 Chemistry
A series of azosalicylic acid analogs were synthesized by coupling of diazonium salt of eight different aryl and hetero aryl amine derivatives with salicylic acid in presence of 10% sodium hydroxide solution (Scheme 1). Diazotization was carried out in presence of nitrosyl chloride and excess nitrous acid was destroyed by the addition of urea. The crude products were recrystallized from absolute ethanol. The structures of prepared compounds have been confirmed by FT/IR, 1H NMR, UV, LC–MS, DSC and elemental analysis.
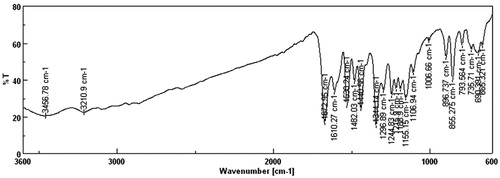
The FT/IR spectrum of the salicylic acid congeners (4a–4h) showed two strong absorption bands at range of 3374–3481 and 1661–1692 cm−1 with respect to ν OH str. and ν C=O str. of salicylic acid. Compound 4h showed strong absorption bands at 1668, 1315, 1170, 928 and 3138 cm−1 due to the presence of ν C=O str; ν SO2 str.; ν S-N str. and ν NH str. respectively. The medium vibration bands at 1473 and 2922 cm−1 which indicates to ν –N=N– and ν CH2 str. of methyl group in compound 4h. Compound 4c showed medium absorption bands at 1482, 1530 and 1344 cm−1 which attributed the presence of ν –N=N– and ν NO2 str. respectively (Fig. 1).
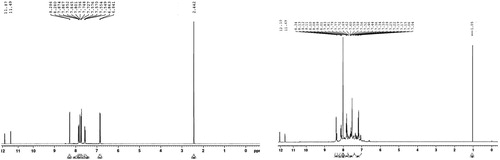
The 1H NMR data of the newly synthesized 5-heteroaryl/arylazo salicylic acid congeners were analyzed in CDCl3 and DMSO d6. All the compounds showed two broad singlet peaks at range of δ 12.08–12.06 ppm and δ 11.09–11.65 ppm which indicates the presence of carboxylic protons and phenolic hydroxyl protons of salicylic acids respectively. The presence of salicylic H-6 protons was observed with sharp singlet peaks at a range of δ 8.29–8.37 ppm in all the congeners. Other aromatic protons of salicylic acid in all the congeners appeared with two sets of doublet signals at a range of δ 6.86–7.36 ppm and δ 7.86–8.13 ppm. In addition to above spectral data, compound 4e appeared with three similar environmental protons at δ 2.44 ppm which attributed the presence of methyl proton. Compound 4f, which appeared with two sharp singlets at δ 2.60 ppm and δ 3.15 ppm, may be due to the presence of N–CH3 and =C–CH3 respectively. The aromatic multiplet signals in the said compound appeared at a range of δ 6.85–7.30 ppm. Compound 4h appeared with a singlet signal at δ 6.17 ppm of isoxazole H-4. The 1H NMR data of compounds 4e and 4a are reported in Fig. 2.
3.1.1 Solvatochromic behavior
The solvatochromic behavior of the products was studied on UV–Visible spectrophotometer. The absorption spectra of the newly synthesized 5-heteroaryl/arylazo salicylic acid congeners (4a–4h) were investigated in different solvents at concentration of 10−5 to 10−6 M. According to illustrated data in , all the synthesized compounds showed red shift in DMSO and DMF except compound 4a with respect to the λmax as compared to the other polar solvents. Introduction of 4-nitro phenylazo substituent on salicylic acid at the C-5 position gives the largest bathochromic shift compared to other azo salicylic acid analogs. The electron withdrawing substituent NO2 on salicylic acid bearing molecules (4c) gives rise to more bathochromic shifts in comparison to compound 4d having electron donating methoxy substituent in all the solvents. All the synthesized compounds observed with λmax at a range of 306–396 nm in all the solvents confirm the formation of –N=N– group. The solvatochromic effect of compound 4h in all the solvents and all the synthesized compounds in DMF is illustrated in Fig. 3.
