Abstract
It is belong to the order Squamata, family, Chamaeleonidae. They have characteristic features of tongue protrusion during capturing prey attracts many research works and assay its velocity during protrusion. Yet little studies touched the anatomical and histological feature of the juvenile tongue and especially the middle tongue region involved in the tongue elongation, the present study aimed to focus on the histological structure of the mid-tongue and clarify its role in projection of the tongue as well as the glandular structure, keratinization of lingual epithelium and proliferation capacity of the fore-tongue region in relation with their feeding habits during the juvenile age. Juvenile Chameleo chameleon are collected from Abu Rawash, north of Giza Governorate, Egypt during summer 2015. Three juvenile developmental stages are used in the present study and categorized according to the gross morphological criteria of head, abdomen and limb lengths. The tongue and hyoid apparatus were removed and photographed. Histological, immunohistochemistry of cytokeratin and stem cell factor and scanning electronic microscopic investigations were carried out on the fore-tongue region, meanwhile only histological studies were done for the median tongue region. Morphometric assessments of number and length of lingual papillae and grades of cytokeratin and stem cell expression were done. Histologically, the dorsal lingual mucosa of the fore-tongue possessed different pattern of lingual papillae including finger-like, club, cubical, biforked and multi-branched papillae. The finger-like papillae are more abundant compared to the other types. The lamina propria of anterior median tongue pad are more glandular and exhibited abundant distribution of PAS-positive tubular glands and moderate alcian blue staining affinity of both alveolar and branched alveolar glands. There is no detected keratinization of the lingual epithelium. Stem cell factor appeared denser on the lingual mucosa and cell boundaries of glandular tissues. The mid-tongue region possessed a central cartilaginous core outlined by double layers of connective tissue coat and longitudinal and circular muscle fibers which give the power of protrusion of tongue. It is the first time to record the presence of cartilaginous elements in the mid-tongue region of chameleon. Finally the authors concluded that the juvenile stages of common chameleon exhibited striking cartilaginous inner compartment of the med-tongue and glandular fore-tongue adapted for capturing prey coincides with high proliferated activities and presence of different kinds of non-keratinized lingual papillae.
1 Introduction
Reptilian species possessed varieties of anatomical and functional diversity of the Squamata tongue structures accomodated their feeding habit and environmental habitats [Citation1,Citation2] . It is served for other functions such as olfaction in combination with the vomeronasal organs [Citation3]. Reilly and McBrayer [Citation4] speculated that the tongue is accommodated for both fluid and food transport.
Keratinization and the glandular structure of the tongue regions varied between vertebrate species such as freshwater [Citation5] and terrestrial turtles [Citation6].
Chameleonidae species is characterized by their high peristaltic movement to capture the prey and their length may exceed the whole body length [Citation7]. Its lingual system is composed of the hyoid body, lingual muscles and other collagenous structures [Citation8,Citation9] . The accelerator muscle creates a great power in contracting and projection of the lingual body [Citation10].
Herrel et al. [Citation11] studied Chamaeleo calyptratus with approximately 3–18 cm SVL (1–200 g) and found that the juvenile ages are less active comparing to the adult age. Many authors illustrated the changes in competitors or predators of the juvenile small sized ages [Citation12]. The feeding habits of chameleon is striking as a result of its rapid growth especially during their first year of life [Citation13], short living and requirements for growth to their adulthood during the first year [Citation14].
Anderson et al. [Citation10] reported that the ballistic tongue apparatus in the juvenile chameleons needed more feeding apparatus compared to the adult age. Juvenile chameleo calyptratus exhibited an increase of the tongue retraction time comparative to their body size due to immature mouth closing until the tongue has been fully retracted [Citation11].
Little studies are known about the anatomical and histological feature of the juvenile tongue and especially the information of the middle tongue structure involved in the tongue elongation. In addition, the pattern glandular structure, keratinization of lingual epithelium and proliferation of lingual structures were subjected for investigation to categorize the capacity of feeding habit in the juvenile age.
