?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
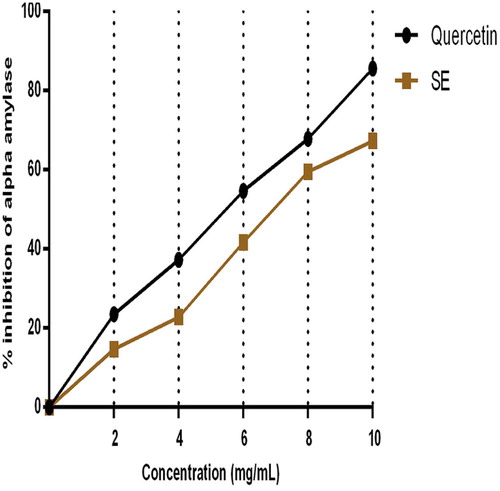
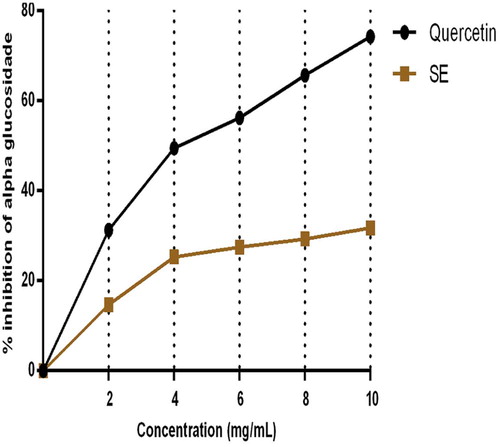
This study investigated the inhibitory effects of Sapium ellipticum (SE) (Hochst) Pax leaf extract on some carbohydrate metabolizing enzymes, pancreatic α-amylase and intestinal α-glucosidase activities in vitro. The inhibitory potential of SE extract was measured against Quercetin. SE extract in a dose dependent pattern significantly inhibited the activity of pancreatic α-amylase by 67.2% at 10 mg/mL. This effect was comparable to that of quercetin which offered 82.6% α-amylase inhibition. In terms of intestinal α-glucosidase activity, the inhibitory effect of the extract was significantly lower than that of quercetin at all investigated concentrations. At 10 mg mL−1 (maximum tested concentration), SE extract exhibited 35.8% inhibitory activity on α-intestinal glucosidase compared to the 74.3% exhibited by Quercetin at the same concentration. Phytochemical analysis results showed that SE contained 74 ± 3.12 milligram Gallic acid equivalents of total phenols and 67.2 ± 2.04 milligram Quercetin equivalents of flavonoids per gram of extract. Fourier Transformed Infra red spectroscopy (FT-IR) of SE extract revealed the presence of active functional groups reminiscence of polyphenols. Alpha amylase and α-glucosidase activities (in vivo) greatly contribute to postprandial hyperglycemia which is a great risk factor for type 2 diabetes mellitus. The inhibitory potential of SE extract on these enzymes as observed in this study suggests a positive and probable role of the extract in the management and treatment of diabetes mellitus, particularly, type 2.
1 Introduction
The effective role of medicinal plants in the management and treatment of diabetes mellitus is becoming increasingly popular. This is not only because of their ability to reverse elevated blood glucose through insulin-tropic functions but also as a result of their inhibitory effects on key carbohydrate metabolizing enzymes including pancreatic α-amylase and intestinal α-glucosidase. Digestion of complex dietary carbohydrates is the primary source of glucose in the blood, and pancreatic α-amylase and intestinal α-glucosidase are key enzymes in carbohydrate catabolism [Citation1,Citation2]. These enzymes contribute enormously to postprandial hyperglycemia, by breaking down complex carbohydrates such as starch into simple and absorbable units like glucose and fructose, leading to increased concentration of simple sugars in the blood. Availability of monosaccharides particularly glucose in the blood is what results in postprandial hyperglycemia which is a great risk factor for type 2 diabetes mellitus (T2DM) [Citation3,Citation4]. Conversely, inhibition of these enzymes significantly decreases the digestion and uptake of carbohydrates, thereby lowering the postprandial blood glucose concentration in T2DM patients [Citation5,Citation6].
