?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A new series of pyrazolo[3,4-d]pyrimidine-3-carbonitrile and pyrazolo[3,4-b]pyridine-3-carbonitrile derivatives was synthesized by the reaction of 5-amino-1-tosyl-1H-pyrazole-3,4-dicarbonitrile as a key starting material with various electrophilic and nucleophilic reagents. All the newly synthesized compounds were structurally confirmed by various modern analytical methods (IR, 1H NMR and MS). All the title compounds have been evaluated for their potential cytotoxicity against human laryngeal epidermoid carcinoma cells (Hep2). Among the screened compounds, 3 and 4 exhibited the highest significant effect.
1 Introduction
Pyrazole derivatives are characterized by their biological and pharmacological activities as potential inhibitors of HIV-1 [Citation1], pesticides [Citation2], fungicides [Citation3], antihypertensive agents [Citation4] and anticancer activity [Citation5]. They are also important and useful precursors for the synthesis of other fused heterocyclic systems, among these pyrazolo[3,4-d]pyrimidine derivatives [Citation6], which have a considerable chemical and pharmacological importance as purine analogues [Citation7–Citation[8]Citation9]. In the literature, it was found that the replacement of 1H of pyrazole of pyrazolo[3,4-d]pyrimidine ring system by some other bioactive moieties drastically alters its pharmacological properties [Citation10]. Also, the pyrazolo[3,4-b]pyridine derivatives represent important building blocks in both natural and synthetic bioactive compounds [Citation11]. They show anxiolytic activity along with xanthine oxidase inhibitors, cholesterol formation inhibitor, and anti-Alzheimer [Citation12]. Moreover, fused heterocyclic containing pyrazolopyridine systems have been described to be associated with several biological and medicinal activities [Citation13–Citation[14]Citation16]. In the present work, some new 1-tosyl-pyrazolo[3,4-d]pyrimidine and 1-tosyl-pyrazolo[3,4-b]pyridine derivatives incorporating the tosyl moiety were synthesized to evaluate their potential cytotoxicity against epidermoid carcinoma of the larynx (Hep2).
2 Experimental
2.1 Materials and methods
All melting points (uncorrected) were determined on an electrothermal Gallenkamp melting point apparatus. The IR spectra were recorded in KBr disks on a Thermo Scientific Nicolet iS 50 FT-IR spectrometer (not all frequencies are reported). The NMR spectra were acquired using a Bruker WP 300 spectrometer at 300 MHz using TMS as an internal standard and DMSO-d6 as solvent. The mass spectra were performed using a LC-MS (Shimadzu-Mass spectrometer) at 70 eV. Elemental analyses were carried out at the Microanalytical Unit, Cairo University, Giza, Egypt; the results were in satisfactory agreement with the calculated values.
2.1.1 5-amino-1-tosyl-1H-pyrazole-3,4-dicarbonitrile (2)
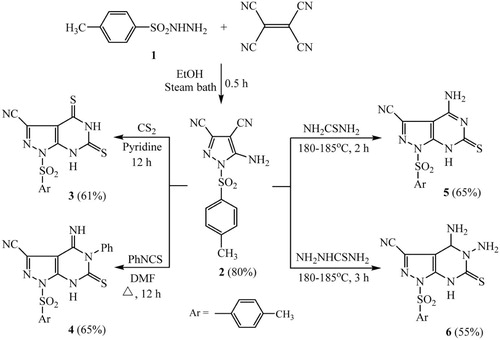
To a cold solution of p-toluenesulfonyl hydrazide (0.05 mol, 9.3 g) in 50 ml ethanol, tetracyanoethylene (0.05 mol, 6.4 g) was added. The reaction mixture was stirred for 1 hour and then heated under reflux on a steam bath for 30 minutes. The mixture was then cooled and the white precipitate was collected and washed with ethanol.