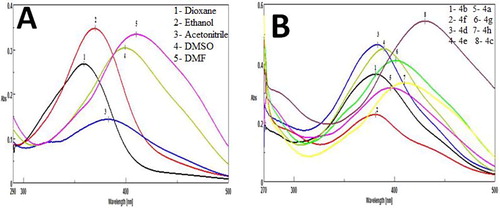
Table 1 UV–Visible spectral data (λmax) of newly synthesized azosalicylic acid analogs (4a–4h).
3.1.2 LC–MS and thermal analysis
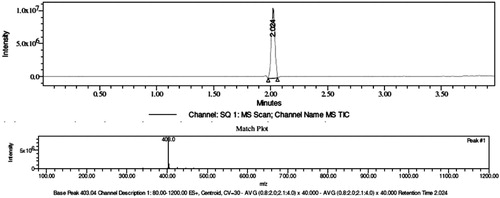
The predicted molecular weight of synthesized compounds was confirmed by LC–MS and strongly reveals their molecular formula. Compounds 4a, 4b, 4c, 4d, 4e, 4f, 4g and 4h having m/z values 320.13, 321.08, 286.12, 273.21, 333.03, 353.07, 284.98 and 403.04 respectively strongly reveal their molecular formula. The LC–MS of compound 4g is given in Fig. 4.
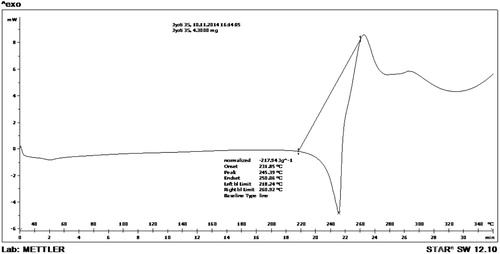
The DSC thermogram reported sharp and narrow endothermic peak by compound 4c evidenced with peak temperature of (245.39 °C) corresponding to its melting point Fig. 5.
3.2 Microbiology
3.2.1 Antimicrobial activity
Most of the synthesized salicylic acid congeners have effective antibacterial activity. The mean ± SD of zone of inhibition for each bacterial strain has been compared by one way-analysis of variance and the resulting p value. The mean zone of inhibition among the different compounds was found to be significantly different with p value of 0.00.
The results of the antibacterial activity of the newly synthesized compounds compared with standard, expressed in mean ± SD, were reported in . The reported results revealed that the compounds 2-hydroxy-5-((4-(N-(5-methylisoxazol-3-yl) sulfamoyl) phenyl) diazenyl) benzoic acid (4h) and 5-((4-bromo-3- methylphenyl)diazenyl)-2-hydroxybenzoic acid (4e) showing excellent significant antibacterial activity in comparison to standard against E. coli, S. ser.typhi, S. typhimurium, S. paratyphi, S. flexneri, V. cholera, K. pneumoniae, M. luteus, S. mitis and B. subtilis may be due to the structural conjugation of 5-methyl isoxazolyl and 4-bromo-3-methyl phenylazo moiety at C-5 position of salicylic acid respectively. The compound 5-((4-carboxyphenyl)diazenyl)-2-hydroxybenzoic acid (4g) showing good significant antibacterial activity against S. flexneri, K. pneumoniae, B. circulans and S. aureus may be due to the structural conjugation of 4-carboxyphenylazo moiety at C-5 position of salicylic acid. The zone of inhibition of salicylic acid analogs (4e–4h) against S. flexneri and B. subtilis is given in Fig. 6. The salicylic acid congeners 4g and 4h showing excellent significant antifungal activity (p < 0.05) against A. niger, T. rubrum and C. glabrata may be due to the structural presence of azo linkage bearing substituent such as 4-carboxy phenylazo and 5-methyl isoxazolylazo at C-5 position of salicylic acid respectively. Compound 4e showed good significant antifungal activity (p < 0.05) against C. albicans and C. glabrata . The graphical interpretation of significant antimicrobial activity of compound 5-((4-bromo-3-methylphenyl) diazenyl)-2-hydroxybenzoic acid (4e) and 2-hydroxy-5-((4-(N-(5-methylisoxazol-3-yl) sulfamoyl) phenyl) diazenyl) benzoic acid (4h) respectively in comparison to standard is illustrated in Fig. 7.