Various orthodox drugs including acarbose and voglibose have been in use as inhibitors of α-glucosidase and α-amylase. However, they have been reported to excessively inhibit pancreatic α-amylase causing abnormal bacterial fermentation of undigested carbohydrates in the colon [Citation7,Citation8], which usually results in undesirable side effects such as abdominal distention, bloating, meteorism, and in some cases, diarrhea [Citation9].
A number of studies have reported the inhibitory ability of medicinal plants with hypoglycemic properties on α-glucosidase and α-amylase [Citation6,Citation10–Citation[11]Citation12]. Sapium ellipticum (Hochst) Pax has a number therapeutic applications in folk medicine [Citation13,Citation14], including treatment of diabetes (ethno botanical survey). It belongs to the family Euphorbiaceae and is commonly referred to as jumping seed tree. S. ellipticum is widely distributed in eastern and tropical Africa. In southwest part of Nigeria, particularly among the Ilorin indigenes, the plant is popularly known as aloko-ạgbọ.
A few scientific investigations have been carried out on it. Adesegun et al. [Citation15] in their in vitro study credited substantial antioxidant properties to the stem bark extract of the plant. Cytotoxicity screening of selected Nigerian plants used in traditional cancer treatment on HT29 (colon cancer) and MCF-7 (breast cancer) cell lines (HeLa cervix adenocarcinoma cells) indicated that S. ellipticum leaf extract expressed the highest cytotoxic activity among other plants with anticancer potential which was comparable to the reference drug, cisplatin [Citation16]. The phythochemical constituents, in vitro antioxidant capacities and antiplasmodial activities of S. ellipticum stem bark extracts were documented by Nana et al. [Citation17]. This present study sought to investigate the effects of the plant leaf extract on the activities of carbohydrate metabolizing enzymes such as pancreatic α-amylase and intestinal α-glucosidase which play significant roles in glucose homeostasis.
2 Materials and methods
2.1 Preparation of S. ellipticum leaf extract
The plant material was freed of extraneous materials; air dried at room temperature and milled to a fine powder, using a Waring blender. Three hundred grams of the powdered sample was macerated in 2.5 liters of the extracting solvent (ethanol). The mixture was allowed to stand for 72 h and stirred intermittently with a glass rod to facilitate extraction. Sieving of the mixture was achieved with a muslin cloth (maximum pore size 2 mm). The resulting filtrate on sieving was further filtered through Whatman filter paper (No 42) and subsequently reduced in volume with a rotary evaporator at 40 °C. Final elimination of solvent and drying was done using a regulated water bath at 40 °C.
2.2 Effect of SE extract on α-amylase activity in vitro
The method described by Bernfield [Citation18] was used to assess the effect of SE extract on α-pancreatic amylase activity. Quercetin was used as a reference compound. Various extract and quercetin concentrations ranging from 2 to10 mg/L were produced. One hundred microliters each of the concentrations was added to 500 µL of 0.02 M sodium phosphate buffer (pH 6.9 with 0.006 M NaCl) containing Porcine pancreatic α-amylase (EC 3.2.1.1) (0.5 mg/mL). The mixture in each case was incubated at 25 °C for 10 minutes. Then, 500 µL of 1% starch solution in 0.02 M sodium phosphate buffer (pH 6.9 with 0.006M NaCl) was added to each tube. The reaction mixture was incubated at 25 °C for 10 minutes and stopped with 1.0 mL of dinitrosalicylic acid color reagent. Thereafter, the mixture was incubated in a boiling water bath for 5 minutes, and cooled to room temperature. The reaction mixture was then diluted by adding 10 mL of distilled water, and absorbance was measured at 540 nm. A complete reaction mixture without quercetin or SE extract was used as control. The α-amylase inhibitory activity was calculated using the formula:
Where Acontrol is the absorbance of the control reaction (containing all reagents except the test extract or quercetin) and Asample is the absorbance of the test material or quercetin. The α-amylase inhibitory activity was expressed as percentage inhibition.
