Abstract
Bisphenol A is one of the anthropogenic chemicals produced worldwide, currently released into the environment and causes endocrine-disruption. The largest environmental compartments of BPA are abiotic associated with water and suspended solids that becomes an integrated part of the food chain. The present study aimed to examine the possible protective role of Ginkgo biloba extract (GBE), melatonin and their combination against BPA-induced liver and kidney toxicity of male rats. Fifty rats were divided into five equal groups: control, BPA, BPA plus GBE, BPA plus melatonin and BPA plus GBE plus melatonin. The elevated activities of plasma ALT and AST in addition to increased levels of urea and creatinine concomitant with the decreased total plasma protein could reflect the injurious effect of BPA. Liver and kidney levels of TBARS were significantly increased, while GSH, SOD and GPX were decreased in BPA-treated rats. Also, CAT and GST activities were significantly disrupted in the liver and kidney of rats treated with BPA. Moreover, BPA significantly increased the proinflammatory cytokine TNF-α in the liver and kidney tissues. The histopathological analysis confirmed these results. All the previous alterations in the liver and kidney could be ameliorated when BPA-treated rats were co-administrated either with GBE, melatonin or their combination. These natural substances could exhibit protective effects against BPA-induced hepato- and nephrotoxicity owing to their antioxidative and anti-inflammatory potentials.
1 Introduction
Bisphenol A (2,2-bis (4-hydroxyphenyl) propane, (BPA) is an environmental chemical contaminant that is widely used in the manufacture of polycarbonate plastics and epoxy resins [Citation1]. Also, BPA is used in the production of thermal stabilizers, plasticides, paints and dental materials [Citation2]. Human extensive exposure to BPA through the food chain is widespread because BPA is released by food and beverage containers [Citation3]. High doses of BPA altered liver weights and decreased the viability of rat hepatocytes [Citation4]. BPA is a nephrotoxic agent due to the accumulation of its toxic metabolites and inability of the kidney to eliminate those metabolites [Citation5]. Also, BPA induce the formation of reactive oxygen species (ROS) which cause tissue injury in the liver, kidney and other organs [Citation6]. Furthermore, the low doses of BPA generate ROS by means of decreasing the activities of the antioxidant enzymes and increasing lipid peroxidation thereby causing oxidative stress [Citation7]. Nowadays, natural supplements are used in the treatment of many diseases either by improving the efficacy of the drug or by minimizing the toxic side effects [Citation8].
Ginkgo biloba leaf extract (GBE) is one of the most widely used herbal supplements in the traditional Chinese medicine for centuries [Citation9]. Several investigators reported that G. biloba polysaccharides (GBP) have different biological actions, such as anti-oxidation, anti-inflammation, immunomodulation and anti-tumor [Citation10]. It could protect the liver from an induced injury by means of reducing lipid peroxidation and GSH depletion concomitant with enhancing gene expression of the antioxidant enzymes [Citation11] and inhibiting TNF-α expression [Citation12]. In vivo results showed that GBL-treatment is a potent protector against uranium-induced toxicity [Citation13].
Melatonin (5-methoxy-N-acetyltryptamine) is natural hormone secreted mainly from the pineal gland [Citation3]. Also, other organs such as eyes, brain, gut, skin and immune cells can synthesize melatonin [Citation14]. Melatonin is also synthesized in the gastrointestinal tract [Citation15]. Melatonin participates in the regulation of many physiological processes. Melatonin can reduce oxidative stress via stimulating the activities of the anti-oxidative enzymes; superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) [Citation16]. Additionally, melatonin can protect DNA, protein and lipids [Citation17]. Moreover, melatonin reduces the level of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), inteleukin-6 (IL-6) and interleukin 1beta (IL-1β) [Citation18]. Consequently, the present study was carried out to evaluate the protective effect of G. biloba extract and/or melatonin against Bisphenol A-induced hepatotoxicity and nephrotoxicity in adult male rats. This is accomplished by the assessment of liver and kidney function tests, apoptotic marker (TNF-α), lipid peroxidation end product (TBARS), enzymatic and non-enzymatic antioxidants in addition to histological examination of the liver and kidney tissues.
2 Materials and methods
2.1 Experimental animals
Adult male rats weighing (180–200 g) were used in the current study. They were obtained from the animal house, Faculty of Medicine, Alexandria University, Egypt. Rats were housed in stainless steel cages and maintained at 25–28 °C with 12-h light-dark cycle. Animals were allowed to food and water ad libitum.
2.2 Experimental design
Rats were randomly distributed into five groups as follows: Control group; rats were used as negative control. Bisphenol A (BPA)-treated group (positive control); rats were orally treated with BPA at a dose of 40 mg/kg body weight [Citation19] and [Citation20]. Bisphenol A + G. biloba extract (BPA + GBE)-treated group; rats of this group were treated with BPA (40 mg/kg b. w.) and GBE used as 100 mg/kg b. w. [Citation21]. Bisphenol A + melatonin (BPA + Mel)-treated group; rats were treated with BPA (40 mg/kg b. w.) and Mel of a dose 10 mg/kg b. w. [Citation22] Bisphenol A + G. biloba extract + melatonin (BPA + GBE + Mel)-treated group; rats were treated with a combination of BPA, GBE and Mel. All the selected doses were orally and daily administered for 70 days.
2.3 Blood collection
At the end of the experimental period, rats were fasted for 12 h then they were scarified by decapitation under diethyl ether anesthesia. Blood samples were collected by heart puncture in tubes containing EDTA (anti-coagulant) and placed immediately on ice. Plasma was obtained by centrifugation of samples at 860 × g for 20 min and stored at −80 °C until used for analyses.
2.4 Tissue preparation
Livers and kidneys were excised and washed using saline solution (0.9%). Parts from the livers and kidneys of each group were fixed in 10% formalin for histological examination. Other parts were minced and homogenized in ice-cold sodium phosphate buffer (0.01 M, pH 7.4) containing 1.15% KCl (10% w/v) using a polytron (Tekmar model TR-10, West Germany) homogenizer. The homogenate was centrifuged at 10,000 × g for 20 min at 4 °C using high speed cooling centrifuge (Universal 32 R, Germany). The resultant supernatants were used for the different investigations.
