?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Cyanobacteria are one of the richest sources of biomedical relevant compounds with extensive therapeutic pharmaceutical applications and are also known as producer of intracellular and extracellular metabolites with diverse biological activities. The genus Anabaena sp. is known to produce antimicrobial compounds, like phycocyanin and others. The goal of this study was to optimize the production of these bioactive compounds. The Plackett–Burman experimental design was used to screen and evaluate the important medium components that influence the production of bioactive compounds. In this present study, eight independent factors including NaNO3, K2HPO4, MgSO4·7H2O, CaCl2, citric acid, ammonium ferric citrate, ethylene diamine tetraacetic acid disodium magnesium salt (EDTA-Na2Mg) and Na2CO3 were surveyed and the effective variables for algal components production of Anabaena oryzae were determined using two-levels Plackett–Burman design. Results analysis showed that the best medium components were NaNO3 (2.25 g l−1); K2HPO4 (0.02 g l−1); MgSO4 (0.0375 g l−1); CaCl2 (0.018 g l−1); citric acid (0.009 g l−1); ammonium ferric citrate (0.009 g l−1) and EDTA-Na2 (0.0015 g l−1) respectively. The total chlorophyll-a, carotenoids, phenol, tannic acid and flavonoid contents in crude extract of Anabaena oryzae were determined. They were 47.7, 4.11, 0.256, 1.046 and 1.83 μg/ml, respectively. The antioxidant capacity was 62.81%.
1 Introduction
Blue-green algae (Cyanobacteria) are group of Gram-negative photoautotrophic prokaryotes that contain a blue-green colored pigment (c-phycocyanin). Cyanobacteria have a fossil history from 3.3 to 3.5 billion years ago [Citation1]. The cyanobacteria can be found in nearly all the imaginable habitats such as fresh and salty water, polluted waters of lakes, ponds, water tanks, soil, rocks and tree barks. Its morphological form varies from unicellular to filamentous or colonial forms [Citation2]. Some cyanobacterial species are thermophilic and growing in thermal springs [Citation3]. Some cyanobacteria are environmentally-friendly and have the ability to fix atmospheric nitrogen and solubilize phosphate [Citation4]. Cyanobacteria are considered as a rich source of primary metabolites such as polysaccharides, proteins, fats, oils, vitamins, pigments and hydrocarbons [Citation5]. They are also a rich source of many secondary metabolites like the antifungal cyclic peptides in Tolypothrix byssoidea [Citation6,Citation7] , the antibacterial diterpenoids compounds in Nostoc commune [Citation8], the algicidal phenolic compound 4,4′dihydroxybiphenyl and the indol alkaloid norharmane in Nostoc insulare and Nodularia harveyana, respectively [Citation9]. There are a lot of intracellular and extracellular metabolites with diverse biological activities as antiviral [Citation10], anticancer, anti-inflammatory, cytotoxic [Citation11] and immunosuppressive agents. The production of extracellular antibiotic metabolites by marine microalgae and screening for other pharmacologically active compounds had been studied in past few decades [Citation12]. In addition to that foregoing, there are many other important compounds with antioxidant activity like ascorbic acid, reduced glutathione, phenolic compounds and flavonoids [Citation13]. Furthermore, photosynthetic pigments which consist of two classes, chlorophylls (α &β chlorophyll) and carotenoids. Carotenoids that contain oxygen known as xanthophylls like lutein, zeaxanthin, canthaxanthin,β-cryptoxanthin and astaxanthin; while that are only hydrocarbons are known as carotenes like β-carotene and lycopene [Citation14]. Sinha et al. [Citation15] reported that the screening programs of cyanobacterial biomass have led to discovery of new compounds with antimicrobial, antineoplastic and cytotoxic activities. The use of non-conventional microorganism in biotechnology had attracted much interest in recent years [Citation16]. The conventional optimization of production factors is usually a time uncontrollable and labor-intensive process. On the contrary, statistically designed two level-factorial experiments, had been proved to be valuable tools for optimizing microbial culture conditions [Citation17]. The objective of this study was to apply Response surface methodology (RSM) to evaluate the effects of the medium composition for attaining the highest bioactive compounds production by cyanobacterium Anabaena oryzae.