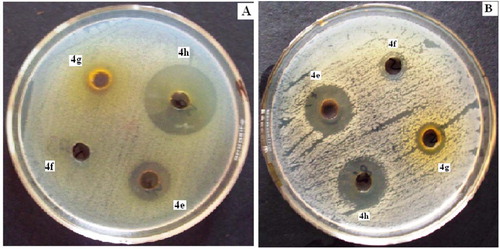
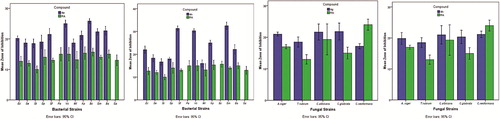
Table 2 Antibacterial activity of azosalicylic acid analogs (4a–4h), Zone of Inhibition (mm).
Table 3 Antifungal activity of azosalicylic acid analogs (4e, 4g and 4h), Zone of Inhibition (mm).
3.2.2 Evaluation of minimum inhibitory concentrations
The inhibitory property of the salicylic acid congeners was determined in terms of MIC (µg/mL). The MIC values of the test analogs against different bacterial strains were investigated to determine the minimum level of concentration at which the compound is able to exert its activity (). All the salicylic acid congeners exhibited potential antibacterial activity by inhibiting the growth of different bacterial strains among which the salicylic acid congeners 4h and 4e inhibited the growth of most of the organisms at a concentration 31.25 µg/mL. The 4-carboxy phenylazo substituted salicylic acid congener 4g comes next in inhibiting the growth of four bacterial pathogens at a concentration 31.25 µg/mL. The reference antibiotic (Ampicillin) is able to exhibit its MIC against all the bacterial strains at 31.25 µg/mL.
Table 4 Minimum inhibitory concentration MIC (µg/mL) of azosalicylic acid analogs (4a–4h) against different bacterial strains.
3.3 Pharmacology
3.3.1 Analgesic activity
The test compounds were safe up to 2000 mg/kg body weight. No toxic symptoms, gross behavioral changes and mortality were observed. The compounds with different functionalities like substituted 4-bromo-3-methyl phenylazo (4e), pyrazolylazo (4f), 4-carboxy phenylazo (4g) and isoxazolylazo (4h) substituted 5-heteroaryl/arylazo salicylic acid congeners were subjected for evaluation of their analgesic activity on the basis of literature.
In the control group, acetic acid produced an average of 83.5 ± 2.40 writhes in 10 min of observation. Standard acetyl salicylic acid showed 66.07% of inhibition with 28.33 ± 1.89*** writhing response at a dose of 50 mg/kg body weight, while the newly synthesized 5-heteroaryl/arylazo salicylic acid congeners (4e, 4f, 4g and 4h) showed 46.10%, 16.97%, 31.13% and 16.76% of inhibition respectively at a dose of 100 mg/kg body weight (). The writhing responses observed at a dose of 100 mg/kg body weight in the compounds (4e, 4f, 4g and 4h) were 45 ± 4.86***, 69.33 ± 5.23, 57.5 ± 5.60** and 69.5 ± 4.12. Azosalicylic acid congener bearing 4-bromo, 3-methyl phenyl and 4-carboxy phenyl substituent (4e and 4g) reported the highest % of inhibition (46.10% and 31.13%) respectively at a dose of 100 mg/kg body weight (). Compounds 4e and 4g showed significant analgesic activity (writhing response) both at a dose of 50 and 100 mg/kg body weight, but compound 4e showed the highest significant analgesic activity 45 ± 4.86*** (p < 0.001) at a dose of 100 mg/kg body weight, which may be due to the structural presence of azolinked 4-bromo,3-methyl phenyl at C-5 position of salicylic acid.
Table 5 Analgesic effect of newly synthesized azosalicylic acid analogs (4e–4h) on acetic acid induced writhing response.