2.5 Assessment of the biochemical parameters
Total plasma protein was determined using biuret reaction [Citation23]. Urea and creatinine plasma levels were also measured [Citation24] and [Citation25], respectively. Plasma and hepatic Aspartate aminotransferase (AST; EC 2.6.1.1) activity was assayed [Citation26]. While, plasma and hepatic alanine aminotransferase (ALT; EC 2.6.1.2) activity was assayed using the methods of International Federation of Clinical Chemistry [Citation27]. Glutathione peroxidase (GPX; EC. 1.1.1.9) activity was assayed in hepatic tissues [Citation28]. Also, reduced glutathione level was determined in both livers and kidneys [Citation29]. Hepatic and renal Glutathione-S-transferase (GST, EC 2.5.1.18) activities were assayed using p-nitrobenzyl chloride in 95% ethanol as a substrate [Citation30]. Moreover, Superoxide dismutase (SOD; EC 1.15.1.1) activity was estimated in Hepatic and renal tissues [Citation31]. Also, Catalase activity (CAT; EC1.11.1.6) was assayed in livers and kidneys [Citation32]. The level of the lipid peroxidation end products, Malondialdehyde, which react with thiobarbituric acid-reactive substances (TBARS), was also evaluated in both livers and kidneys [Citation33]. The pro-inflammatory cytokine TNF-α (K0331196) level was quantified by the enzyme-linked immunosorbent assay (ELISA) using ELISA kit specific for rat cytokines (Biosource International, Nivelles, Belgium).
2.6 Histological section preparation
Liver and kidney sections were fixed in formalin (10%), treated with alcohol and xylol then embedded in paraffin and sectioned at thickness of 4–6 μm. These sections were stained with Haematoxylin and Eosin stain (H&E, X 400) for the examination of histopathological changes [Citation34].
2.7 Statistical analysis
Results were reported as means ± SE and data were statistically analyzed [Citation35]. The statistical significant difference in values of the experimental animals was calculated by (F) test at 5% significance level. All parameters were calculated using the general linear model produced by Statistical Analysis Systems Institute [Citation36]. Beside, Duncan’s New Multiple Range Test was used in assessment of the significant differences between groups. Values of P < 0.05 were considered statistically significant.
3 Results
3.1 Assessment of biochemical parameters
The current study indicated that plasma activities of ALT and AST were significantly (p < 0.05) increased while hepatic ALT and AST specific activities were significantly (p < 0.05) decreased in BPA-treated group compared to the control. On the other hand, plasma ALT and AST activities were significantly (P < 0.05) decreased whereas hepatic ALT and AST specific activities were significantly (p < 0.05) increased in groups treated with BPA + GBE, BPA + Mel and their combination compared to BPA-treated ones ().
Table 1 The effect of BPA, GBE and melatonin administration on the liver function tests and the activities of the antioxidant enzymes.
It was observed that hepatic GST and CAT activities were significantly (p < 0.05) increased in BPA-treated rats when compared to control, while significantly (p < 0.05) decreased after BPA co-treatment with GBE, Mel and their combination in comparison to BPA-treated ones (). Liver activities of GPx and SOD were significantly (p < 0.05) decreased in BPA-treated group as compared to control. On the contrary, treatment with GBE, Mel and their combination together with BPA resulted in significant (p < 0.05) increases in the activities of these enzymes ().
In addition, our results indicated that renal activities of GST, SOD and CAT showed significant (p < 0.05) decreases with BPA treatment compared to control. While, these enzyme activities were significantly (p < 0.05) increased when BPA co-administered with GBE, Mel and their combination ().
Furthermore, the results revealed that BPA induction caused significant (p < 0.05) increases in the plasma urea and creatinine levels compared to the controls. While, the co-administration of GBE, Mel and their combination together with BPA significantly (p < 0.05) reduced these elevations ().
Table 2 The effect of BPA, GBE and melatonin administration on the levels of urea, creatinine, TBARS and GSH in the plasma samples and the hepatic and renal extracts of male rats.
Comparing to control, the hepatic TBARS level was significantly (p < 0.05) increased next to BPA-treatment. GBE, Mel and their combination diminished this significant increase that induced by BPA. Conversely, BPA caused significant (p < 0.05) decrease in the hepatic GSH level as compared with the control group. However, the co-administration of GBE, Mel and their combination with BPA significantly increased (p < 0.05) GSH to control level ().
Similarly, renal TBARS level showed significant (p < 0.05) increase in the BPA-induced rats concomitant with significant (p < 0.05) decrease in the renal GSH level when both compared with the control. But, co-administration of GBE, Mel and their combination attenuated the increase in renal TBARS while caused significant (p < 0.05) increase in the renal GSH ().