2 Materials & methods
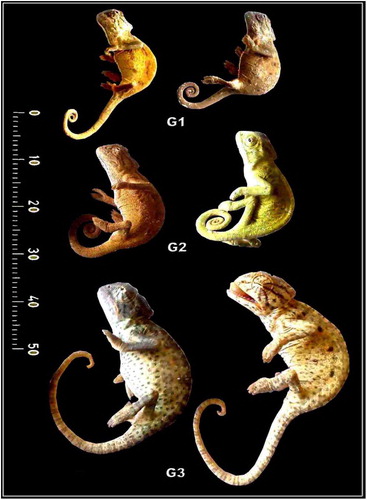
Juvenile individuals of common chameleon, Chameleo chameleon, Linnaeus (1758) (Class: Reptilia; Order, Squamata; Suborder Iguania; Family, Chameleonida; Genus, Chameleo; Species, chameleon) [Citation15] were captured during spring 2015 from the green area of Abou-Rawash, north Giza of Egypt. The protocol of the study approved by the Research Ethical committee of Mansoura University, Egypt. Their ages were identified on the base of the length and width of abdomen, head, tail and limb ( and ). They were sacrificed by overdose of ether and categorized according their length and photographed. Their head regions were decapitated and tongue regions connected with the hyoid apparatus were fixed in 10% neutral phosphate buffered formalin and photographed. The tongue regions were processed as follows:
Table 1 Morphometric analysis of different developing stages of Chameleo chameleon in (mm).
2.1 Scanning electron microscopy
Fore tongue of the developing ages were fixed in 2.5% glutaraldhyde in cacodylate buffer pH (7.4), dehydrated in ascending percentages of alcohol, critically drying in critical point dried in Polaron apparatus and coated with gold in Ion Sputter model 3 Pelco 91000. The samples were examined and photographed at different angles with Jeol scanning electron microscope at accelerating voltages of 5 or 10 kV.
2.2 Histological, histochemical and immunohistochemical studies
The specimens were fixed in 10% phosphate buffered formalin (pH 7.4), dehydrated in ascending percentages of ethyl alcohol, cleared in xylene and mounted in molten paraplast at 58–62 °C. Serial 5 µm sections were cut and stained with hematoxylin and eosin. For alcian blue PAS for glycogen and mucins, the hydrated paraffin sections were treated with 0.5% periodic acid for liberated aldehyde group from polysaccharides and stained with Schiff reaction for preserving glycogen. The specimens were further stained with alcian blue pH 2.3 for acid mucopolysaccharides and subsequently processed for permanent preparation [Citation16] and investigated under olympus light microscope.
Also, the hydrated tissue sections were immunohistochemical staining of cytokeratin (CK) and stem cell factor (SCF) by incubating for overnight with the primary monoclonal mouse anti-human antibody of SCF (Cat. sc-13126, 1:100, mouse, Santa Cruz) and CK (Cat. sc-25280, 1:50, mouse, Santa Cruz). These were followed by carefully washing with distilled water and incubated with the secondary biotin linked anti-mouse antibody. At finished the specimens treated with streptoavidin-peroxidase complex and incubated with developing diaminobenzidine-hydrogen peroxide (DAKO), for developing the dark-brown reaction and investigated under the bright field light microscope with a digital canon camera. The grades of immunohistochemical staining was categorized as −, Negative; +, Weak; ++, Moderate; +++, Intense immunoreactions.