3.3.2 In vitro antioxidant screening
The structural elucidation of the titled synthesized compounds showed the presence of phenolic groups. In general, phenolic compounds and nitrogen-bearing heterocyclic rings have free radical scavenging activity. DPPH radicals accept the hydrogen atom or electron from the organic molecules and can form stable diamagnetic molecules. Scavenging effect of newly synthesized 5-heteroaryl/arylazo salicylic acid congeners (4e, 4f, 4g and 4h) showed 50% of inhibition (IC50) at a concentration level of 47.47 ± 0.02, 37.34 ± 0.02, 39.3 ± 0.05 and 59.7 ± 0.03 µg/mL respectively, whereas standard BHT showed at 33.5 ± 0.05 µg/mL (). The results of percentage of inhibition of the compounds included for evaluation of their antioxidant activity revealed that the test compound 4g showed significant DPPH scavenging activity (>20%) at a concentration of 10 µg/mL in comparison to standard, whereas compound 4f showed significant DPPH scavenging activity (>79%) at a concentration of 60 µg/mL. The free radical scavenging activity of synthesized analogs 4e, 4f, 4g and 4h is mentioned in Fig. 8. The azosalicylic acid analog 4f showed IC50 at lowest concentration in comparison to other three compounds which may be due to the structural presence of 4-antipyrinylazo functionality at the C-5 position of salicylic acid.
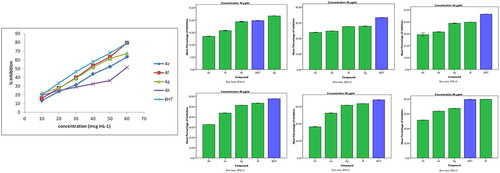
Table 6 Antioxidant activity of newly synthesized azosalicylic acid analogs (4e–4h).
4 In-silico studies
Docking is widely used in modern drug discovery process and is an effective tool capable of quickly and accurately predicting biomolecular conformations with binding energies of protein–ligand complexes. The molecular docking work is aimed at finding out the effective synthetic compounds against individual target protein viz. NDM-1 and COX-2 ().
Table 7 Converted synthesized structures (4e–4h) to K. pneumoniae PDB ID: 3SPU NDM-1 (structure of enzyme-β-lactamase-1) and Mos musculus PDB ID: 1CX2 of COX-2 (structure of enzyme-Cyclooxygenase-2).
Individually the ligands 4e–4h were docked with protein NDM-1 of K. pneumoniae. The docking energies of the salicylic acid congeners were obtained in negative value out of which 4h and 4e are potent inhibitors of β-lactamase-1. The protein–ligand interaction of compound 4h against NDM-1 is reported in Fig. 9. The ligand 4h binds to the amino acids of NDM-1 protein such as, ASP 34, ARG 52, GLN 53, TRP 59, ARG 81, TRP 104, GLU 108, ILE 109. Individually the ligands 4e–4h were docked with COX-2. Compounds 4e and 4h are potent inhibitors of cyclooxygenase-2. The ligand 4e binds to the amino acids of COX-2 protein viz. VAL 295, TRP 387, HIS 388, LEU 391, PHE 395, PHE 404, PHE 407, LEU 408. The protein–ligand interaction of compound 4e against COX-2 is reported in Fig. 9.
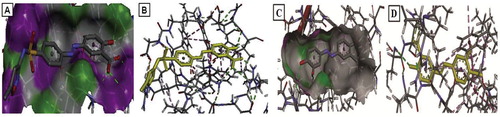
The salicylic acid congeners exhibited with significant potential antibacterial and analgesic effect (4e–4h) were subjected to molecular docking. Docking results between salicylic acid congeners and selected receptor β-lactamase-1 enzyme of K. pneumoniae and Cyclooxygenase-2 of M. musculus are reported in . The ligand receptor fits best with the highest binding energy for compounds 4h and 4e at docking energy value (−9.64) and (−12.00) with β-lactamase-1 and Cyclooxygenase-2 respectively. The ligand 4f showed least binding energy at energy value of −8.23 and −9.64 against β-lactamase-1 and Cyclooxygenase-2 respectively which strongly reveals the results of biological action.
Literature survey indicated that bromine substituted molecules have analgesic effect [Citation7]. The results of analgesic activity of salicylic acid congeners also revealed that the substituted bromo-compound 4e (4-bromo-3-methyl phenyl substituted azosalicylic acid) has highest significant analgesic activity. The in silico investigation of azosalicylic acid congeners (4e–4h) also predicted that the 4-bromo-3-methyl phenylazo substituted salicylic acid analog (4e) has highest binding energy i.e. −12.00 kcal/mol against Cyclooxygenase-2 which also supports the results obtained by acetic acid induced method.