2 Materials and methods
2.1 Culture preparation and isolation of micro-organism
Cyanobacterium Anabaena oryzae was isolated from the rice fields in Egypt. The soil samples were collected, air-dried, ground to pass a 2-mm sieve. Soil samples were transferred into Petri-dishes, wetted by sterilized distilled water and incubated under fluorescent illumination for three weeks. The formed mats were inculcated into modified (BG-11) medium [Citation18], and the mat was re-cultured several times to new solid BG-11 plates and incubated under continuous illumination using white fluorescent light intensity of 2000–2500 lux for three weeks at 26 ± 2 °C, with regular microscopic examination.
Modified BG-11 medium was used in the present study and its final pH after autoclaving and cooling was 7.4.
2.2 Alga identification
Cyanobacterium Anabaena oryzae was identified according to Desikachary [Citation19] and the samples were prepared according to the procedure described by Cronberg [Citation20] using light microscope at 100× oil immersion lens.
2.3 Maintenance medium for alga
The Anabaena oryzae alga was maintained on BG-11 medium, 250 ml of algal culture was transferred to 500 ml Erlenmeyer flasks. The flasks were exposed to a light/dark cycle of 14/10 h, respectively at room temperature, left to grow and harvested after 2 weeks by centrifugation at 1000 rpm for 10 min.
2.4 Optimization of the bioactive compounds production based on multi-factorial experiments
The Plackett–Burman experimental design, [Citation21] an efficient technique for optimization of the factors affecting active compounds' production was used to reflect the relative importance of various chemical factors on bioactive compounds production.
This model does not describe the interaction between the factors, but was used to screen and evaluate the important media components that influence the production of antibacterial compounds.
Eight independent variables () were screened in thirteen runs organized according to the Plackett–Burman design matrix as indicated in . The studied factors were: NaNO3, K2HPO4, MgSO4, CaCl2, citric acid, ammonium ferric citrate, ethylene diaminetetraacetic acid disodium magnesium salt (EDTA-Na2Mg) and Na2CO3. For each variable, a high level (+) and low level (−) were tested, which represents two different nutrient concentrations. The trials were performed in triplicate using 250 ml Erlenmeyer flasks and final data was calculated as the mean of triplicate.
Table 1 Factors and coded levels examined as independent variables affecting bioactive components production by Anabaena oryzae and their levels in the Plackett–Burman design experiment.
Table 2 Randomized Plackett–Burman experimental design for the eight tested BG-11 medium factors.
2.5 Cell growth and determination of cell dry weight
The growth of Anabaena oryzae was determined by measuring the optical density of the algal suspension at wavelength 660 nm for 7 days using spectrophotometer (UV-200-RSLW scientific)[Citation22]. The algal biomass can be determined by measuring its dry weight. The pellets were washed with distilled water, recentrifuged and kept for drying at 60 °C till constant weight [Citation23].
2.6 Preparation of alga extract
One hundred mg of alga was soaked in 5 ml petroleum ether (60–80 °C) for 48 h and filtrated; the precipitate was soaked in dichloromethane for the same period and repeated for the last time in methanol.
2.7 Analysis of bioactive compounds
The methanol extracts of Anabaena oryzae were used for the tannin, flavonoids, total phenol content, antioxidant, ascorbic acid and pigments analyses.
2.8 Total phenolic content (TPC)
Total phenolic content was performed by Folin-Ciocalteu method [Citation24]. Distilled water (3.16 ml) was mixed with the 40 μl of sample and then 200 μl of Folin Ciocalteu reagent was added. After 5 min, 600 μl of 20% sodium carbonate solution (Na2CO3) was added and solution mixed again. The solution was left at room temperature for 2 h. The color intensity was measured using spectrophotometer at wavelength 750 nm. The total phenol content was compared to with gallic acid standard curve according to the formula:where;
C = concentration of the gallic acid equivalent from standard curve (mg/mL),
V = volume of the extract used (ml),
g = weight of extract (g).