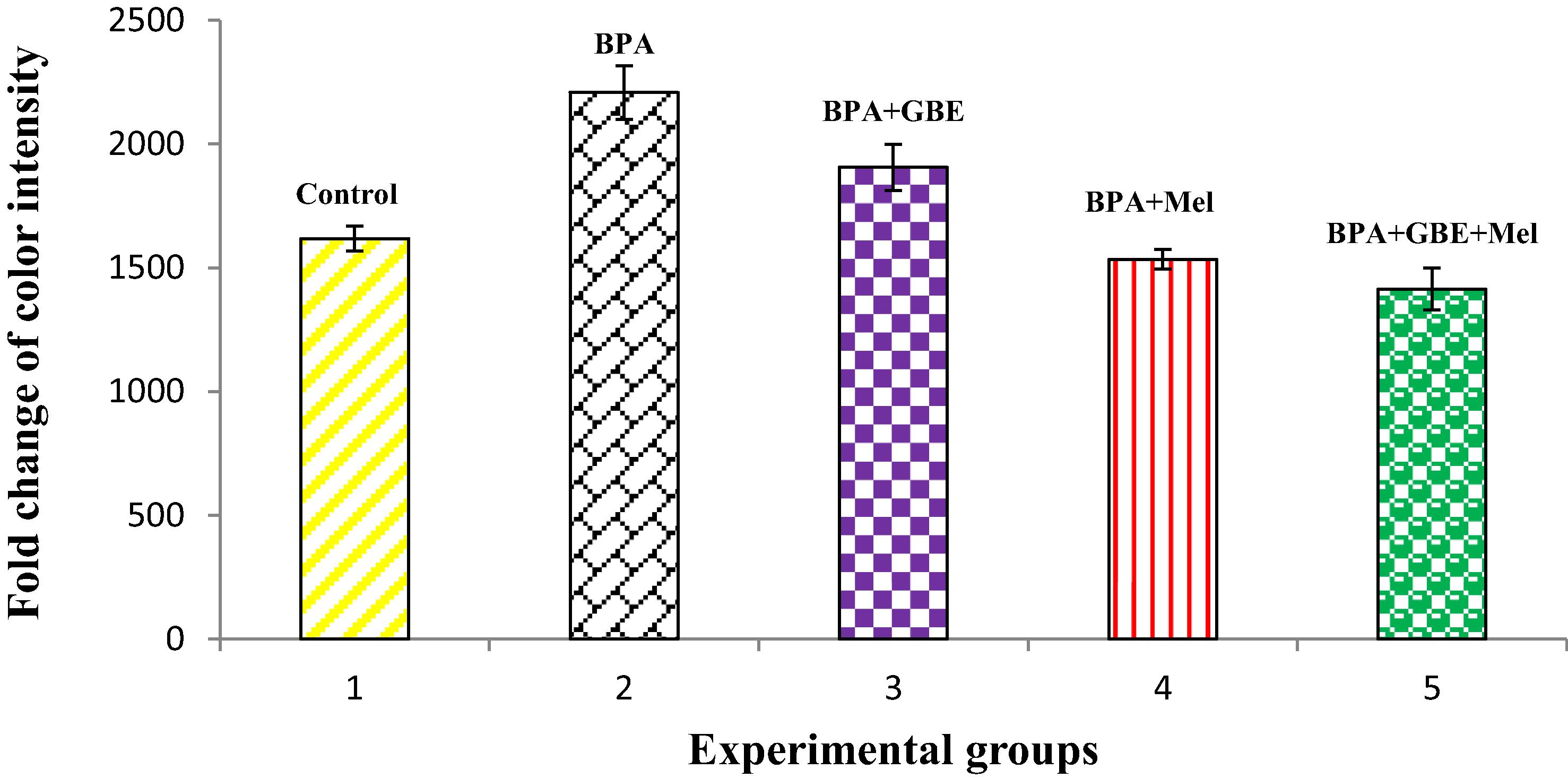
The level of TNF-α in both livers and kidneys of BPA-treated groups showed significant (P 0.05) increase when comparing its level with controls. This increase was diminished after the co-treatment of BPA with GBE, Mel and their combination as in and
Fig. 1 Changes in the hepatic level of TNF-α in the adult male rats treated with BPA, BPA + GBE, BPA + Mel and BPA + GBE + Mel.
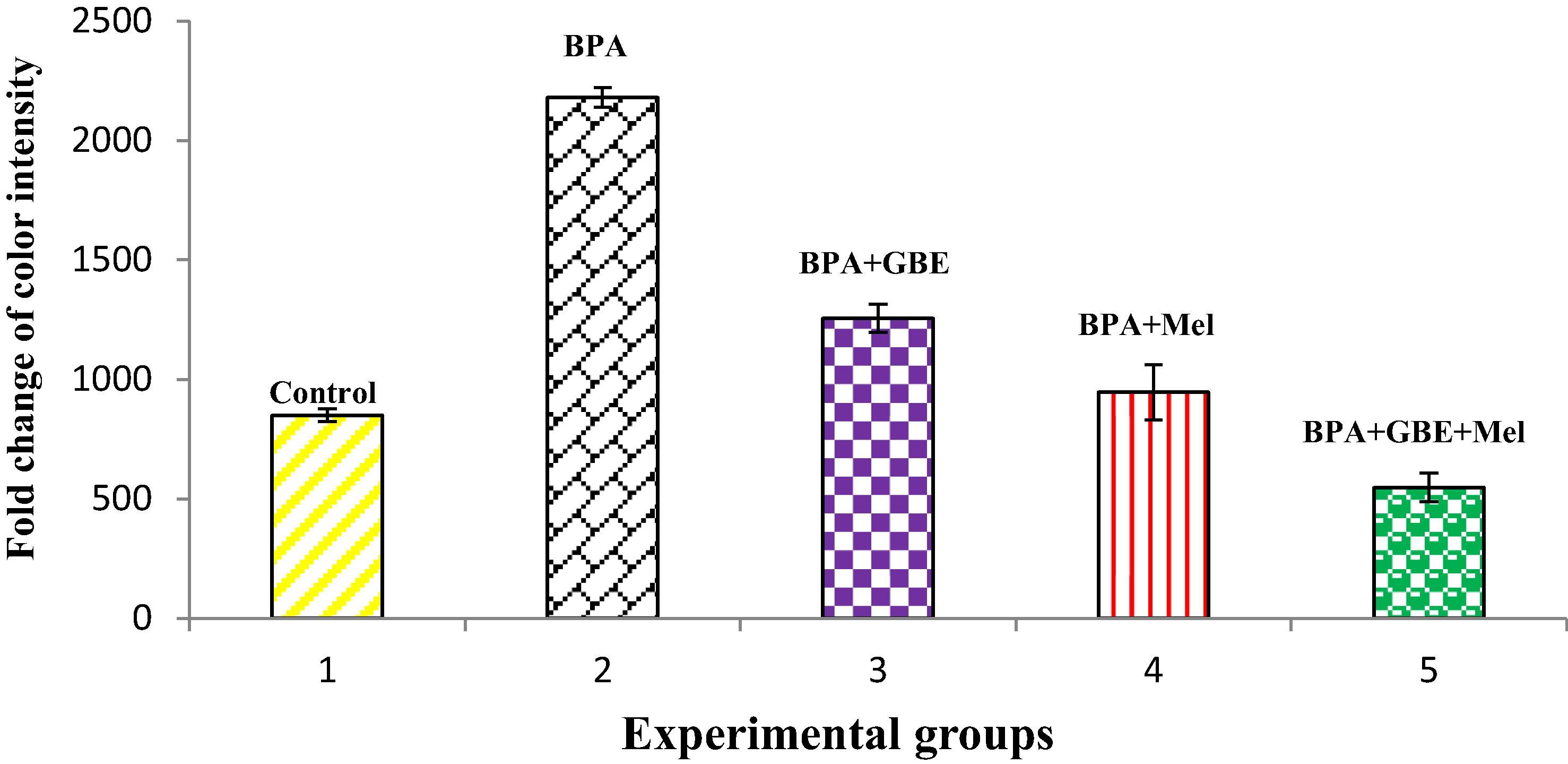
Fig. 2 Changes in the renal level of TNF-α in the adult male rats treated with BPA, BPA + GBE, BPA + Mel and BPA + GBE + Mel.