2.3 Effect of SE extract on α-glucosidase activity in vitro
Evaluation of the effect of SE extract on α-glucosidase activity was performed according to the procedure described by Apostolidis et al. [Citation19]. Quercetin was used as a reference compound. Various extract and quercetin solutions with concentrations ranging from 2 to 10 mg/L were prepared. Twenty microliters each of the solutions and 100 µL of α-glucosidase (EC 3.2.1.20) solution in 0.1M phosphate buffer (pH 6.9) were incubated at 25oC for 10 minutes. Then, 50 µL of 5 mMp-nitrophenyl-α-D-glucopyranoside solution in 0.1M phosphate buffer (pH 6.9) was added. The mixtures were incubated at 25 °C for 5 minutes, before reading the absorbance at 405 nm in the spectrophotometer. A complete reaction mixture without quercetin or SE extract was used as control. The α-glucosidase inhibitory activity was expressed as percentage inhibition and calculated using the formula:
2.4 Total phenol estimation
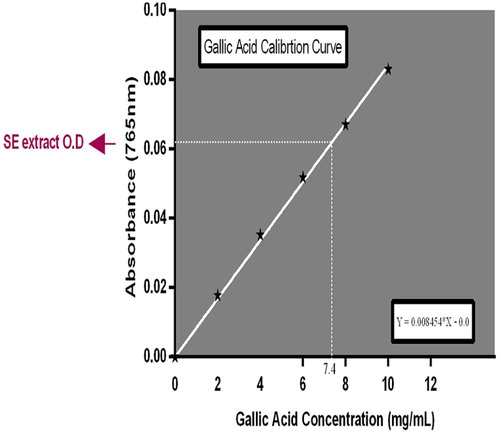
The total phenol content of SE extract was estimated using the Folin–Ciocalteu reagent method as described by Singleton et al. [Citation20] and Demiray et al. [Citation21]. The plant sample (100 mg mL−1, 1.0 mL) was mixed thoroughly with 5 mL Folin–Ciocalteu reagent (diluted ten-fold) and after 5 minutes, 4.0 mL of sodium carbonate (0.7 M) was added and the mixture was allowed to stand for 1 h with intermittent shaking; for color development. The absorbance was measured at 765 nm in a spectrophotometer against a blank. The blank solution contained the solvent used to dissolve the plant extract. Gallic acid was used as a standard. Serial dilution of 10 mg/mL of the standard was made to obtain a calibration curve. Total phenol contained in SE extract was calculated as gallic acid equivalents (GAE) using the following equation: C = (c × V)/m. Where C = Total Phenol, mg/g SE extract, in GAE, c = the concentration of gallic acid established from the calibration curve (mg/mL), V = the volume of extract in mL, and m = the weight of crude plant extract in g.
2.5 Total flavonoids estimation
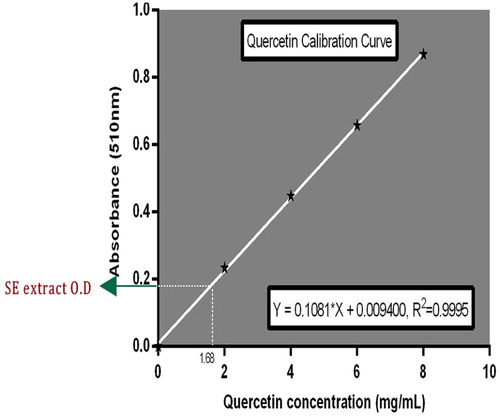
The total flavonoid content of SE extract was determined using a colorimetric method described by Zhishen et al. [Citation6] with slight modifications. SE (100 mg) was dissolved in 10 mL of 80% methanol and the resultant homogenous mixture was allowed to stand for 20 minutes at room temperature. This was followed by filtration through Whatman filter paper (No 42). An aliquot of 0.4 mL of the filtrate was mixed with 0.6 mL distilled water and 5% NaNO2 solution (0.0 6 mL). The mixture was allowed to stand for 5 minutes at room temperature. After 6 minutes 10% AlCl3 solution (0.0 6 mL) was added to the mixture. This was immediately followed by the addition of 1M NaOH (0.4 mL) and 0.45 mL distilled water to the mixture and allowed to stand for another 30 minutes. Absorbance of the mixture was determined at 510 nm. Quercetin calibration curve was prepared at the same wavelength (510 nm) and used for the quantification of total flavonoid content. The total content of flavonoid compounds in SE extract was calculated by the following equation: C = (c × V)/m. The result was expressed as milligram of Quercetin equivalents per gram (dry weight) of SE.