3 Results
3.1 Gross morphology
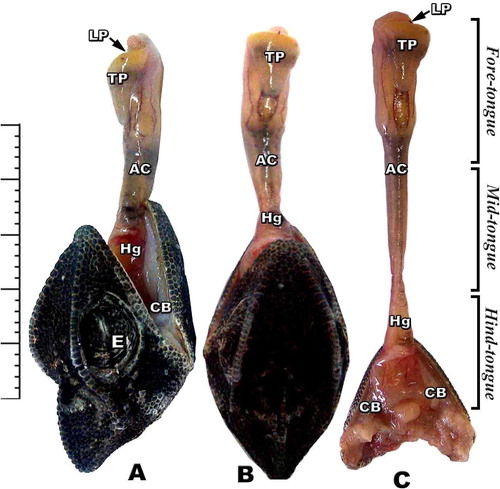
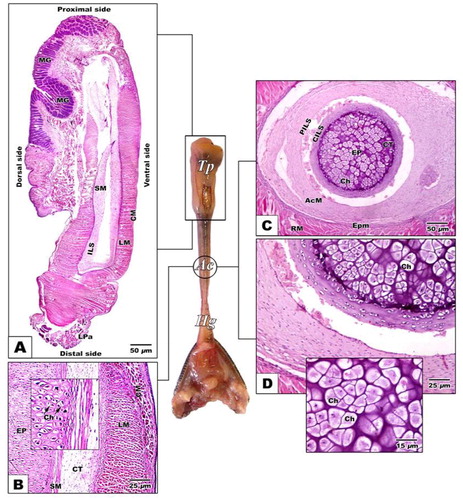
The tongue is a muscular fleshy organ divided into three distinct regions; fore-, middle and hind-tongue region. The fore-tongue is composed of flattened conical muscular pad have a characteristic anterior lingual pouch immersed in the tongue pad. The medium tongue region is a long narrow cylindrical apparatus protruded out of the mouth during the tongue protrusion. The median accelerator muscle connected with the enteroglossal processes of the hyoid region. The hind part is the tongue hypoglossal complex which is inserted posteriorly on the connective tissue sheet surrounding the accelerator muscle of the hypoglossus complex. The hyoid skeletal compartment is located in the throat region by a group of strap-like muscles attached to the mandible anteriorly (A, B & C).
3.2 Scanning electron microscopy observations
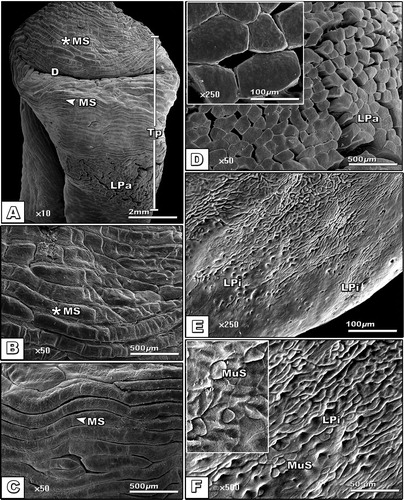
The dorsum lingual apex of the fore-tongue region is characterized by the presence of dimple-like structure of infolding lingual papillae (A). The lingual mucosa is composed of lingual strands regularly oriented and interconnected with each other (B & C). The lingual strand surfaces carried different lingual papillae (D). The entire surface of the tongue infiltrated by numerous glandular pores containing mucous secretion (E & F).
3.3 Morphometric observations
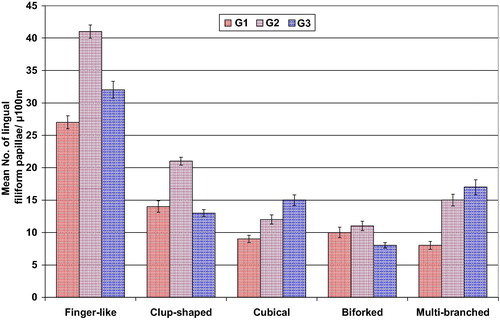
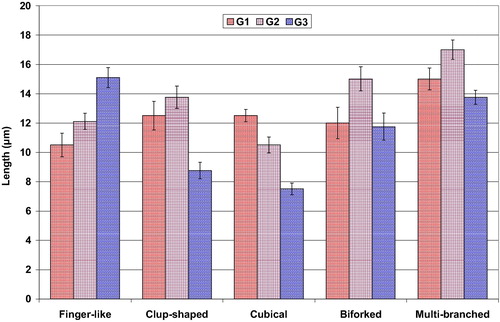
Five different types of mechanical lingual papillae are observed in the dorsum tongue surface. These are club-shaped, cubical, biforked, multi-branched and finger-like structures. The finger-like papillae are more abundant compared to the other types. The club-shaped, biforked and multi-branched types are wider, meanwhile the finger-like papillae are the more elongated in the 3rd age (, & ).