2.9 Pigments estimation
A known volume of culture was centrifuged at 8000 rpm for 10 minutes, after that the algal pellets were treated with known volume of methanol and kept in water bath for 30 min at 55 °C, then centrifuged again. The color of pellets must be white to ensure complete extraction of pigments. Absorbance was measured at 666, 653 and 470 nm. Calculations were made according to the formula devised by Costache [Citation25] for chlorophyll (a) and carotenoids estimation.
Chlorophyll (a) = 15.65 Ab 666-7.340 Ab653
Carotenoids = 1000 Ab 470-2.860 Chlorophyll (a)
2.10 Flavonoid content determination
The flavonoid content of Anabaena oryzae extract samples were determined according to the aluminum chloride method described by Barku [Citation26]. One ml of sample solution of alga extract was added to 0.5 ml of distilled water. About 0.075 ml of 5% sodium nitrite solution was then added to the mixture followed by incubation for 6 min after which 0.15 ml of 10% aluminum chloride was added, shaked and left to stand for 6 min, 0.5 ml of 1 M sodium hydroxide was finally added and the mixture was diluted with 0.275 ml distilled water, shaked and left to stand for 15 min before absorbance reading, using the sample solution without coloration as reference solution. The absorbance of the reaction mixture was measured at 500 nm.
Flavonoid content was expressed as mg of quercetin equivalent (QE)/g dry weight.
2.11 Antioxidant capacity
Antioxidant activity of Anabaena oryzae extract was determined by the ability of extract to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH)radicals [Citation27]. One hundred μl of test extract was mixed with 1 ml of methanolic solution of 0.1 mM DPPH. The mixture was vortexed and incubated at 37 °C for 30 min in dark. The absorbance of the samples was measured at 517 nm.
Lower absorbance of the reaction mixture indicated higher free radical scavenging activity which was calculated using the following equation:where: A° is the absorbance of the control reaction and A1 is the absorbance of reaction mixture.
Percentage of the DPPH scavenging activity was plotted versus the different concentrations of sample extract used and the concentration causing a 50% reduction of DPPH absorbance was expressed as the half maximal inhibitory concentration (EC50) value.
2.12 Vanillin – HCL assay
Sample (0.2 g) of dried Anabaena oryzae biomass was extracted with 10 ml of methanol for 24 h at 30 °C. One ml of the resulting extract was reacted with 5 ml of vanillin reagent 50:50 (mixture of 1% vanillin:8% HCl in methanol) for 20 min at 30 °C, and absorbance was measured at 500 nm. For the control sample, vanillin reagent was replaced with 5 ml of 4% HCl in methanol. Blank value was subtracted from experimental value to give adjusted data.
Tannic acid standard curve from 0.0–1.0 mg/ml was used in calculating tannin levels [Citation28].
2.13 Ascorbic acid content
Ascorbic acid content of Anabaena oryzae was estimated according to the method of Loeffler and Ponting [Citation29]. Ten ml of 2,6-dichlorophenol indophenol dye solution was added to 5 ml of 100 mg of dried Anabaena oryzae biomass then was extracted by 6% HPO3, vortexed well and the red color was measured within 15–20 second at 518 nm.
Ascorbic acid standard curve was used in calculating mg of ascorbic acid per g of dry sample according to the formula:
2.14 Statistical analysis
Statistical were carried by using Minitab 16 software.
3 Results and discussion
In the present study screening of basic medium compositions influencing active constituents production by Anabaena oryzae were analyzed by Plackett–Burman experimental design under shaking conditions.
The data shown in () revealed a wide variation in total phenol concentrations. The maximum phenol production was found in experimental trial no. 10 (0.256 mg/ml), whereas minimum concentration of phenol was in trial no. 8 (0.097 mg/ml).
Table 3 Randomized Plackett–Burman experimental design for the eight tested BG-11 medium factors affecting bioactive components production by Anabaena oryzae.