2.6 Fourier transformed infrared (FT-IR) spectroscopic analysis of SE crude extract
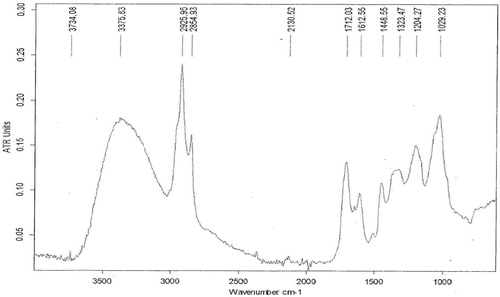
Dried ethanol extract was used for FT-IR analysis according to the combined methods of Hussein et al. [Citation22] and Huda et al. [Citation23]. 2 mg of SE sample was mixed with 100 mg potassium bromide (KBr)| (FT-IR grade) and then compressed to prepare a translucent sample disc (3 mm diameter). The disc was immediately kept in the sample holder. The sample was scanned and the FT-IR spectra were recorded in the absorption range between 1000 and 3500 nm. FT-IR analysis was performed using Perkin Elmer Spectrophotometer system, which was used to detect the characteristic peaks, types of chemical bonds and probable functional groups present in SE sample. The peak values of the FT-IR were recorded and the analysis was repeated twice for the spectrum confirmation.
2.7 Effect of SE on intestinal glucose absorption in normoglycemic rats
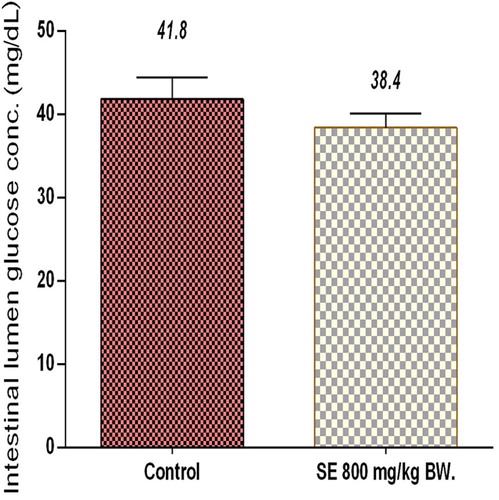
Twelve rats were fasted for 12 h and assigned randomly to 2 groups, each containing 6 rats. The control animals received corn oil (0.5 mL, p.o) while the experimental animals were administered SE (800 mg/kgBW, p.o). Thirty minutes, after the administrations, all rats in both groups were orally loaded with 10 mL 50% (w/v) of glucose solution per kilogram body weight. Thereafter, the rats were sacrificed and their small intestines were excised. Fifty milliliters of distilled water was then infused from one cut end of the intestine and the content was collected at the other end. The content was centrifuged at 3000 rpm for 5 minutes. Glucose level in the supernatant was then estimated by the glucose oxidase method using a Randox kit. The animals were not allowed access to laboratory chow and water ad libitum during the experiment.
3 Results
3.1 Effects of SE on α-amylase and α-glucosidase activities
The inhibitory ability of SE extract and quercetin on pancreatic α-amylase and α-glucosidase activities in vitro are represented by Figs. 1 and 2. Fig. 1 depicts that SE extract in a dose dependent pattern significantly inhibited the activity of pancreatic α-amylase affording 67.2% inhibition at 10 mg/mL. This effect was comparable to that of quercetin which offered 82.6% α-amylase inhibition. In terms of intestinal α-glucosidase activity, the effect of the extract was significantly lower than that of quercetin at all investigated concentrations. At 10 mg mL−1 (maximum tested concentration), SE extract exhibited 35.8% inhibitory activity on α-intestinal glucosidase compared to the 74.3% exhibited by Quercetin at the same concentration as summarized in Fig. 2.