Yield 80%; mp (°C); 213–215 (Lit. m.p. = 214–216 °C) [Citation17]; IR (): 3313, 3246 (NH2), 2238 (C–N), 1639 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.30 (s, 3H, CH3), 7.10 (d, 2H, Ar-H, J = 7.95 Hz), 7.50 (d, 2H, Ar-H, J = 1.80 Hz), 7.85 (s, 2H, NH2); MS (EI): m/z (%) = 287 (molecular ion, 19), 272 (14), 242 (7), 215 (33), 195 (8), 173 (15), 155 59), 139 (6), 114 (37), 91 (base peak, 100), 77 (23), 65 (40). Analysis for C12H9N5O2S (287.30): Calcd.%: C, 50.17; H, 3.16; N, 24.38; Found%: C, 50.28; H, 3.11; N, 24.45.
2.1.2 4,5,6,7-tetrahydro-4,6-dithioxo-1-tosyl-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (3)
To a stirred suspension of compound 2 (2 mmol, 0.57 g) in pyridine (20 ml), carbon disulfide (4 mmol, 0.3 ml) was added dropwise. The reaction mixture was then heated on water bath for 12 hrs. The reaction mixture was cooled at room temperature, then poured into ice cold water, and neutralized with hydrochloric acid. The precipitated product was filtered off, washed and recrystallized from EtOH-DMF mixture (1:1) to afford the corresponding product 3 as dark green crystals.
Yield 61%, mp (°C); > 300; IR (): 3437 (NH), 2221 (C–N), 1638 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.25 (s, 3H, CH3), 7.10 (d, 2H, Ar-H, J = 7.95 Hz), 7.50 (d, 2H, Ar-H, J = 1.80 Hz), 11.22 (s, 1H, NH), 13.69 (s, 1H, NH); MS (EI): m/z (%) = 363 (molecular ion, 20.1), 200 (21.2), 172 (17.7), 133 (48.1), 91 (188.7), 80 (base peak, 100), 64 (63.7). Analysis for C13H9N5O2S3 (363.44): Calcd.%: C, 42.96; H, 2.50; N, 19.27; Found%: C, 42.82; H, 2.56; N, 19.35.
2.1.3 4-Imino-5-phenyl-6-thioxo-1-tosyl-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (4)
A mixture of compound 2 (2 mmol, 0.57 g) and phenyl isothiocyanate (2 mmol, 0.24 ml) in 15 ml DMF was refluxed for 12 hrs. The reaction mixture was allowed to cool at room temperature and then poured into ice cold water. The precipitate that formed was filtered off, dried and then recrystallized from EtOH to afford 4 as yellowish brown crystals.
Yield 65%; mp (°C); 155–157; IR (): 3374 (NH), 3180 (NH), 2222 (C–N), 1632 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.35 (s, 3H, CH3), 6.90–7.35 (m, 7H, Ar-H), 7.65 (d, 2H, Ar-H, J = 1.85 Hz), 10.25 (s, 1H, NH), 12.45 (s, 1H, NH); MS (EI): m/z (%) = 422 (molecular ion, 6), 372 (17), 345 (20), 329 (31), 311 (41), 297 (44), 282 (35), 268 (46), 253 (base peak, 100), 236 (65), 210 (19), 182 (18), 155 (11), 133 (26), 107 (33), 93 (60), 77 (65), 65 (20). Analysis for C19H14N6O2S2 (422.48): Calcd.%: C, 54.01; H, 3.34; N, 19.89; Found%: C, 54.18; H, 3.41; N, 19.78.
2.1.4 4-Amino-6-thioxo-1-tosyl-6,7-dihydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (5)
A mixture of compound 2 (2 mmol, 0.57 g) and thiourea (2 mmol, 0.16 g) was heated together in oil bath at 180–185 °C for 2 hrs. After cooling, the resulting solid was dissolved in dilute sodium hydroxide and then acidified with dil. HCl to give the crude product 5. The solid product that formed was filtered off, dried and then recrystallized from EtOH to furnish 5 as dark yellow crystals.
Yield 65%; mp (°C); > 300; IR (): 3337, 3193 (NH and NH2), 2221 (C–N), 1640 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.35 (s, 3H, CH3), 6.40 (s, 2H, NH2), 7.25 (d, 2H, Ar-H, J = 8.05 Hz), 7.65 (d, 2H, Ar-H, J = 1.80 Hz), 11.25 (s, 1H, NH); MS (EI): m/z (%) = 346 (molecular ion, 9.1), 246 (40.2), 172 (21.0), 133 (19.9), 107 (32.6), 91 (base peak, 100), 77 (32.0), 65 (41.7). Analysis for C13H10N6O2S2 (346.39): Calcd.%: C, 45.08; H, 2.91; N, 24.26; Found%: C, 45.29; H, 2.80; N, 24.38.