Table 2 Length (μm) and numbers (No./100 μm) of different types of lingual filiform papillae of different developing stages of Chameleo chameleon.
3.4 Light microscopic observations
The proximal tongue pad characterized by abundant lingual papillae and mucous gland in their dorsal surface comparing with muscular structure and missing of glands in the ventral region (A & B). Following investigation of cross section of the medium cylindrical tongue region, there is no indication of the presence of lingual papillae on the surface of the mid-tongue region. It is appeared in the form of long cylindrical structure with smooth surface. Its inner central compartment is composed central cartilaginous core outlined by a perichondrium sheath. It is the first time to record the presence of cartilaginous elements in the mid-tongue region of chameleon. The cartilage element is further surrounded by central and peripheral connective tissue sheath in a circular manner. The external surface of the mid-tongue region is composed of muscular tissue of both longitudinal and circular muscle fibers (C & D).
Fig. 6 (A-D): Photomicrograph of histological sections of tongue of Chameleo chameleon. HX-E A. Gross sagittal histological section of tongue pad showing dense distribution of dorsal lingual papillae and lingual glands. B. Sagittal section of medium tongue region. C-D. Transverse histological section of middle cylindrical tongue core showing central cartilaginous compartment ensheathed by connective tissue layer followed by longitudinal and circular muscle layers. (Abbreviations: AcM, Accelerator muscles; Ch, chondrocyte; CILS, central intralingual sheath; CM, circular muscle; CT, connective tissue sheat; EP, enteroglossal process; Epm, epimysium; Hg, hyoglossus muscle; ILS, intralingual sheath; LM, longitudinal muscle; LPa, lingual papillae; MG, mucous gland; PILS, peripheral intralingual sheath; RM, retractor muscle; SM, smooth muscle; Tp, tongue pad).
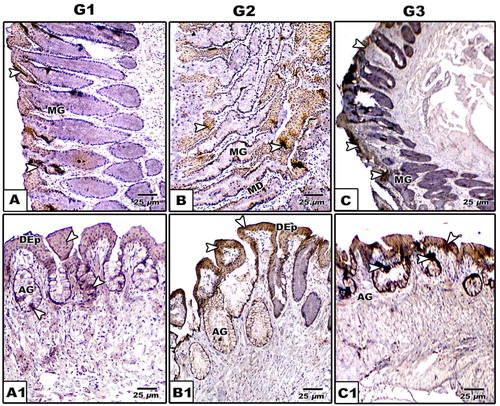
The dorsal surface carries different forms of lingual papillae such as club-shaped, cubical, biforked, multi-branched papillae and finger-like structures. There is no presence of gustatory papillae (A1–C1). The dorsal region is rich in mucous glands compared to the ventral muscular one. Alveolar mucous glands are more abundant in the proximal tongue region compared to the tubular ones in the medium and posterior region (A–C). Following alcian periodic acid Schiff staining, there are different forms of lingual glands such as tubular, alveolar and branched alveolar. In stage 1, the branched alveolar mucous glands appeared faintly alceophilic staining (A–A1). In the subsequent stages 2 & 3, there are varying degree of staining affinities with either alcian blue or PAS, reflecting the varying degrees of mucins and glycogen derivative substances (B–C2). The intertubular glandular sepses are rich in the blood capillaries (B–C2).
Fig. 7 (A-C1): Photomicrograph of histological sections of tongue of Chameleo chameleon. HX-E (Abbreviations: BF, Biforked; C, clup-shaped; Cu, cubical; DEp, Dorsal epithelium; F, Finger-like; LPa, lingual papillae; MB; Multi-branched MD, mucous duct; MF, muscle fibers; MG, mucous gland).