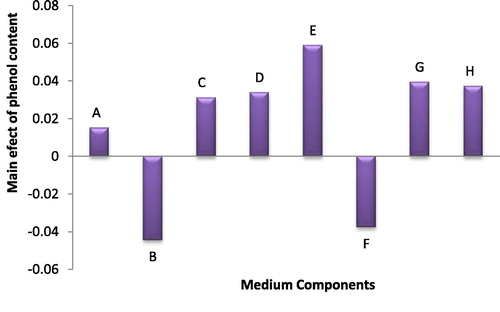
Statistical results of eight tested medium variables in case of the phenol production revealed that K2HPO4 and ferric ammonium citrate had a negative effect, other variables had a positive effect and in general there was no significant effect between medium variables on phenol production by Anabaena oryzae at P ≤ 0.05 ().
Fig. 1 The main effects of different medium variables on the total phenol content of Anabaena oryzae.
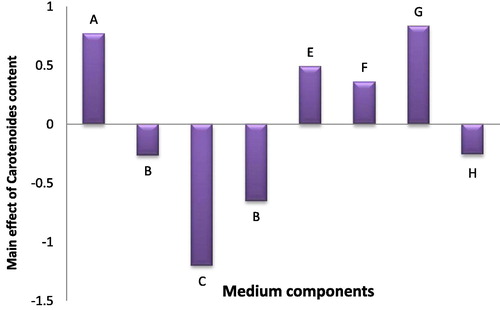
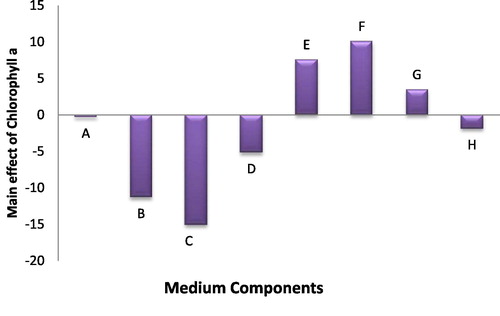
In case of chlorophyll (a) and carotenoid pigments production, maximum concentration of chlorophyll (a) was found in trial no. 10 (47.7 µg/ml) and minimum concentration was found in experimental trial no. 6 (1.7 µg/ml), while maximum carotenoid pigment production also was present in trial 10 (4.11 µg/ml). Statistical results of eight tested medium variables in case of the chlorophyll (a) revealed that ferric ammonium citrate and citric acid had a significant positive effect, while in carotenoid pigment production, NaNO3, citric acid and ferric ammonium citrate had a significant positive effect. The results revealed that MgSO4, K2HPO4 and CaCL2 variables had a significant negative effect in chlorophyll (a) production while MgSO4 and CaCL2 had that effect in production of carotenoid, respectively (Figs. 2 and 3).
Fig. 2 The main effects of different medium variables on the Chlorophyll content of Anabaena oryzae.
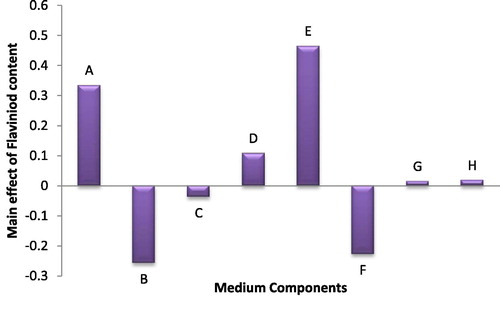
The highest record of total flavonoid content was observed in trial no. 10 (1.83 µg/ml). () shows that both NaNO3 and citric acid variables had a significant positive effect on the production of flavonoids.
Fig. 4 The main effects of different medium variables on the total flavonoid production of Anabaena oryzae.
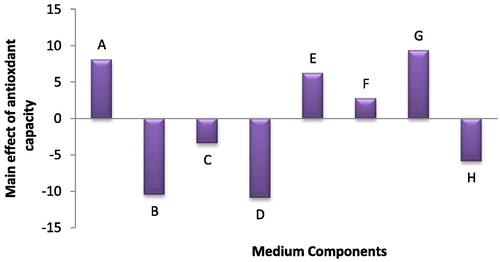
All experimental trials have a scavenging activity toward the DPPH reagent; however the trial no. 10 exhibited the highest scavenging activity (62.81%). It was found that among the eight investigated medium variables, EDTA, citric acid and NaNO3 had positive main effects on antioxidant capacity (); while the K2HPO4 and CaCL2 variables had negative main effects. The differences between both positive and negative variables were significant on antioxidant capacityat P ≤ 0.05.