3.2 Total phenolic and flavonoid content of SE
The total phenol content of SE leaf extract was estimated as 74.0 mg GEA/g of extract as shown in Fig. 3. The extract afforded total flavonoid content (C) of 65.2 mg QE/g of extract (Fig. 4).
From C = (c × V)/m, total flavonoid content = (1.68 × 0.4)/0.01 (10 mg) = 67.2 mg QE per gram of SE.
3.3 Effect of SE extract on intestinal glucose absorption
The effect of SE on glucose absorption is shown in Fig. 5. Compared to the control group, SE did not significantly influence intestinal glucose absorption in the experimental animals.
3.4 FT-IR spectra of SE extract
Fig. 6 shows the result of the Fourier Transform Infra Red (FT-IR) spectroscopic study of SE extract. The FT-IR spectra depict different characteristic peak values with various functional groups of compound (s) in SE extracts. Broad O–H stretching around 3734 cm−1 and 3375 cm−1 respectively depict the presence of an alcohol and amide functional groups. This justifies the presence of compound (s) with alcohol and amide characteristics in SE. Moderately to strong intense stretches around 2854 cm−1 and 2925 cm−1 show O–H stretching which respectively depict an amide and carboxylic acid functional groups. The stretching and bending around 1448 cm−1 and 1323 cm−1 respectively reveals an SP3 functionality which is indicative of the presence of saturated compounds in SE The stretch around 1712 though not of a strong absorption band is indicative of the presence of a carbonyl (C=O) in the crude extract. This carbonyl stretch suggest the presence of aldehydes, acyclic esters or cyclic esters and carboxylic acids in the crude extract.1612 cm−1 gives a clue as to the presence of a C=C functional group. The peak values, ATR units, chemical bonds, functional groups and possible groups of compounds present in SE are summarized in .
Table 1 FT-IR peak values and functional groups of ethanol extract of SE.
4 Discussion
Carbohydrate catabolism, release of glucose and transport across the intestinal brush border membrane down to the blood stream has in recent times attracted serious attention as potential targets to controlling postprandial hyperglycemia. According to Rains and Jain [Citation24] control of blood glucose concentration is a crucial factor in the management of diabetes and its associated complications. Inhibitors of α-amylase and α-glucosidase such as acarbose are known to delay carbohydrate breakdown and extend total carbohydrates digestion time, causing a reduction in the rate of glucose uptake and ultimately lowering postprandial blood glucose upsurge, especially in type 2 diabetes mellituspatients [Citation4,Citation25,Citation26]. Besides, Scorpiglione et al. [Citation27] had established that long-term inhibition of α-glucosidase partly decreased the level of HbA1c in diabetic patients, resulting in a significant reduction in the incidence of chronic vascular complication such as macro and micro vascular diseases.
In the same vein, inhibitors of α -amylase and α-glucosidase contained in plants have been found to largely suppress postprandial hyperglycemia in diabetes [Citation28–Citation[29]Citation30].
SE extract in the current study, significantly inhibited the activity of pancreatic α-amylase in vitro in a measure comparable to that offered by quercetin. The extract however failed to replicate the same level of activity when assessed with intestinal α-glucosidase. Its inhibitory effect on this enzyme was approximately half of its effect on pancreatic α-amylase. This observation is noteworthy, knowing that alpha-amylase catalyzes the first step in the hydrolysis of starch and other related polysaccharides by cleaving the internal α-1, 4-glycosidic bonds linking the monomers of these compounds, while α-glucosidase completes the hydrolysis to yield monosaccharides. Increased monosaccharide level (glucose) in the blood pool results in postprandial hyperglycemia which is an underlining factor in all forms of diabetes mellitus, particularly type 2 [Citation3,Citation4]. In light of this, the above findings suggest that SE extract will be more effective in delaying carbohydrate digestion into absorbable units than reducing the rate of intestinal glucose uptake. This postulation is substantiated by the fact that the extract did not significantly affect glucose absorption in the experimental rats relatively to their control counterparts (Fig. 5).