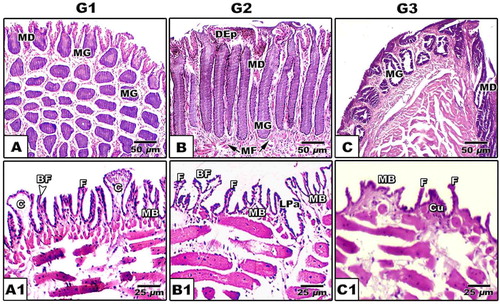
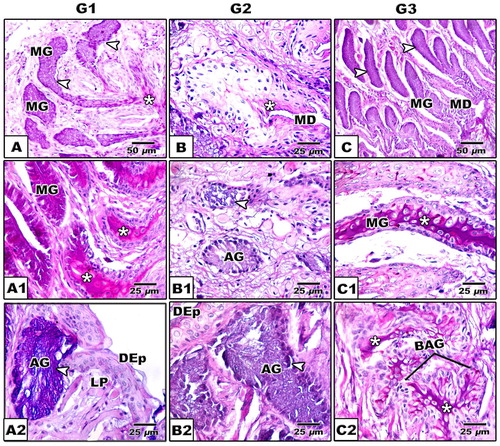
Fig. 8 (A-C2): Photomicrograph of histological sections of tongue of Chameleon chameleon. Alcian-PAS. A-A2. G1 showing alcian positive branched alveolar glands distributed in lamina properia (A), PAS positive alveolar and tubular glands (MG) (A1) & alcian positive alveolar gland (A2). B-B2. G2 Showing negative staining alveolar gland (B) and alcian positive alveolar glands (B1 & B2). C-C2. G3 showing alcian positive branched alveolar gland (C) and PAS positive staining of both tubular and branched alveolar glands (C1 &C2). (Abbreviations: AG, alveolar gland; BAG, branched alveolar gland; DEp; Dorsal epithelium; LP, lamina proporia; MD, mucous duct; MG, mucous gland). (*), Positive PAS staining; The arrow head (
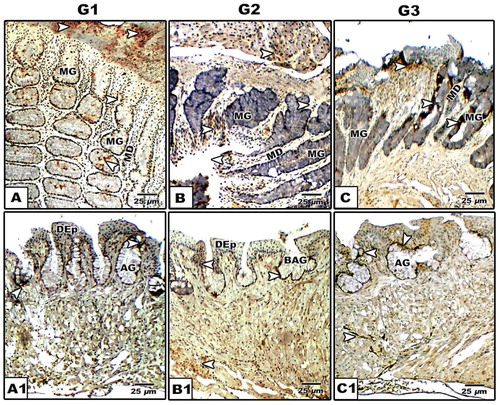
Following cytokeratin immunohistochemical staining, there is a detectable weak expression in the lingual epithelial cells. However, moderate staining affinity was remarked in the epithelial cells lining the alveolar glands of the developing juvenile ages (, ).
Table 3 Immunoreactivity of CK, SCF and AB/PAS of tongue of G1, G2 and G3 of Chameleo chameleon.
Fig. 9 (A-C1): Photomicrographs of formalin-fixed, paraffin-embedded tongue of Chameleo chameleon, immunohistochemically stained with anti-CK. A-A2. G1. B-B2. G2. C-C2. G3. Note mild cytokeratin reaction in G1 and intense staining in G2 & G3 in lingual mucosa. (Abbreviations; AG, alveolar gland; DEp; Dorsal epithelium; MD, Mucous duct; MG, mucous gland).
Concerning stem cell factor antibody, over-expression is detected in the lingual and glandular epithelial cells especially in the young ages compared to the advanced ones (, ).