Fig. 5 The main effects of different medium variables on the antioxidant capacity of Anabaena oryzae.
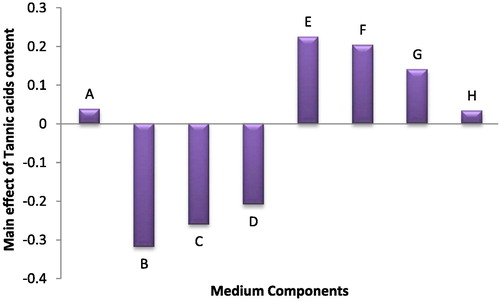
Also, in case of tannin production, the highest tannin content measured at the end of the experimental period was 1.046 mg/ml in trial no. 10, whereas the lowest content was 0.003 mg/ml in the trial no. 3. Statistical results of eight tested medium variables in tannin production revealed that K2HPO4 and MgSO4 variables had a significant negative effect; and citric acid, ferric ammonium citrate and EDTA had a positive effect, respectively ().
Fig. 6 The main effects of different medium variables on the tannic acid production of Anabaena oryzae.
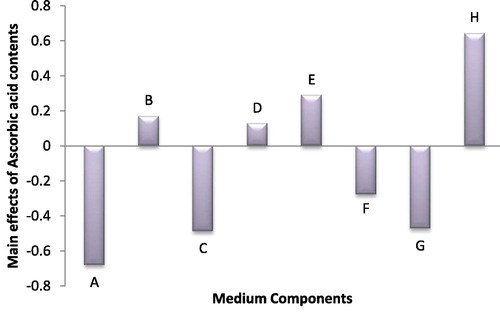
The maximum concentration of ascorbic acid was 4.82 mg/ml in trial no. 12, from the statistical results represented in (). The main effect of the eight different factors in the Plackett -Burman experimental design, the p-value and the significant of the different factors that affect ascorbic acid production showed that, NaNO3, MgSO4 and EDTA have negative main effects on the ascorbic acid content, while the other factors have positive main effects on the ascorbic acid content. Statistical analysis also estimated that NaNO3, MgSO4, EDTA and Na2CO3 were significant variables on production of ascorbic acid by Anabaena oryzae at P ≤ 0.05.
Fig. 7 The main effects of different medium variables on the ascorbic acid production of Anabaena oryzae.Medium components: NaNo3(A), K2HPO4(B), MgSO4(C), CaCl2(D), Citric acid(E), Ammonium ferric citrate(F), EDTA-Na2Mg(G) and Na2CO3(H).
Among the eight nutrient components used in this study, K2HPO4, MgSO4 and citric acid had contributed to a large extent for most bioactive compounds production.
The chlorophyll (a) and carotenoid pigments, antioxidant capacity, flavonoid content and tannin content yields were increased in the presence of high concentration of citric acid and low concentrations of K2HPO4 and MgSO4.
NaNO3 was effective in higher level in increasing concentration of carotenoid pigments, flavonoid content and antioxidant capacity; while ferric ammonium citrate was effective in case of chlorophyll (a), carotenoids and tannic acid production. On the other hand the CaCl2 was the most important medium variable in lower level in increasing of chlorophyll (a), carotenoid pigments and percentage of antioxidant activity.