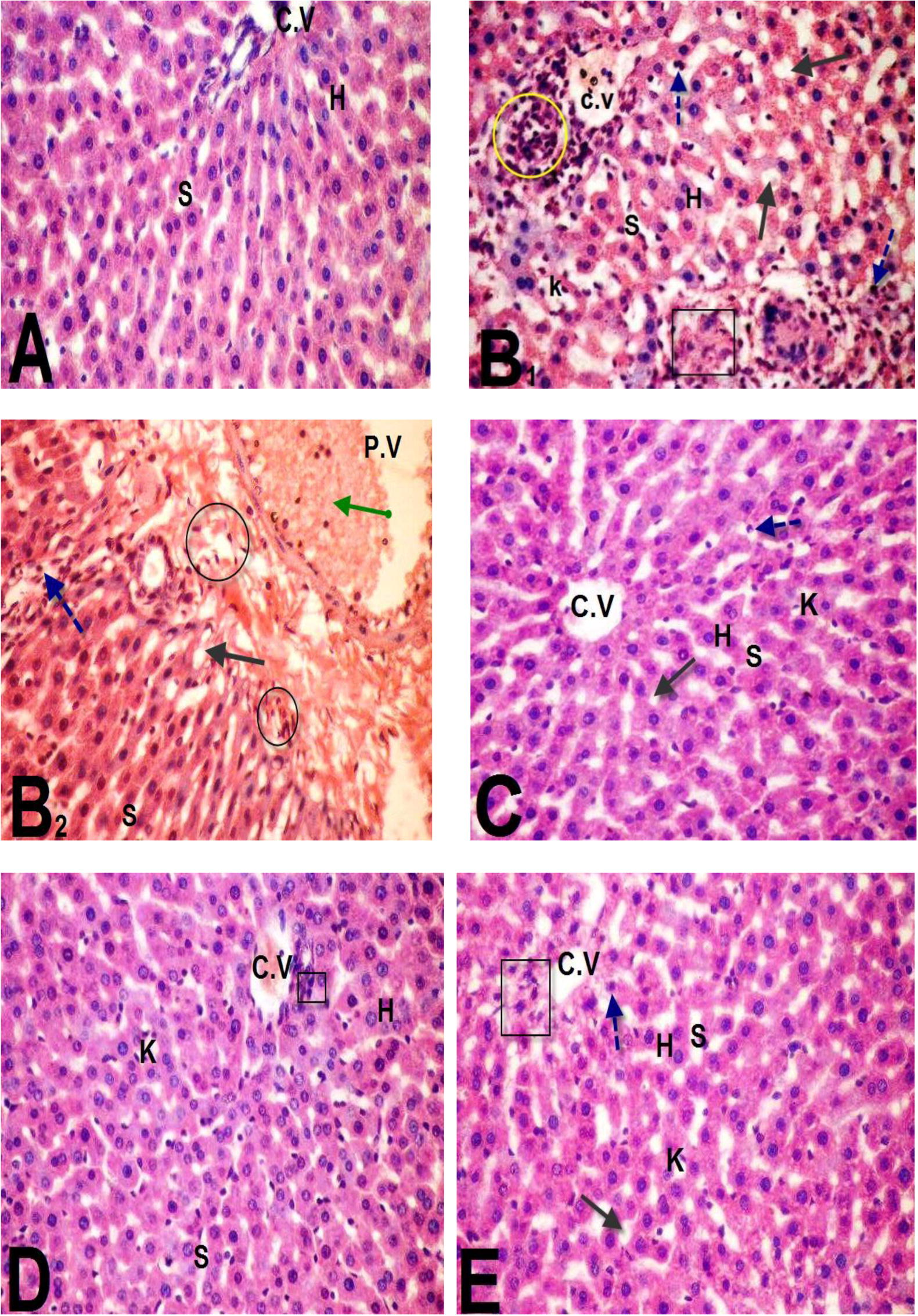
Liver tissues examination of the different experimental groups was illustrated in . Sections of the control group showed normal cellular architecture A with distinct hepatic cells (H), sinusoidal spaces (S) and central vein (C.V). Whereas, liver sections of BPA-induced group indicated remarkable histopathological alteration as in B1 and B2. This alteration was indicated from the loss of normal hepatocytes architecture, degenerated hepatocytes (Black Square) with pyknotic nuclei (blue dotted arrows). In addition, hepatocyte vacuolization (black arrow), dilation and hemorrhage of hepatic sinusoids (S) were seen. Also, diffusion of kupffer cells (k) in dilated sinusoids (S) between the hepatocytes as well as lymphocytes aggregation (yellow circle) was observed. Moreover, distention and hemorrhage in the central vein beside dilatation and congestion of the portal veins (green arrow) with inflammatory cellular infiltration (black circle). However, most of the histological alterations induced by BPA were markedly reduced and attenuated in liver sections of rats treated with either BPA + GBE, BPA + Mel or BPA + GBE + Mel as shown in C, D & E, respectively.
Fig. 3 Microscopic examination of liver sections of rats treated as follow: control group (A), BPA-treated rats (B), BPA + GBE-treated rats (C), BPA + Mel-treated rats (D), BPA + GBE + Mel-treated rats (E).