Fig. 10 (A-C1): Photomicrographs of formalin-fixed, paraffin-embedded tongue of Chameleo chameleon, immunohistochemically stained with anti-SCF. A-A2. G1. B-B2. G2. C-C2. G3. Note mild stem cell reaction in G1 and moderate staining in G2 & G3 in both lingual mucosa and lingual glands. (Abbreviations: AG, alveolar gland; BAG, branched alveolar gland; DEp; dorsal epithelium; MD, mucous duct; MG, mucous gland).
4 Discussion
The present findings declare that the chameleon tongue is a striking pattern structure. It is composed of functional fore tongue for capturing the prey and mid & hind tongue for pumping the muscular force for elongation and prehension prey. Anatomically, the mid-tongue region is composed of a central cartilaginous element which provide the tongue solidification during protrusion. It is the first time to record the presence of cartilaginous elements in the mid-tongue region of chameleon. The surrounding connective tissue coat softens and allow external muscular coat for more contraction and sliding along the interior connective tissue which facilitated more protrusion of the tongue. The hyoid play an integral in tongue projection. It is a movable skull components and located in the thoracic region by a system of strap-like muscles attached anteriorly the mandible and posteriorly the sternum and shoulder.
The present findings agree with the work of Herrel et al. [Citation8] who attributed chameleon muscle elongation to the helical arrangement of the collagen fibers in the accelerator epimysium.
De Groot and van Leeuwen [Citation9] attributed the tongue prehension of Chamaeleo melleri and Cantherhines pardalis, to combined helical arrays of collagen fibers and hydrostatic lengthening of the accelerator muscle.
Many authors attributed the extreme chameleon tongue projection to the generation of suction activities of the intrinsic tongue muscles that pull the tongue pad inwards [Citation17] or to the forces produced by the accelerator muscle [Citation8,Citation9] .
The present findings illustrated the presence of cartilaginous elements as an extra skeletal element besides the connective tissue sheath and lingual muscle strengthens tongue elongation in juvenile chameleon. These clarify the development of tongue apparatus which enable these developing ages for capturing prey.
The investigated glandular structures in the dorsal lingual mucosa with characteristic variations of alcian and periodic acid Schiff reaction reflected the diversity of acid mucins and glycogen derivatives. The increased numbers of mucous glands and their secretion all over the tongue surface facilitate the capturing of prey and its swallowing. The moisture secreted by the lingual glands may be necessary to maintain the structural integrity of the lingual papillae. Also, the discharge of glandular secretions may facilitate the promotion of lingual muscle contraction.
The present findings agree with the work of Bell [Citation18] and Bels et al. [Citation19] whom reported that chameleons possessed serous and mucous secretions which hold the prey on the tongue after capture [Citation20] as well as sterile the lingual structure against infection [Citation21].
Rabinowitz and Tandler [Citation22] reported the presence of tubular salivary glands inbetween the papillae in the posterior zone of the tongue of the American chameleon Anolis carolinensis.
Similar pattern of lingual glands were reported in the Eumeces schneideri [Citation21], Acanthodactylus boskianus [Citation23] and in the iguanid lizard Oplurus cuvieri [Citation20], toad Bufo marinus [Citation24] and Agama Laudakia stellio [Citation25].
Different forms of filiform papillae were identified. They took different shapes such as club-shaped, cubical, biforked, multi-branched papillae and finger- like structures. These seemed to be a rigid tooth filaments helping in capturing of the prey and swallowing of the food items.
Similar pattern structures of lingual papillae such as feather-like structure, cuboidal and leaf-like papillae were identified in adult and juvenile form of Eublepharis macularius (Squamata: Gekkonidae) [Citation26].
The connective tissue core of this pattern of lingual papillae is rich in elastic fibers aligned perpendicularly to the epithelial surface protecting against mechanical stress and allow tongue movement during mastication [Citation27]. The thready filiform papillae may represent a heat-releasing organ and be involved in the control of body temperature [Citation28].