The Plackett–Burman experimental design has been used to evaluate the components of BG-11 medium, affecting the production of active constituents like the phycocyanin. MgSO4 and ferric ammonium citrate were found to be the effective components affecting the phycocyanin production [Citation30]. Bloor and England [Citation31], studied the increasing of nitrate from its base level of 8.8 mM to 26.4 mM which increased the antibiotic production by Nostoc muscorum. However, increasing the concentration of iron from its base level of 5 mM to15 mM caused a dramatic decrease in antibiotic production. Similar results obtained by Ohta et al. [Citation32] who reported that the increase in magnesium and phosphate concentrations cause increase in antibiotic production from Chlorococcum strain HS-101, whereas, the antibiotic production from Chlorococcum strain HS-101 increased by 1.8- fold in the optimized BG-11 medium than in the standard BG-11 and also the antibiotic production from the alga Dunaliella primolecta increased by 2.3-fold in the optimized BG-11 medium. Yin et al. [Citation33] stated that changes in phosphate, nitrate, calcium, irradiance and temperature all caused quantitative, but not qualitative, changes in toxin composition produced by the Cyanobacterium Lyngbya wollei where, lower phosphate, nitrate and higher calcium levels gave rise to higher biomass and toxicity. Lower calcium or phosphate deficient medium and high nitrate or phosphate caused a large decrease in dry weight and toxicity. The results obtained from Plackett–Burman experimental design stated that the highest main effect and t-value obtained by NaCl was significant variable within seven independent variable, so the effect of NaCl on the antifungal activity of Spirulina platensis is the highest and positive [Citation34].
4 Conclusion
Blue-green algae Anabaena oryzae was used in this work for bioactive compounds production using modified blue-green medium (BG-11). Eight independent factors including NaNO3, K2HPO4, MgSO4, CaCl2, citric acid, ammonium ferric citrate, ethylene diamine tetra acetic acid disodium magnesium salt (EDTA-Na2Mg) and Na2CO3 surveyed considered as more and effective variables for the production of chlorophyll (a) and carotenoid pigments, phenols, flavonoids, tannins. Also increased the antioxidant capacity of the algal extract. The Plackett–Burman experimental design, an efficient technique for optimization of the factors affecting active constituent's production was used to reflect the relative importance of various chemical factors on bioactive compounds production.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgement
Authors thank Dr. Asmaa Abdella lecturer of pharmaceutical industries, genetic engineering and biotechnology research institute (current residence: Lincoln Nebraska) for her constructive comments and English revision.
References
- W.SchopfB.PackerEarly archaen microfossils from warrawoona group, AustraliaScience23719877073
- R.K.SinghS.P.TiwariA.K.RaiT.M.MohapatraCyanobacteria: emerging source for drug discoveryJ Antibiot642011401412
- P.KaushikA.ChauhanG.ChauhanP.GoyalAntibacterial potential and UV-HPLC analysis of laboratory-grown culture of Anabaena variabilisInternet J Food Saf1120091118
- N.ThajuddinG.SubramanianCyanobacterial biodiversity and potential application in biotechnologyCurr Sci8920054757
- M.A.BorowitzkaVitamins and fine chemicals from micro-algaeM.A.BorowitzkaL.J.BorowitzkaMicro-algal biotechnology1988Cambridge univ. pressCambridge211217
- M.A.BorowitzkaFats, oils and hydrocarbonsM.A.BorowitzkaL.J.BorowitzkaMicro-algal biotechnology1988Cambridge univ. pressCambridge257287
- B.JakiO.ZerbeJ.HeilmannO.SticherTwo novel cyclic peptides with antifungal activity from the cyanobacterium Tolypothrix byssoidea (EAWAG 195)J Nat Prod642001154158