Quercetin is a well known flavonoid and its choice as a benchmark in this study is hinged on the fact that flavonoids are popular and efficient inhibitors of pancreatic α-amylase and intestinal α-glucosidase in vitro [Citation31,Citation32]. The hydroxyl group in the chemical structure of these polyphenolic compounds has been implicated in the inhibitory process. It is suggested to form hydrogen bonds with the polar side chains of the amino acids which line the allosteric site of the enzymes. This interaction readily results in alteration of an enzyme's molecular conformation as well as its hydrophilic and hydrophobic properties, leading to a decrease in enzyme activity [Citation32,Citation33], as observed with SE in this study. This may be associated with the high phenol and flavonoid contents of the extract (74 ± 3.12 milligram Gallic acid equivalents of total phenols and 67.2 ± 2.04 milligram Quercetin equivalents of flavonoids per gram of extract) as shown by the phytochemical analysis results (Figs. 3 and 4). Moreover, a number of peak values revealed by FT-IR spectroscopic analysis of SE extracts demonstrated the presence of functional groups which are indicative of flavonoids and polyphenols among other compounds. Besides, HPLC-MS analysis of SE fractions in a yet to be published study unveiled an array of polyphenols and flavonoids in the form Luteolin-7-O-glucoside and Amentoflavone. The presence of these compounds in SE leaf extract underscores its ability to inhibit the activities of pancreatic α-amylase and intestinal α-glucosidase in vitro as noted in this investigation.
5 Conclusion
The data obtained in this study indicate that SE extracts inhibited key enzymes involved in carbohydrate catabolism and thus suggestive of the possible role the plant extract in controlling postprandial blood glucose rise in the diabetics.
References
- B.MayurS.SandeshS.ShrutiS.Sung-YumAntioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides LJ Med Plants Res415201015471553
- L.J.ShaiP.MasokoM.P.MokgothoS.R.MaganoA.M.MogaleN.Boaduoet alYeast alpha glucosidase inhibitory and antioxidant activities of six medicinal plants collected in Phalaborwa, South AfricaS Afr J Bot762010465470
- M.F.Lopes-VirellaG.VirellaThe role of immune and inflammatory processes in the development of macrovascular disease in diabetesFront Biosci8Suppl.2003750768
- M.B.NarkhedeInvestigation of in vitro α-amylase and α-glucosidase inhibitory activities of polyherbal extractInt J Pharm Res Dev38201197103
- A.Fred-JaiyesimiA.KioW.Richardα-Amylase inhibitory effect of 3β-olean-12-en-3-yl (9Z)-hexadec-9-enoate isolated from Spondias mombin leafFood Chem1162009285288
- Y.ZhishenT.MeugchengW.JianmingDetermination of flavonoids content in mulberry and their scavenging effect on superoxide radicalsFood Chem641999555559
- H.BischoffPharmacology of alpha-glucosidase inhibitionEur J Clin Invest241994310
- S.KumarS.NarwalV.KumarO.Prakashα-Glucosidase inhibitor from plants. A natural approach to treat diabetesPharmacogn Rev520111929
- B.KimmelS.InzucchiOral agents for type 2 diabetes: an updateClin Diabetes2320056476
- M.I.KazeemO.G.RaimiR.M.BalogunA.L.OgundajoComparative study on the α-amylase and α-glucosidase inhibitory potential of different extracts of Blighia sapida KoenigAm J Res Commun172013178192
- G.N.KimJ.G.ShinH.D.JangAntioxidant and antidiabetic activity of Dangyuja (Citrus grandis Osbeck) extract treated with Aspergillus saitoiFood Chem11720093541