2.1.5 4,5-Diamino-6-thioxo-1-tosyl-4,5,6,7-tetrahydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (6)
A mixture of compound 2 (2 mmol, 0.57 g) and thiosemicarbazide (2 mmol, 0.19 g) was fused in oil bath at 180–185 °C for 3 hrs under dry conditions. The fused mixture was heated in ethanol (15 ml) and the precipitate that formed on cooling was collected by filtration, dried and recrystallized from EtOH:DMF mixture (1:1) to furnish compound 6 as brown crystals.
Yield 55%; mp (°C); > 300; IR (): 3417, 3332, 3195 (NH and NH2), 2218 (C–N), 1632 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.35 (s, 3H, CH3), 5.20 (s, 1H, CH), 6.15 (s, 2H, NH2), 7.25 (d, 2H, Ar-H, J = 8.10 Hz), 7.70 (d, 2H, Ar-H, J = 1.80 Hz), 8.35 (s, 2H, NH2), 11.10 (s, 1H, NH); MS (EI): m/z (%) = 363 (molecular ion, 20.0), 304 (29.2), 266 (45.4), 246 (68.4), 190 (64.8), 172 (28.7), 133 (17.0), 107 (34.6), 91 (100.0), 77 (35.6), 65 (44.8). Analysis for C13H13N7O2S2 (363.42): Calcd.%: C, 42.97; H, 3.61; N, 26.98; Found%: C, 43.14; H, 3.72; N, 26.86.
2.1.6 4-Oxo-1-tosyl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (7)
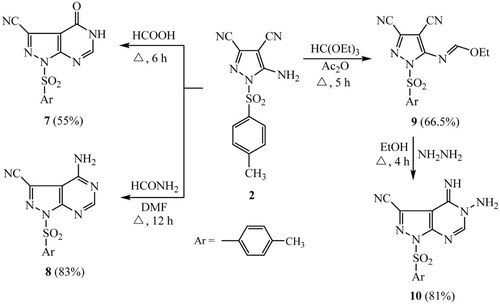
A suspension of compound 2 (2 mmol, 0.57 g) in excess of formic acid (10 ml) was refluxed on sand bath for 6 hrs. After cooling, the mixture was diluted by cooled water (10 ml) and the precipitate that formed was filtered off, dried and purified by recrystallization from EtOH to give 7 as yellowish brown powder.
Yield 55%; mp (°C); 266–268; IR (): 3253 (NH), 2240 (C–N), 1668 (C–O); 1H NMR (DMSO-d6): δ/ppm = 2.40 (s, 3H, CH3), 7.23 (d, 2H, Ar-H, J = 7.90 Hz), 7.64 (d, 2H, Ar-H, J = 1.80 Hz), 8.20 (s, 1H, pyrimidine CH–N), 9.45 (s, 1H, NH); MS (EI): m/z (%) = 315 (molecular ion, 6.8), 203 (55.7), 179 (base peak, 100), 161 (13.3), 136 (28.0), 79 (33.5), 52 (31.0). Analysis for C13H9N5O3S (315.31): Calcd.%: C, 49.52; H, 2.88; N, 22.21; Found%: C, 49.71; H, 2.93; N, 22.27.
2.1.7 4-Amino-1-tosyl-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (8)
A mixture of pyrazole 2 (2 mmol, 0.57 g) and formamide (10 ml) in DMF (10 ml) was heated under reflux for 12 hrs. The reaction mixture was allowed to cool at room temperature and poured onto 200 ml ice-cooled water. The yellow precipitate that formed was filtered off, dried and recrystallized from EtOH to give 8 as a yellow powder.
Yield 83%; mp (°C); 133–135; IR (): 3323, 3166 (NH2), 2235 (C–N), 1636 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.35 (s, 3H, CH3), 7.30 (d, 2H, Ar-H, J = 8.05 Hz), 7.55 (s, 2H, NH2), 7.75 (d, 2H, Ar-H, J = 1.75 Hz), 8.25 (s, 1H, pyrimidine CH–N). Analysis for C13H10N6O2S (314.32): Calcd.%: C, 49.67; H, 3.21; N, 26.74; Found%: C, 49.48; H, 3.13; N, 26.82.