Although there is no obvious keratinization of lingual epithelium, cytokeratin expression in the epithelial cells of the dorsum lingual mucosa. However, moderate staining affinity was identified in the epithelial cells of the alveolar glands of the juvenile chameleon stages. These epithelial cells exhibited over expression of the antibody of stem cell factor.
Similar identification of stem cells were identified in the anterior and posterior column base of the filiform papilla in the tongue of the mammals especially hamster [Citation29].
The present finding of lack keratinization were reported previously in adult chameleon species [Citation30] and Pogona vitticeps lizard [Citation31].
Shimada et al. [Citation32] attributed the expression of cytokeratin expression to the increase of sulphur in filiform papillae, the markers of product of keratininzation cystine and cysteine amino acids.
Iwasaki et al. [Citation33] reported over expression of cytokeratin 13 and 14 in the filiform papillae during 13, 15, 17 and 21 days embryonic development of rats predicting early keratinization.
Different studies correlated between cytokeratin expression and stem cells identification. Tanaka et al. [Citation34] identified that the lingual epithelial stem cells over expressed cytokeratin 14 and 5. Increased expression of stem cells in lingual epithelium during juvenile ages revealed marked proliferation and differentiation of the lingual mucosa.
Finally the authors concluded that the juvenile ages of common chameleon exhibited the characteristic feature of the developing tongue system which is adapted for capturing prey coincides with glandular structure of fore-tongue, pattern of lingual papillae and stem cell contents.
References
- S.IwasakiEvolution of the structure and function of the vertebrate tongueJ Anat20112002113
- A.HerrelM.CanbekU.OzelmasM.UyanogluM.KarakayaComparative functional analysis of the hyolingual anatomy in lacertid lizardsAnat Rec A2842005561573
- J.C.GillinghamD.L.ClarkSnake tongue-flicking: transfer mechanics to Jacobson’s organCan Zool59198116511657
- S.M.ReillyL.McBrayerPrey capture and prey processing behavior and the evolution of lingual and sensory characteristics: Divergences and convergences in lizard feeding biologyS.M.ReillyL.D.McBrayerD.B.MilesThe evolutionary consequences of foraging mode2007Cambridge University PressCambridge302333
- C.J.BeisserJ.WeisgramH.HilgersH.SplechtnaFine structure of the dorsal lingual epithelium of Trachemys scripta elegans (Chelonia: Emydidae)Anat Rec25021998127135
- R.M.WinokurThe buccopharyngeal mucosa of the turtles (testudines)J Morphol196119883352
- T.E.HighamC.V.AndersonFunction and adaptation of chameleonsK.A.TolleyA.HerrelThe Biology of Chameleons2014University of California PressBerkeley, CA6383
- A.HerrelJ.J.MeyersK.C.NishikawaF.De VreeMorphology and histochemistry of the hyolingual apparatus in chameleonsJ Morphol24922001154170
- J.H.De GrootJ.L.van LeeuwenEvidence for an elastic projection mechanism in the chameleon tongueProc Roy Soc London B27115402004761770
- C.V.AndersonT.SheridanS.M.DebanScaling of the ballistic tongue apparatus in chameleonsJ Morphol27311201212141226
- A.HerrelC.L.ReddingJ.J.MeyersK.C.NishikawaThe scaling of tongue projection in the veiled chameleon, Chamaeleo calyptratusZool (Jena)11742014227236
- A.HerrelA.C.GibbOntogeny of performance in vertebratesPhysiol Biochem Zool79200616
- P.C.WainwrightA.F.BennettKinematics of prey processing in Chameleo jacksonii: conservation of function with morphological specializationJ Zool22619924764