- G.ObohA.O.AdemosunO.V.OdubanjoI.A.AkinbolaAntioxidative properties and inhibition of key enzymes relevant to type-2 diabetes and hypertension by essential oils from black pepperAdv Pharmacol Sci5201317
- H.M.BurkillThe useful plants of west tropical Africa2nd edFamilies ADvol. 11985Royal Botanic GardensKew
- H.M.Burkill2nd edThe useful plants of west tropical Africavol. 1–31995Royal Botanic GardensKew
- S.A.AdesegunN.A.ElechiH.A.CokerAntioxidant activities of methanolic extract of Sapium ellipticumPak J Biol Sci112008453457
- A.SowemimoM.Van de VenterL.BaatjiesT.KoekemoerCytotoxicity evaluation of selected Nigerian plants used in traditional cancer treatmentJ Med Plants Res511201124422444
- O.NanaJ.MomeniR.Nzangué TepongningM.B.NgassoumPhythochemical screening, antioxydant and antiplasmodial activities of extracts from Trichilia roka and Sapium ellipticumJ Phytopharmacol2420132229
- H.BernfieldEnzymes of starch degradation and synthesisAdv Enzymol Relat Subj Biochem121951379428
- E.ApostolidisY.I.KwonK.ShettyInhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertensionInnov Food Sci Emerg Technol8120074654
- P.SingletonBacteria in biology, biotechnology and medicine4th ed1999John Wiley and Sons LtdNew York324337
- S.DemirayM.E.PintadoP.M.CastroEvaluation of phenolic profiles and antioxidant activities of Turkish medicinal plants: Tilia argentea, Crataegi folium leaves and Polygonum bistorta rootsWorld Acad Sci Eng Technol542009312317
- J.HudaO.H.AmeeraH.H.ImadA.K.MuhannedCharacterization of alkaloid constitution and evaluation of antimicrobial activity of Solanum nigrum by using (GC-MS)J Pharmacogn Phytother7420155672
- A.O.HusseinI.H.HameedH.JasimM.A.KareemDetermination of alkaloid compounds of Ricinus communis by using gas chromatography-mass spectroscopy (GC-MS)J Med Plants Res9102015349359
- J.L.RainsS.K.JainOxidative stress, insulin signaling, and diabetesFree Radic Biol Med5052011567575
- M.ShimabukuroN.HigaI.ChinenK.YamakawaN.TakasuEffects of a single administration of acarbose on postprandial glucose excursion and endothelial dysfunction in type 2 diabetic patients: a randomized crossover studyJ Clin Endocrinol Metab912006837842
- ZhangL.LiJ.S.HoganH.ChungG.E.WelbaumZhouK.Inhibitory effect of raspberries on starch digestive enzyme and their antioxidant properties and phenolic compositionFood Chem1192010592599
- N.ScorpiglioneM.BelfiglioF.CarinciD.CavaliereA.De CurtisM.Franciosiet alThe effectiveness, safety and epidemiology of the use of acarbose in the treatment of patients with type II diabetes mellitus. A model of medicine-based evidenceEur J Clin Pharmacol5541999239249
- S.FrantzL.CalvilloJ.TillmannsI.ElbingC.DieneschH.Bischoffet alRepetitive postprandial hyperglycemia increases cardiac ischemia/reperfusion injury: prevention by the alpha-glucosidase inhibitor acarboseFASEB J192005591593
- S.LordanT.J.SmythA.Soler-VilaC.StantonR.P.RossThe α-amylase and α-glucosidase inhibitory effects of Irish seaweed extractsFood Chem1413201321702176
- P.McCueD.VattemK.ShettyInhibitory effect of clonal oregano extracts against porcine pancreatic amylase in vitroAsia Pac J Clin Nutr1342004401408
- G.GriffithsL.TruemanT.CrowtherB.ThomasB.SmithOnions – a global benefit to healthPhytother Res1672002603615
- K.TaderaY.MinamiK.TakamatsuT.MatsuokaInhibition of alpha-glucosidase and alpha-amylase by flavonoidsJ Nutr Sci Vitaminol (Tokyo)5222006149153
- WangY.MaL.LiZ.DuZ.LiuZ.QinJ.et alSynergetic inhibition of metal ions and genistein on alpha-glucosidaseFEBS Lett57620044650