2.1.8 Ethyl N-3,4-dicyano-1-tosyl-1H-pyrazol-5-yl formimidate (9)
To a mixture of triethyl orthoformate (5 mmol, 0.75 ml) and acetic anhydride (20 ml), the pyrazole 2 (5 mmol, 1.44 g) was added. The reaction mixture was refluxed for 5 hrs and the solvent was removed under reduced pressure. The separated solid was recrystallized from ethanol to afford 9 as a yellow powder.
Yield 66.5%; mp (°C); 145–147°; IR (): 2233 (C–N), 1638 (C–N); 1H NMR (DMSO-d6): δ/ppm = 1.20 (t, 3H, CH3, J = 7.15 Hz), 2.35 (s, 3H, CH3), 4.40 (q, 2H, OCH2, J = 7.15 Hz), 7.30 (d, 2H, Ar-H, J = 8.10 Hz), 7.55 (s, 1H, N–CH), 7.80 (d, 2H, Ar-H, J = 1.75 Hz). Analysis for C15H13N5O3S (343.36): Calcd.%: C, 52.47; H, 3.82; N, 20.40; Found%: C, 52.63; H, 3.88; N, 20.53.
2.1.9 5-Amino-4-imino-1-tosyl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (10)
To a solution of compound 9 (2 mmol, 0.68 g) in absolute ethanol (50 ml), hydrazine hydrate (4 mmol, 0.2 ml) was added. The reaction mixture was refluxed for 4 hrs, concentrated and cooled. The solid product that separated out was filtered off and recrystallized from EtOH to yield 10 as a yellow powder.
Yield 81%; mp (°C); 186–188; IR (): 3342, 3216 (NH and NH2), 2236 (C–N), 1635 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.35 (s, 3H, CH3), 7.30 (d, 2H, Ar-H, J = 7.95 Hz), 7.45 (s, 2H, NH2), 7.80 (d, 2H, Ar-H, J = 1.80 Hz), 8.15 (s, 1H, pyrimidine CH), 11.35 (s, 1H, NH). Analysis for C13H11N7O2S (329.34): Calcd.%: C, 47.41; H, 3.37; N, 29.77; Found%: C, 47.58; H, 3.29; N, 29.86.
2.1.10 5-Acetyl-4-amino-6-hydroxy-1-tosyl-1H-pyrazolo[3,4-b]pyridine-3-carbonitrile (12)
A mixture of compound 2 (4 mmol, 1.36 g), potassium carbonate (6 mmol, 0.83 g) and ethyl acetoacetate (4 mmol, 0.52 ml) in 15 ml DMF was heated under reflux for 12 hrs. The reaction mixture was allowed to cool at room temperature, poured into ice-cooled water and then neutralized by drops of dilute HCl. The solid product that obtained by filtration was purified by recrystallization from EtOH to obtain 12 as brown crystals.
Yield 65%; mp (°C); 305–308; IR (): 3293, 3201, 3140 (NH2 and OH), 2240 (C–N), 1702 (C–O), 1648 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.35 (s, 3H, CH3), 2.50 (s, 3H, CH3), 6.02 (s, 2H, NH2), 7.35 (d, 2H, Ar-H, J = 8.05 Hz), 7.75 (d, 2H, Ar-H, J = 1.80 Hz), 11.55 (s, 1H, OH); MS (EI): m/z (%) = 371 (molecular ion, 43), 199 (base peak, 100), 170 (19), 158 (11), 77 (5), 67 (20). Analysis for C16H13N5O4S (371.37): Calcd.%: C, 51.75; H, 3.53; N, 18.86; Found%: C, 51.62; H, 3.61; N, 18.94.
2.1.11 4,6-diamino-1-tosyl-1H-pyrazolo[3,4-b]pyridine-3,5-dicarbonitrile (13)
A mixture of compound 2 (4 mmol, 1.36 g) and malononitrile (4 mmol, 0.27 g) in 15 ml DMF containing few drops of piperidine was refluxed for 12 hrs. The reaction mixture was allowed to cool to room temperature, diluted with cold water and then neutralized by few drops of dilute HCl. The resulting solid was filtered off, washed several times with cold water, dried and then recrystallized from EtOH:DMF mixture (1:1) to afford 13 as brown crystals.