- B.JakiJ.OrjalaJ.HeilmannA.LindenB.VoglerO.SticherNovel extracellular diterpenoids with biological activity from the cyanobacterium Nostoc communeJ Nat Prod632000339343
- R.B.VolkF.H.FurkertAntialgal, antibacterial and antifungal activity of two metabolites produced and excreted by cyanobacteria during growthMicrobiol Res612006180186
- T.HayashiK.HayashiCalcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga, Spirulina platensisJ Nat Prod5919968387
- H.LueschW.Y.YoshidaE.MoorRV.J.PaulS.L.MooberryIsolation, structure determination and biological activity of Lyngbya bellin A from the marine cyanobacterium Lyngbya majusculaJ Nat Prod632000611615
- J.L.ReicheltM.A.BorowitzkaAntimicrobial activity from marine alga: Results of a large scale screening programmeHydrobiologia1161171984158168
- S.C.WuF.J.WangC.L.PanThe comparison of antioxidative properties of seaweed oligosaccharides fermented by two lactic acid bacteriaJ Mar Sci Tech1842010537545
- E.Kotake-NaraA.NagaoAbsorption and metabolism of xanthophyllsMar Drugs9201110241037
- R.P.SinhaS.YadavM.B.TyagiAntimicrobial activity of some cyanobacteriaInt J Pharm Pharm Sci432012631635
- J.F.T.SpenceA.L.Ragout de SpencerC.LaluceNonconventional yeastsAppl Microbiol Biotechnol582002147156
- L.P.HooijkaasE.C.WilkinsonJ.TramperA.A.BuitelMedium optimization for spore production of Conithyriumminitans using statistically based experimental designsBiotechnol Bioeng64199892100
- R.RippkaJ.DeruellesJ.B.WaterburyM.HerdmanR.Y.StainerGeneric assignments, strain histories and properties of pure cultures of cyanbacteriaJ Gen Microbiol1111979161
- T.V.DesikacharyCyanophyta1959Indian Council of Agricultural ResearchNew Delhi
- G.CronbergPhytoplankton changes in Lake Trummen induced by restorationFolia Limnol Scand1819821119
- R.L.PlackettJ.P.BurmanThe design of optimum multifactorial experimentsBiometrika331946305325
- APHA.Standard methods for the examination of water and wastewater, 21st Edition 2005.
- V.K.NigamR.VermaA.KumarS.KunduP.GhoshInfluence of medium constituents on the biosynthesis of cephalosporin-CElectron J Biotechnol200710.2225/vol10-issue2-fulltext-8
- K.SlinkardV.L.SingletonTotal phenol analyses: automation and comparison with manual methodsAm J Enol Vitic2819774955
- M.A.CostacheG.CampeanuG.NeataStudies concerning the extraction of chlorophyll and total carotenoids from vegetablesRom Biotechnol Lett1752012
- V.Y.A.BarkuY.Opoku-BoahenE.Owusu-AnsahE.F.MensahAsian J Plant Sci Res3120136974
- Blois MS. Antioxidant determinations by the use of a stable free radical, Nature, Barku, V.Y.A., Y. Opoku-Boahen, E. Owusu-Ansah and E.F. 1958; 181: 1199–1200.
- C.F.EarpJ.O.AkingbalaS.H.RingL.W.RooneyEvaluation of several methods to determine tannins in sorghums with varying kernel characteristicsCereal Chem581981234238
- H.J.LoefflerJ.D.PontingAscorbic acid, a rapid determination in fresh, frozen or dehydrated fruits and vegetablesIndonesian English Chem Anal Educ141942846848
- Devendra V.DeshmukhAshwini A.ManePallavi S.VhareSanjay N.HarkeOptimization of media component affecting phycocyanin production from microcystsis sp. isolated from salimalilake, AurangabadJ Algal Biomass Utln4220134246
- S.BloorR.R.EnglandElucidation and optimization ofthe medium constituents controlling antibiotic production by the cyanobacterium Nostoc muscorumEnzyme Microb Technol1319917681
- S.OhtaY.ShiomiA.KawashimaO.AozasaT.NakaoT.Nagataet al.Antibiotic effect of linolenic acid from Chlorococcum strain HS-101 and Dunaliella primolectaon methicillin- resistant Staphylococcus aureusJ Appl Phycol71995121127
- Q.YinW.W.CarmichaelW.R.EvansFactors influencing growth and toxin production by cultures of the fresh water cyanobacterium Lyngbya Wolleifar low ex GomontJ Appl Phycol919975563
- R.M.A.AbedinH.M.TahaAntibacterial and antifungal activity ofcyanobacteria and green microalgae. Evaluation of media components by Plackett–Burman design for antimicrobial activity of Spirulina platensisGlobal J Biotechnol Biochem3120082231