5 Conclusion
In this research, a series of azosalicylic acid analogs were synthesized. The structures and their composition were confirmed by means of different spectral analysis. The 4-bromo-3-methyl phenylazo and isoxazolylazo substituted salicylic acid analogs 4e and 4h showed highest potent antibacterial activity, whereas the 4-carboxy phenylazo substituted salicylic acid analog 4g showed good significant antibacterial activity which justifies the prediction by in silico studies. No compounds exhibited significant antifungal activity against C. neoformans. However, the 4-bromo-3-methyl phenylazo and 4-carboxy phenylazo substituted salicylic acid analogs 4e and 4g showed highest significant analgesic activity which also justifies the in silico prediction. The antipyrinylazo and 4-carboxy phenylazo substituted salicylic analogs 4f and 4g showed potential antioxidant activity.
Acknowledgements
The authors acknowledge the Dean, School of Pharmaceutical Sciences, Siksha ‘O’ Anusandhan University, Director of NISER, IMMT and Deputy Director Regional Institute for Planning, Applied Economics and Statistics, Bhubaneswar, India. Sincere thanks to the Faculty in English, IHSE, S'O'A University for the kind cooperation in proof-reading.
References
- D.B.JackOne hundred years of aspirinLancet3501997437439
- M.M.Al-DabbasT.SuganumaK.KitaharaHouD.X.M.FujiiCytotoxic, antioxidant and antibacterial activities of Varthemia iphionoides Boiss. extractsJ Ethnopharmacol1082006287293
- E.DjurendicS.D.VujaskovicM.SakacJ.AjdukovicA.GakovicV.Kojicet alSynthesis and biological evaluation of some new 2-oxazoline and salicylic acid derivativesARKIVOCii201183102
- A.Q.M.MohsenD.MulveyJ.D.PriddleD.S.ParsonsD.P.JewellEffects of olsalazine in the jejunum of the ratGut281987346352
- A.BrukeE.SmythG.FitzA.GarretGoodman and Gilman's the pharmacological basis of therapeutics11th ed2006McGraw-HillNew York
- P.P.CliveJ.C.MichaelS.MorleyW.MichaelH.BrianFarmacogia integrada1998ElsevierEspaña
- S.M.MosaadM.K.MohsenM.M.K.EmadA.NagehM.KhedrF.A.MarwaSynthesis, biological evaluation and molecular docking of quinazoline-4(1H)-one derivatives as anti-inflammatory and analgesic agentsActa Pol Pharm6852011665675
- B.P.MariappanB.P.SahaL.SutharsonA.HaldarSynthesis and bioactive evaluation of pyrazolone derivativesInd J Chem49B201016711674
- B.SamirR.RamyA.E.HassanA.F.AhmedSynthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moietyEur J Med Chem43200821222129
- S.KeinenenS.SimilaK.KouvalainenOral antipyretic therapy: evaluation of the N-aryl-anthranilic acid derivatives mefenamic acid, tolfenamic acid and fulfenamic acidEur J Clin Pharmacol131978331334
- J.SahooS.K.MekapP.Sudhir KumarSynthesis, spectral characterization of some new 3-Heteroaryl azo 4-Hydroxy coumarin derivatives and their antimicrobial evaluationJ Taibah Univ Sci2014http://dx.doi.org/doi:10.1016/j.jtusci
- B.PradhanS.K.DashS.SahooScreening and characterization of extracellular L-asparaginase producing Bacillus subtilis strain hswx88, isolated from Taptapani hot spring of Odisha, IndiaAsian Pac J Trop Biomed32013936941
- A.H.ShridharJ.KeshavayyaS.K.PeethambarH.Joy HoskeriSynthesis and biological activities of Bis alkyl 1, 3, 4-oxadiazole incorporated azo dye derivativesArab J Chem2012http://dx.doi.org/10.1016/j.arabjc.2012.04.018
- M.AndrewsDetermination of minimum inhibitory concentrationsJ Antimicrob Chemother482001516
- A.G.JagtapB.B.FernandesAnalgesic potential of Pterocarpus indicusInd Drug4582008649654
- K.DustinS.NatalieCrystal structure of New Delhi metallo-b-lactamase reveals molecular basis for antibiotic resistanceProtein20201114841491