Yield 67%; mp (°C); > 300; IR (): 3410, 3335, 3245 (NH2), 2216 (C–N), 1651 (C–N); 1H NMR (DMSO-d6): δ/ppm = 2.40 (s, 3H, CH3), 6.45 (s, 2H, NH2), 7.42 (d, 2H, Ar-H, J = 8.05 Hz), 7.61 (d, 2H, Ar-H, J = 1.80 Hz), 8.47 (s, 2H, NH2); MS (EI): m/z (%) = 353 (molecular ion, 8.2), 199 (57.3), 143 (58.5), 115 (33.2), 80 (49.5), 55 (base peak, 100). Analysis for C15H11N7O2S (353.36): Calcd.%: C, 50.99; H, 3.14; N, 27.75; Found%: C, 50.78; H, 3.22; N, 27.65.
2.2 In vitro antitumor activity
2.2.1 Cell line
Epidermoid carcinoma (larynx) Hep2 was obtained from ATCC via a holding company for biological products and vaccines (VACSERA), Cairo, Egypt. 5-Fluorouracil was used as a standard anticancer drug for comparison.
2.2.2 Chemical reagents
The reagents were RPMI-1640 medium, MTT, DMSO and 5-fluorouracil (Sigma Co., St. Louis, USA), and fetal bovine serum (GIBCO, UK).
2.2.3 MTT assay [Citation18]
The cell line mentioned above was utilized to determine the inhibitory effects of compounds on cell growth utilizing the MTT test. This colorimetric test is based on the transformation of the yellow 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to a purple formazan derivative by mitochondrial succinate dehydrogenase in practical cells. Hep2 was refined in RPMI-1640 medium with 10% fetal bovine serum. The anti-toxins included were 100 units/ml penicillin and 100 µg/ml streptomycin at 37 °C in a 5% CO2 incubator. The cell line was seeded in a 96-well plate at a density of 1 × 104 cells/well [Citation19] at 37 °C for 48 hours under 5% CO2. After incubation the cells were treated with distinctive concentration of compounds and incubated for 24 hours. After 24 hours of medication treatment, 20 µl of MTT solution at 5 mg/ml was added and incubated for 4 hours. Dimethyl sulfoxide (DMSO) in volume of 100 µl is added into each well to dissolve the purple formazan formed. The colorimetric test is measured and recorded at the absorbance of 570 nm utilizing a plate reader (EXL 800, USA). The relative cell viability in percentage was ascertained as: (A570 of treated samples/A570 of untreated sample) × 100.
3 Results and discussion
3.1 Chemistry
The key starting material 5-amino-1-tosyl-1H-pyrazole-3,4-dicarbonitrile (2) was synthesized in 80% yield by refluxing p-toluenesulfonyl hydrazide (1) with tetracyanoethylene in absolute ethanol [Citation17]. The ease synthesis and expected biological activity of the starting compound prompted us to prepare some 3-cyano-1-tosyl-pyrazolo[3,4-d]pyrimidine and 3-cyano-1-tosyl-pyrazolo[3,4-b]pyridine derivatives starting from available and inexpensive compounds such as carbon disulfide, thiourea, formic acid and ethyl acetoacetate. Thus, heating of the pyrazole compound 2 with carbon disulfide in pyridine for 12 hours furnished the corresponding 4,6-dithioxo-1-tosyl-1H-pyrazolo[3,4-d]pyrimidine derivative 3 in good yield (Scheme 1). The chemical structure of 3 was secured by its correct elemental analysis and spectral data. Its IR spectrum showed absorption bands at 3437 and 2221 cm−1 for the imino (NH) and nitrile (C–N) functions. The 1H NMR spectrum revealed singlet signal at 2.25 ppm for methyl protons (CH3), two doublet signals at 7.10 and 7.50 ppm for para-disubstituted benzene ring, and two singlet signals at NH at 11.22 and 13.69 ppm for two imine protons (NH). The reaction of pyrazole compound 2 with phenyl isothiocyanate proceeded by heating under reflux in dimethyl formamide to afford the corresponding 4-imino-5-phenyl-6-thioxo-1-tosyl-1H-pyrazolo[3,4-d]-pyrimidine derivative 4. The chemical structure of 4 was established on the basis of elemental and spectroscopic analyses. The IR spectrum showed two absorption bands at 3374 and 3180 cm−1 for two (NH) functions, 2222 cm−1 for the nitrile function (C–N), and 1632 cm−1 for the (C–N) function. The 1H NMR spectra of 4 revealed the presence of singlet signal at 2.35 ppm for methyl protons (CH3), and two singlet signals at 10.25 and 12.45 ppm of two NH protons, in addition to the aromatic protons as multiplet (7H) and doublet (2H) in the range 6.90–7.65 ppm. The mass spectrum showed the molecular ion peak at m/z = 422 corresponding to the molecular weight of its chemical formula C19H14N6O2S2.