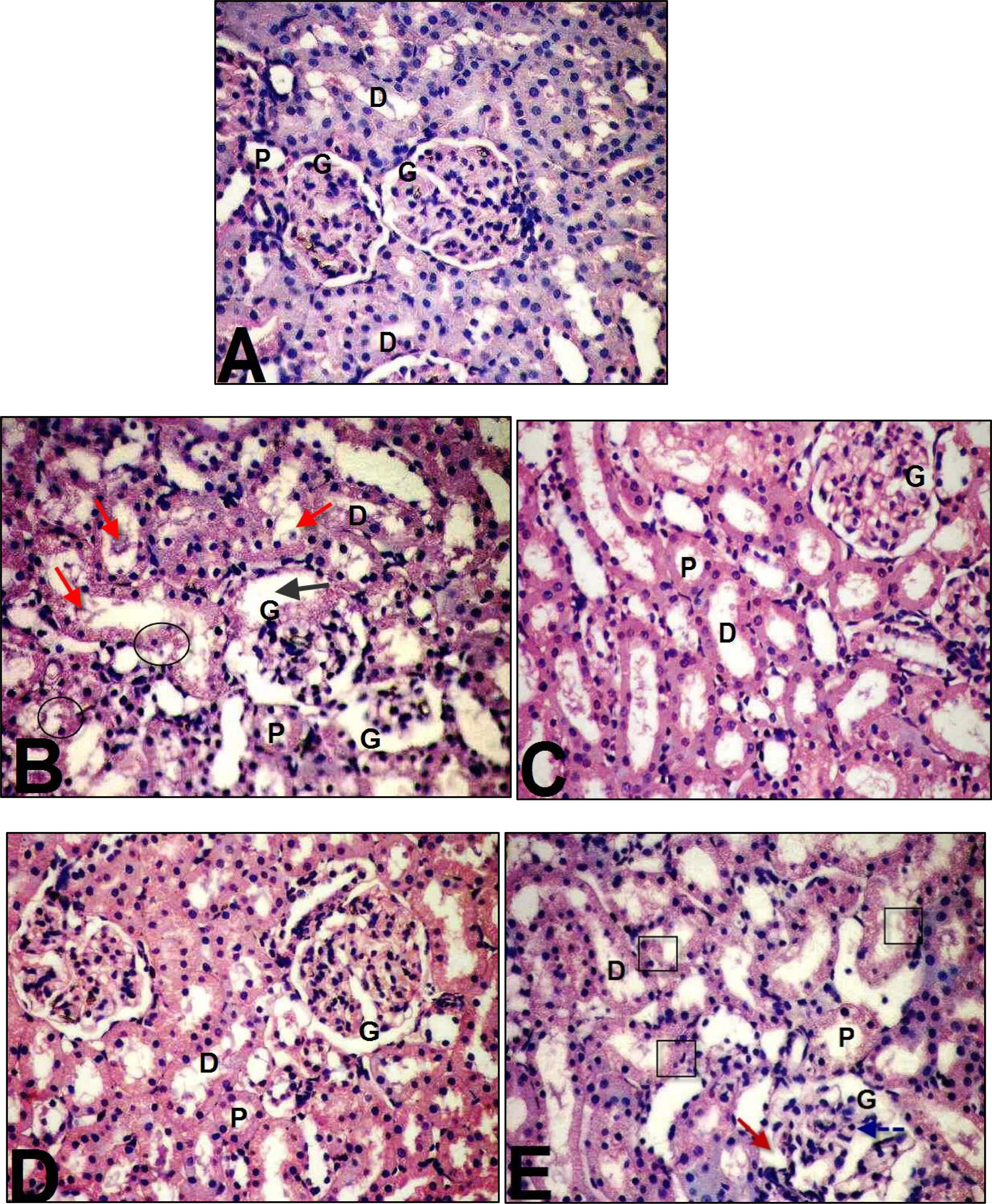
Histological examination of kidney tissues of the different experimental groups was represented in . Kidney sections of the control group revealed normal cortical architecture with normal glomerular tuft (G), proximal (P) and distal (D) convoluted tubules (A). On the other hand, BPA induced kidneys histopathological changes (B). These changes represented by shrinkage of capillaries in the glomerulus (G) with the capsular space (black arrow), considerable degeneration in the epithelial cells of both proximal and distal tubules (black circle) with pyknotic nuclei and cellular debris in the renal tubules (red arrow). Co-administration of GBE and/or Mel in combination with BPA showed evident improvement in the histopathological alterations compared with those indicated in BPA-treated group (C, D & E).
4 Discussion
This study was intended to examine the antioxidative effect of GBE and/or Mel against BPA-induced oxidative damage in the liver and kidney tissues of adult male rats. The present data showed that BPA caused significant increases in the plasma AST and ALT activities indicating liver damage. These two enzymes are the most indicative markers of hepatocellular damage [Citation37]. The enhanced activities of the plasma liver markers point out the cellular leakage and the loss of the functional integrity of membrane architecture [Citation38]. The BPA-induced hepatotoxicity was ameliorated after the administration of Mel and/or GBE as indicated from the significant decreases in the hepatic activities of both AST and ALT as shown in. It was reported that GBE ameliorated the toxic effect of CCL4 [Citation39]. Pre-treatment with Mel prior to thioacetamide induction in rats resulted in the decrease of both AST and ALT activities. Also, melatonin tends to restrain liver damage, reserves the integrity of the plasma membranes and hence restores these enzymes [Citation40].
TBARS, GSH, GST, CAT, SOD and GPx are important biomarkers of oxidative damage [Citation41]. Our results indicated the increase in the level of TBARS and the decrease in the GSH level in the BPA-treated rats. The significant increase in the levels of hepatic TBARS was concomitant with significant decrease in the GSH level at a dose of 50 mg/kg BPA [Citation42].
Conversely, our results indicated that the hepatic TBARS level was significantly decreased while hepatic GSH level was significantly increased in the GBE plus BPA-administered group. Flavonoids in the GBE can discharge the tissue damage caused by oxygen-derived free radicals consequently reduces lipid peroxidation [Citation39].