- K.B.KarstenL.N.AndriamandimbiarisoaS.F.FoxC.J.RaxworthyA uniquelife history among tetrapods: an annual chameleon living mostly as an eggProc Natl Acad Sci USA105200889808984
- J.A.SchulteIIJ.P.ValladaresA.LarsonPhylogenetic relationships within Iguanidae inferred using molecular and morphological data and a phylogenetic taxonomy of iguanian lizardsHerpetologica592003399419
- J.A.KiernanHistological and histochemical methods: theory and practicePergamon press1981169189
- A.HerrelJ.J.MeyersP.AertsK.C.NishikawaThe mechanism of prey prehension in chameleonsJ Exp Biol203200032553263
- D.A.BellFunctional anatomy of the chameleon tongueZool J Biol Anat1191989313336
- V.L.BelsM.ChardonK.V.KardongBiomechanics of the hyolingual system in SquamataV.L.BelsM.ChardonP.VandewalleAdvances in Comparative and Environmental Physiologyvol. 181994SpringerBerlin197240
- V.DelheusyG.ToubeauV.BelsTongue structure and Function in Oplurus cuvieri (Reptilia: Iguanidae)Anat Rec2381994263276
- I.T.WassifM.S.El-HawaryScanning electron microscopy of the dorsal lingual epithelium of the golden lizard, Eumeces schneideri (Reptilia: Scincidae)J Egypt Ger Soc Zool26C19981130
- T.RabinowitzB.TandlerUltrastructural of lingual salivary glands in the American chameleon: Anolis carolinensisAnat Rec22941991489494
- M.B.H.MohammedStructure and function of the tongue and hyoid apparatus in Acanthodactylus boskianus (Lacertidae: Reptilia)J Egypt Ger Soc Zool26B19927590
- M.González-ElorriagaThe glands of the dorso-distal mucosa of the toad Bufo marinus (Amphibia: Anura) tongue: histology and ultrastructureActa Cient Venez53120024959
- Y.B.KocaE.O.OğuzE.OsançMorphology, and muscle- and papilla-volume ratios, of the tongue of Laudakia stellio (Agamidae, Squamata): a histological and stereological studyZoolog Sci2492007899905
- H.A.JamniczkyA.P.RussellM.K.JohnsonS.J.MontuelleV.L.BelsMorphology and histology of the tongue and oral chamber of eublepharis macularius (Squamata: Gekkonidae), with special reference to the foretongue and its role in fluid uptake and transportEvol Biol362009397406
- T.YamamotoS.KondoH.NagaiOn the distribution of elastic fibers in the filiform papillae of Suncus murinusShika Kiso Igakkai Zasshi3141989379384
- T.NagatoM.NagakiM.MurakamiH.TaniokaMorphological studies of rat lingual filiform papillaeOkajimas Folia Anat Jpn6641989195209
- G.E.SaleR.F.RaffR.StorbStem cell regions in filiform papillae of tongue as targets of graft-versus-host diseaseTransplantation5811199412731275
- L.N.ZghikhE.VangyselD.NonclercqA.LegrandB.BlaironC.BerriT.BordeauC.RémyC.BurtéaS.J.MontuelleV.BelsMorphology and fibre-type distribution in the tongue of the Pogona vitticeps lizard (Iguania, Agamidae)J Anat22542014377389
- Y.A.FoudaD.A.SabryD.F.Abou-ZaidFunctional Anatomical, Histological and Ultrastructural Studies of three Chameleon Species: Chamaeleo Chamaeleon, Chamaeleo Africanus, and Chamaeleon VulgarisInt J Morphol333201510451053
- K.ShimadaI.SatoA.YokoiT.KitagawaM.TezukaT.IshiiThe fine structure and elemental analysis of keratinized epithelium of the filiform papillae analysis on the dorsal tongue in the American alligator (Alligator mississippiensis)Okajimas Folia Anat Jpn6661990375391
- S.IwasakiH.YoshizawaH.AoyagiImmunohistochemical expression of keratins 13 and 14 in the lingual epithelium of rats during the morphogenesis of filiform papillaeArch Oral Biol5152006416426
- T.TanakaY.KomaiY.TokuyamaH.YanaiS.OheK.OkazakiH.UenoIdentification of stem cells that maintain and regenerate lingual keratinized epithelial cellsNat Cell Biol1552013511518