Fusion of pyrazole 2 with thiourea furnished one product on TLC, which identified as 4-amino-6-thioxo-1-tosyl-6,7-dihydro-1H-pyrazolo[3,4-d]-pyrimidine-3-carbonitrile (5) on the basis of its elemental analysis and spectral data. The IR spectrum showed absorption bands at 3337 and 3193 cm−1 for the amino function (NH2) and 2221 cm−1 for the nitrile function (C–N). The 1H NMR spectrum exhibited singlet signal at 2.35 ppm referring to three protons of methyl group (CH3), singlet signal at 6.40 ppm for two protons of amino group (NH2), two doublet signals at 7.25 and 7.65 ppm for para-disubstituted benzene ring, and a further singlet signal appear downfield at 11.25 ppm corresponding to (NH) proton.
In the same way with compound 5, 4,5-diamino-6-thioxo-1-tosyl-1H-pyrazolo[3,4-d]pyrimidine derivative 6 was prepared by fusion of the starting compound 2 and thiosemicarbazide. The IR spectrum showed absorptions bands at 3417, 3332, and 3195 cm−1 referring to (NH) and (NH2) groups, 2218 cm−1 for the nitrile function (C–N), and absorption band at 1632 cm−1 for the (C–N) function. The 1H NMR spectrum revealed singlet signal at 2.35 ppm for methyl protons (CH3), singlet signal at 5.20 ppm for (CH) proton, two singlet signals of two amino (NH2) groups at 6.15 and 8.35 ppm, two doublet signals at 7.25 and 7.70 ppm for the aromatic protons, and singlet signal at 11.10 ppm corresponding to imino (NH) group.
When compound 2 reacted with formic acid at reflux temperature afforded 4-oxo-1-tosyl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile 7 (Scheme 2). The structure of 7 was confirmed by elemental analysis and spectral data. IR spectrum showed absorption band at 3253 cm−1 referring to imino group (NH), band of nitrile group (C–N) was detected at 2240 cm−1, and a sharp peak appeared at 1668 cm−1 for the amidic carbonyl group (C–O). The 1H NMR spectrum of 7 revealed singlet signal at 2.40 ppm corresponding to protons of methyl group (CH3), two doublet signals at 7.23 and 7.64 ppm for the para-disubstituted benzene ring, singlet signal at 8.20 ppm referring to proton of (CH–N) function, and singlet signal at 9.45 ppm for imino (NH) group proton.
Heating of pyrazole compound 2 with formamide in dimethyl formamide (DMF) as a solvent afforded the corresponding 4-amino-1-tosyl-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile (8). The structure of 8 was established on the basis of elemental analysis and spectral data. The IR spectrum showed absorption bands at 3323 and 3166 cm−1 referring to the amino (NH2) group, 2235 cm−1 for the nitrile group (C–N) and 1636 cm−1 for the (C–N) function. The 1H NMR spectrum of 8 revealed singlet signal at 2.35 ppm for three protons of methyl group (CH3), and two doublet signals at 7.30 and 7.55 ppm for the aromatic protons, in addition to singlet signal at 8.25 ppm characteristic to the pyrimidine proton (CH–N).