Moreover, the presence of melatonin with BPA caused significant decrease in the hepatic TBARS level. A significant decrease in the hepatic TBARS level was observed in melatonin plus lead-treated group [Citation38]. In our study, the treatment with Mel significantly attenuated the decreased level of GSH by BPA [Citation43]. GSH is an essential intracellular hydrophilic antioxidant that participates in the catalytic cycles of many antioxidant enzymes such as glutathione peroxidase, glutathione reductase and glutathione-S-transferase [Citation44,Citation45] . The decreases in the specific activities of GPx and SOD in the liver of BPA-treated rats were recorded [Citation42]. Glutathione peroxidase catalyzes the dismutation of the superoxide anion (O2−) into hydrogen peroxide (H2O2) which is then converted into water [Citation42]. In this manner, it effectively removes toxic peroxides and protects living cells from ROS [Citation46]. In addition, superoxide dismutase protects the mitochondrial lipids, proteins and DNA from the attack of superoxide [Citation47]. However, the treatments of the BPA-intoxicated rats with GBE and/or melatonin ameliorated the oxidative stress. Thus, the hepatic activities of SOD and GPx were significantly increased after GBE or Mel co-treatment with BPA. Previous findings indicated significant increases in the hepatic SOD and GPx activities after administration of either GBE or melatonin [Citation48,Citation45] . Our results showed significant increases in the activities of both hepatic GST and CAT in the BPA-treated rats. GST activity was significantly increased at the expense of the content of GSH that acts as a catalyst for GST after induction with BPA [Citation49]. When GPx is present at low concentration, it is responsible for the detoxification of H2O2, whereas CAT plays its role when GPx reaches the saturation state with its substrate [Citation50]. This could explain the significant increase in the hepatic CAT activity concomitant with the highly significant decrease in the hepatic GPx activity that was observed in the present study. Catalase has a higher km than GPx. This recommends a major role for CAT at higher levels of H2O2 and minor role at the physiological H2O2 levels [Citation51]. On the other hand, we observed significant decreases in the hepatic GST and CAT activities upon co-administration of GBE with BPA as compared with BPA-treated group as in . An ameliorative effect of GBE on the hepatic GST and CAT induced by MnCl2 has been indicated in adult male rats [Citation52]. Moreover, the significant decreases in the activities of the hepatic CAT and GST enzymes in BPA + Mel-treated rats. Mel attenuated the hepatic increase in CAT induced by gamma-irradiation in adult male rats [Citation53]. Regarding the BPA induction of renal toxicity, our study showed significant increase in the urea and creatinine levels in BPA-treated group. The accumulation of BPA-toxic metabolites and the inability of the kidney to eliminate them resulted in nephrotoxicity [Citation54]. However, our study revealed that the treatment with BPA + GBE, BPA + Mel and BPA + GBE + Mel caused significant decrease in the urea and creatinine levels as compared with the BPA-treated group. These results are supported by previous studies that showed the ameliorative role of GBE against nephrotoxicity [Citation56]. Also, Mel prohibited acetaminophen-induced nephrotoxicity in adult male rats [Citation55]. Our study revealed that there is a significant decrease in the renal GSH level in the BPA-treated group compared with the control. While, the renal TBARS level was significantly increased after BPA administration. The increased TBARS and decreased GSH levels indicate an increased ROS generation and hence lipid peroxidation in the kidney. However, our results indicated that there is a significant decrease in renal TBARS accompanied with a significant increase in the renal GSH in the BPA + GBE-treated group as compared with BPA-treated group. The protective role of GBE in nephro-hepato toxicity induced in male rats was investigated [Citation56]. Also, the present study showed that co-administration of BPA + Mel exhibited a significant decrease in the renal TBARS and this was accompanied with a significant increase in the renal GSH level. The treatment of male rats intoxicated by CCl4 with Mel leads to a significant decrease in the renal TBARS and increase in GSH levels [Citation57]. The depletion of GSH diminishes the overall kidney’s antioxidant potential as a result of increased lipid peroxidation after BPA-treatment. The activities of the renal GST, SOD and CAT were significantly decreased after administration of BPA as compared with the control It is well known that SOD and CAT possess sequential functions in ROS removing, by O2 dismutation, followed by H2O2 decomposition to H2O and O2 [Citation49]. However, the renal activities of GST, SOD and CAT were significantly increased in BPA + GBE-treated group compared to BPA-treated one. There was an increase in the renal SOD activity when adult male rats were administered with GBE plus gentamycin compared with gentamycin-administration [Citation56]. Also, the current study showed that there were significant increases in the activities of the renal GST, SOD and CAT in the BPA + Mel-treated group compared with BPA-treated one. Furthermore, it has been indicated that these renal antioxidant enzymes were significantly increased in CCl4 plus melatonin treated-rats [Citation57].
In different inflammatory processes, TNF-α is considered as one of the key released cytokines, which activates signaling pathways associated with inflammatory response. In response to inflammation and infection, TNF-α is produced mainly by the immune system [Citation58]. Our study showed significant increase in the level of TNF-α following BPA-treatment in both liver and kidney tissues . TNF-α level was increased significantly in the liver tissues when the mice were treated with different doses of BPA [Citation4]. While, our results indicated that Mel co-treatment along with BPA resulted in a significant decrease in the hepatic and renal TNF-α level as compared with BPA-treated group [Citation58]. Melatonin significantly reduced hepatic TNF-α level in thioacetamide-induced rats [Citation40]. Also, our data demonstrate that there is a significant decrease in both hepatic and renal TNF-α level in rats co-treated with BPA + GBE [Citation59].
Histopathological alterations in livers and kidneys indicated tissue injury after Bisphenol A administration accompanied by degenerative changes in the hepatic cells [Citation4]. The cell rupture and membrane damage of human erythrocytes as a result of the oxidative damage was induced by Bisphenol A [Citation4]. Moreover, our histological examination revealed markers of inflammatory cellular infiltration, hepatocytes vacuolations and congestion in central and portal veins with the elevation in numbers of Kupffer cells [Citation49]. Moreover, BPA-treated rats exhibited morphological changes in the kidney tissue compared to the control group. It caused dilation in Bowman’s space and hypercellularity of glomerulus. Also, it induced degeneration of proximal tubules’ epithelial cells [Citation60,Citation61] . In the present study, co-administration of GBE and/or Mel in combination with BPA revealed remarkable improvement in the liver and kidney tissues compared to BPA-treated group [Citation62]. They demonstrated that the histopathological score of fibrosis together with liver function were improved in rats treated with CCl4 plus GBE. Furthermore, our results showed appreciable improvement in the liver and kidney tissues after supplementation with Mel which serves as a powerful, naturally occurring antioxidant [Citation63].