Heating of compound 2 with triethyl orthoformate in acetic anhydride afforded ethyl N-(3,4-dicyano-1-tosyl-1H-pyrazol-5-yl)formimidate 9, which underwent heterocyclization with hydrazine hydrate in absolute ethanol, afforded 5-amino-4-imino-1-tosyl-4,5-dihydro-1H-pyrazolo[3,4-d]pyrimidine-3-carbonitrile 10 in good yield. The IR spectrum showed absorption bands at 3342 and 3216 cm−1 for the (NH and NH2) groups, 2236 cm−1 due to the presence of nitrile (C–N) function and 1635 cm−1 for the (C–N) group. The 1H NMR of 10 showed singlet signal at 2.35 ppm for three protons of methyl group (CH3), two doublet signals of the aromatic protons at 7.30 and 7.80 ppm, and singlet signal at 8.15 ppm due to pyrimidine C-2 proton, and it also revealed two singlet signals of NH2 and C–NH protons at 7.45 and 11.35 ppm respectively.
Heating of compound 2 with ethyl acetoacetate in dimethyl formamide in the presence of an equimolar amount of potassium carbonate gave one spot on TLC using ethyl acetate:petroleum ether mixture (2:3) as eluent, the obtained product was identified as 5-Acetyl-4-amino-6-hydroxy-1-tosyl-1H-pyrazolo-[3,4-b]pyridine-3-carbonitrile (12) on the basis of elemental and spectral analyses (Scheme 3). The formation of 12 was proposed to be continued through heterocyclization of the expected intermediate 5-acetyl-4-amino-6-hydroxy-1-tosyl-1H-pyrazolo[3,4-b]pyridine-3-carbonitrile (11). The IR spectrum showed absorption bands at 3293, 3201 and 3140 cm−1 for (OH and NH2) groups, 2240 cm−1 corresponding to nitrile function (C–N), 1702 cm−1 due to C–O group and 1648 cm−1 referring to (C–N) function. The 1H NMR revealed two singlet signals at 2.35 and 2.50 ppm for six protons of two methyl groups (2CH3), singlet signal at 6.02 ppm referring to the (NH2) protons, two doublet signals at 7.35 and 7.75 ppm for para-disubstituted benzene ring protons, and singlet signal at 11.55 ppm corresponding to (OH) proton. The mass spectrum showed the molecular ion peak at m/z = 371 corresponding to the molecular weight of the molecular formula C16H13N5O4S.
Heating of compound 2 with malononitrile in refluxing DMF containing piperidine produces a sole product that identified as 4,6-diamino-1-tosyl-1H-pyrazolo[3,4-b]pyridine-3,5-dicarbonitrile 13, in 67% isolated yield. Presumably, ionized malononitrile attacks the cyano group in compound 2 before the amino group of the latter attacks one of the malononitrile cyano groups resulting in ring formation. The IR spectrum showed absorption peaks at 3410, 3335 and 3245 cm−1 due to the presence of amino groups (NH2), 2216 cm−1 due to the nitrile function (C–N) and 1651 cm−1 corresponding to (C–N) function. The 1H NMR spectrum exhibited singlet signal at 2.40 ppm corresponding to the methyl protons (CH3), two singlet signals at 6.42 and 8.47 ppm for the two amino groups (NH2), while the doublet signals at 7.42 and 7.61 ppm were assigned for the aromatic protons.
3.2 In vitro antitumor activity
The antitumor activity of the synthesized compounds were evaluated against human laryngeal epidermoid carcinoma cell line (Hep2) using the MTT assay method. The results acquired in demonstrated that compounds 3 (IC50 36.9 µM) and 4 (IC50 21.3 µM) have the highly significant effect compared to the standard anticancer drug 5-fluorouracil (IC50 41.5 µM). This may be because of the presence of such NH-C–S moiety in addition to the N-tosyl-pyrazolo-pyrimidine moiety in the two compounds. The other compounds showed low impact against epidermoid carcinoma (Hep2).
Table 1 Cytotoxic activity of the synthesized compounds against epidermoid carcinoma cell line (Hep2).
Acknowledgment
The authors would like to thank Mr. Ahmed Abas, the researcher at Drugs Department, Faculty of Pharmacy, Mansoura University, Egypt, for kindly providing his technical assistance during the antitumor activity testing.