In conclusion, the wide use of BPA in the different plasticizers and other industries has a hazard effects on human today. Thus, we should limit the consumption of its products. GBE and Mel were observed as potent natural antioxidants. Owing to their antioxidant capacities; GBE, Mel and their combination exhibited protective effect on liver and kidney tissues against BPA-induced injury in rats. They could restore the liver and kidney functions, enzymatic and nonenzymatic antioxidants to control values. They also attenuated the lipid peroxidation and the proinflammatory status in livers and kidneys which was evidently appeared in the histopathological investigations. Thus, consumption of GBE and Mel could introduce health benefits to human against the hazards of BPA.
References
- J.I.EidS.M.EissaA.A.El-GhorBisphenol A induces oxidative stress and DNA damage in hepatic tissue of female rat offspringJ Basic Appl Zool7120151019
- W.M.S.AhmedW.A.MoselhyT.M.NabilBisphenol A toxicity in adult male rats: hematological, biochemical and histopathological approachGlobal Vet142015228238
- M.A.El-MissiryA.I.OthmanM.A.Al-AbdanA.A.El-SayedMelatonin ameliorates oxidative stress, modulates death receptor pathway proteins, and protects the rat cerebrum against Bisphenol-A-induced apoptosisJ Neurol Sci3472014251256
- M.K.MoonM.J.KimI.K.JungY.D.KooH.Y.AnnK.J.Leeet al.Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect levelJ Korean Med Sci272012644652
- N.P.SangaiR.J.VermaM.H.TrivediTesting the efficacy of quercetin in mitigating Bisphenol A toxicity in liver and kidney of miceToxicol Ind Health302012581597
- H.KabutoS.HasuikeN.MinagawaT.ShishiboriEffects of Bisphenol A on the metabolisms of active oxygen species in mouse tissuesEnviron Res9320043135
- V.BindhumolK.C.ChitraP.P.MathurBisphenol A induces reactive oxygen species generation in the liver of male ratsToxicology1882003117124
- H.SalemA.MohamedE.SalehK.ShalabyInfluence of Hesperidin combined with Sinemet on genetical and biochemical abnormalities in rats suffering from Parkinson’s diseaseLife Sci J92012930945
- P.C.ChanQ.XiaP.P.FuGinkgo biloba leave extract: biological, medicinal, and toxicological effectsJ Environ Sci Health252007211244
- Z.YanR.FanS.YinX.ZhaoJ.LiuL.Liet al.Protective effects of Ginkgo biloba leaf polysaccharide on nonalcoholic fatty liver disease and its mechanismsInt J Biol Macromol802015573580
- G.RimbachA.M.MinihaneJ.MajewicA.FischerJ.PallaufF.Virgliet al.Regulation of cell signalling by vitamin EProc Nutr Soc612002415425
- G.YuanZ.GongJ.LiX.LiGinkgo biloba extract protects against alcohol-induced liver injury in ratsPhytother Res212007234238
- K.YaparK.CavuşoğluE.OruçE.YalçinProtective role of Ginkgo biloba against hepatotoxicity and nephrotoxicity in uranium-treated miceJ Med Food132010179188
- J.SehajpalT.KaurR.BhattiA.P.SinghRole of progesterone in melatonin-mediated protection against acute kidney injuryJ Surg Res1912014441447
- G.CzechowskaK.CelinskiA.KorolczukG.WojcickaJ.DudkaA.Bojarskaet al.Protective effects of melatonin against thioacetamide-induced liver fibrosis in ratsJ Physiol Pharmacol662015567579
- C.Tomas-ZapicoA.Coto-MontesA proposed mechanism to explain the stimulatory effect of melatonin on antioxidative enzymesJ Pineal Res39200599104
- H.J.WuC.LiuW.X.DuanS.C.XuM.D.HeC.H.Chenet al.Melatonin ameliorates Bisphenol A-induced DNA damage in the germ cells of adult male ratsMutat Res75220135767
- S.SidhuP.PandhiS.MalhotraK.VaipheiK.L.KhandujaMelatonin treatment is beneficial in pancreatic repair process after experimental acute pancreatitisEur J Pharmacol6282010282289
- AIHA, American Industrial Hygiene AssociationBisphenol AAm Ind Hyg Assoc J281967301304
- NIOSH, National Institute for Occupational Safety and HealthRegistry of toxic effects of chemical substances1987U. S. Department of Health, Education and Welfare, Public Health Service85718582
- X.F.YangN.P.WangW.H.LuF.D.ZengEffects of Ginkgo biloba extract and tanshinone on cytochrome P-450 isozymes and glutathione transferase in ratsActa Pharmacol Sin24200310331038
- A.A.SulaimanN.N.Al-shawiH.A.JwaiedM.D.MahmoodA.S.HussainProtective effect of melatonin against chlorpromazine-induced liver disease in ratsSaudi Med J27200614771482
- A.G.GornallC.J.BardawillM.M.DavidDetermination of serum proteins by means of the biuret reactionJ Biol Chem1771949751766
- J.K.FawcettJ.E.ScottA rapid and precise method for the determination of ureaJ Clin Pathol131960156159
- D.L.FabinyAutomated reaction-rate methods for determination of serum creatinineClin Chem171971696700
- S.ReitmanS.FrankelA colorimetric method for the determination of serum glutamic oxalocetic and glutamic pyruvic transaminasesAm J Clin Pathol2619575663
- IFCCMethods for the measurement of catalytic concentrations of enzymes. Part 3: IFCC method for Alanine aminotransferaseJ Clin Chem Clin Biochem11986497510
- D.T.Y.ChiuF.H.StultsA.L.TappelPurification and properties of rat lung soluble glutathione peroxidaseBiochim Biophys Acta4451976558566
- D.J.JollowJ.R.MichellN.ZampaglionicJ.R.GilleteBromobenzene-induced Liver necrosis: protective role of glutathione and evidence for 3, 4-Bromobenzene oxide as hepatotoxic metabolitePharmacology111974151169
- W.H.HabigM.J.PabstW.B.JakobyGlutathione S-transferases. The first enzymatic step in mercapturic acid formationJ Biol Chem197471307139
- H.P.MisraI.FridovichThe role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutaseJ Biol Chem247197231703175
- H.LuckCatalaseM.V.BergmayerMethod of enzymatic analysis1974Verlag Chemic Academic PressNew York885888
- A.L.TappelH.ZalkinInhibition of lipid peroxidation in mitochondria by vitamin EArch Biochem Biophys801959333336
- R.D.LillieHistopathologic technic and practical histochemistry3rd ed.1965McGraw Hill Book Co.New York
- R.G.D.SteelJ.TorriePrinciples and procedures of statistics. A biometric approach2nd ed.1981Mc Graw Hill International Book Co.Singapore City
- Institute SASSAS/QC user’s guide1986SAS Institute Inc.Cary, NC version 5 edition
- W.A.MoselhyW.M.S.AhmedT.M.NabilBisphenol A toxicity in adult male rats: hematological, biochemical and histopathological approachGlobal Vet142015228238
- D.GhoshE.MitraM.DeyS.B.FirdausA.GhoshD.Mukherjeeet al.Melatonin protects against lead-induced oxidative stress in rat liver and kidneyAsian J Pharm Clin Res62013137145
- B.S.AljadaaniA.A.BakrA.H.HamzaEffect of Ginkgo biloba and Commiphora opobalsamum extracts on liver fibrosis and kidney injury induced by carbon tetra chloride in experimental modelsWorld J Pharm Sci42016148152
- A.A.AbdalfattahA.A.El-EbiaryE.M.HantashStudy of the effects of melatonin on experimentally induced hepatic fibrogenesis in ratsInt J Adv Res4201611871197
- K.EriksonA.DobsonD.DormanM.AschnerManganese exposure and induced oxidative stress in rat brainSci Total Environ3342004409416
- Z.K.HassanM.A.ElobeidP.VirkS.A.OmerM.ElAminM.H.Daghestaniet al.Bisphenol A induces hepatotoxicity through oxidative stress in rat modelOxid Med Cell Longevity201216
- R.A.SaadM.Fath El-BabA.A.ShalabyAttenuation of acute and chronic liver injury by melatonin in ratsJ Taibah Univ Sci20138896
- S.MelovAnimal models of oxidative stress, aging, and therapeutic antioxidant interventionsInt J Biochem Cell Biol34200213951400
- S.K.BiswasI.RahmanEnvironmental toxicity, redox signaling and lung inflammation: the role of glutathioneMol Aspects Med3020096076
- M.M.Sayed-AhmedA.M.AleisaS.S.Al-RejaieA.A.Al-YahyaO.A.Al-ShabanahM.M.Hafezet al.Thymoquinone attenuates diethylnitrosamine induction of hepatic carcinogenesis through antioxidant signalingOxid Med Cell Longevity32010254261
- A.K.HolleyV.BakthavatchaluJ.M.Velez-RomanD.K.St.ClairManganese superoxide dismutase: guardian of the powerhouseInt J Mol Sci12201171147162
- D.ChengB.LiangY.LiAntihyperglycemic effect of Ginkgo biloba extract in streptozotocin-induced diabetes in ratsBiomed Res Int162724201317
- I.M.MouradY.A.KhadrawThe sensitivity of liver, kidney and testis of rats to oxidative stress induced by different doses of Bisphenol AInt J Life Sci Pharma Res220121928
- A.L.HussainJ.M.JakoniukLiver catalase, glutathione peroxidase and reductase activity, reduced glutathione and hydrogen peroxide levels in acute intoxication with chlorfenvinphos, an organophosphate insecticidePol J Environ Stud132004303309
- B.HalliwellOxidants and human diseasesFASEB J11987441445
- S.M.MahmoudH.I.BahrPotential protective effects of Ginkgo biloba and rosemary on hepato encephalopathy and chromosomal aberrations induced by manganese chloride in ratsInt J Adv Res32015483497
- V.K.BhartiR.S.SrivastavaH.KumarS.BagA.C.MajumdarG.Singhet al.Effects of melatonin and epiphyseal proteins on fluoride-induced adverse changes in antioxidant status of heart, liver, and kidney of ratsAdv Pharmacol Sci532969201416
- N.P.SangaiR.J.VermaM.H.TrivediTesting the efficacy of quercetin in mitigating Bisphenol A toxicity in liver and kidney of miceToxicol Ind Health302012581597
- Y.O.Z.IlbeyE.OzbekM.CekmenA.SomayL.OzcanA.Otünctemuret al.Melatonin prevents acetaminophen-induced nephrotoxicity in ratsInt Urol Nephrol412009 695–02
- M.M.HamdyD.A.El-SersM.M.AbdelhamidProtective effect of curcumin and Ginkgo biloba l. Extract against gentamicin-induced nephrotoxicity in ratsAssiut Med J372013135146
- S.O.AdewoleA.A.SalakoO.W.DohertyT.NaickerEffect of melatonin on carbon tetrachloride-induced kidney injury in wistar ratsAfr J Biomed Res102007153164
- M.E.AhmedH.I.AhmedE.M.El-MorsyMelatonin protects against diazinon-induced neurobehavioral changes in ratsNeurochem Res38201322272236
- G.TablA.M.ElwyEvaluation of Ginkgo biloba as alternative medicine on ova-induced eotaxin and eosinophilia in asthmatic lungLife Sci J10201321312136
- O.RahimiF.FarokhiS.M.KhojastehS.A.OziThe effect of Bisphenol A on serum parameters and morphology of kidney’s tissueBiol Int J720157990
- H.BhattacharyaQ.XiaoL.LunToxicity studies of nonylphenol on rosy barb (Puntius conchonious): a biochemical and histopathological evaluationTissue Cell402008243249
- F.A.MahboubH.A.LamfonProtective effect of Ginkgo biloba extract on carbendazim-induced hepatotoxicity in albino ratsFood Nutr Sci42013866872
- D.FouadAntioxidant and modulatory effect of melatonin on hepatotoxicity and oxidative stress induced by orange yellow S in male ratsPak J Zool472015383391