References
- A.M.Kamal El-DeanA.M.ElkhawagaS.RadwanM.M.AhmedSynthesis of some pyridothienopyrazolopyrimidopyrimidine and mercaptomethylpyrazolopyrimidine derivativesPhosphorous Sulfur Silicon184200920342048
- MinG.C.EricY.LihuA general method for the preparation of 3-acyl-4-cyano-5-amino-pyrazolesTetrahedron Lett47200657975799
- A.E.HassanM.M.AbdellaA.I.AhmedStudies on the synthesis and cyclization reactions of 2-(5-Amino-3-arylpyrazol-1-yl)-3-methylquinoxalinesJ Chem Res (S)1997322323
- A.A.AlySynthesis of polyfunctionally substituted pyrazolonaphthyridine, pentaazanaphthalene, and heptaazaphenanthrene derivativesPhosphorous Sulfur Silicon181200623952409
- E.R.AymnI.H.MohamedE.A.M.RandaF.NahedM.E.A.M.FaroukSynthesis and anti-HSV-1 evaluation of some pyrazoles and fused pyrazolopyrimidinesEur J Med Chem44200932853292
- A.M.ElkhawagaA.M.Kamal El-DeanM.RadwanM.M.AhmedSynthesis of some imidazopyrazolopyrimidines, pyrazolopyrimidopyrimidines and pyrazolopyrimidothiazinesBull Korean Chem Soc302009561566
- S.SchenoneO.BrunoM.RadiM.Botta4-amino-substituted pyrazolo[3,4-d]pyrimidines: synthesis and biological propertiesMini-Rev Org Chem62009220233
- I.A.EljaziA.A.SamerHeterocyclic o-aminonitriles: preparation of pyrazolo[3,4-d]-pyrimidines with modification of the substituents at the 1-positionMolecules62001621638
- M.S.AbdellatifM.F.O.AnaM.R.LigiaHeterocyclic synthesis with nitriles: synthesis of pyrazolopyrimidine and pyrazolopyridine derivativesSynth Commun39200911861195
- B.S.HollaM.K.ShivanandaP.M.AkberaliM.S.ShenoySynthesis and antibacterial activities of some fluorine containing arylfurylpropenones, pyrazolines and N-acetylpyrazolineIndian J Chem39B2000440447
- C.MouradS.ElenaS.AbdelouahidJ.Marco-ContellesStudies on the acetylation of 3,6-diamino-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile derivativesJ Heterocyclic Chem472010861872
- L.A.ZhmurenkoG.M.MolodavkinT.A.VoroninaV.P.LezinaSynthesis and antidepressant and anxiolytic activity of derivatives of pyrazolo[4,3-c ]pyridine and 4-phenylhydrazinonicotinic acidsPharm Chem J4620121519
- A.E.MohamedF.R.HalaM.B.DohaY.E.IbrahimMicrowave-assisted synthesis of some new pyrazolopyridines and their antioxidant, antitumor and antimicrobial activitiesEur J Med Chem662013415422
- M.A.A.FaragSynthesis and antimicrobial evaluation of some novel Bis-α,β-unsaturated ketones, nicotinonitrile, 1,2-dihydropyridine-3-carbonitrile, fused thieno[2,3-b]pyridine and pyrazolo[3,4-b]pyridine derivativesInt J Mol Sci14201329672979
- W.SteveA.A.KateriJ.B.AlexF.BainianL.G.SusanG.Stefanet alPyrazolopyridine inhibitors of B-RafV600E. Part 2: structure–activity relationshipsBioorg Med Chem Lett21201155335537
- Q.JunWeiZ.H.XianhaiD.PawanP.AnandanA.Robertet alDiscovery of a potent pyrazolopyridine series of γ-secretase modulatorsACS Med Chem Lett22011471476
- C.L.DickinsonJ.K.WilliamsB.C.McKusickAminocyanopyrazolesJ Org Chem29196419151919
- M.TimRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods6519835563D.FrancoisL.RitaRapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliabilityJ Immunol Methods891986271277
- J.M.HelenaN.H.NaderA.B.MichealH.G.DavidJ.S.MaryA.S.Kerriet alCombined effects of angiostatin and ionizing radiation in antitumour therapyNature3941998287291