Abstract
Arsenic is a neurotoxic substance that makes the brain susceptible to free radicals. Thymoquinone (TQ) is a potent antioxidant extracted from Nigella sativa seeds. It scavenges free radicals and prevents the cell damage resulted from oxidative substances. In this study, the ameliorative effect of TQ in arsenic-induced neurotoxicity was investigated. Rats were treated for 21 days with: distilled water, 20 mg/kg sodium arsenate, 10 mg/kg TQ, and arsenate followed by TQ. Cerebral cortex, cerebellum and brain stem were removed for the measurements of different physiological parameters. Cerebelli were prepared for histopathological studies. Arsenate treatment caused a decrease in the levels of norepinephrine (NE), dopamine (DA), acetylcholine esterase (AChE) and Na+-K+-ATPase activities in cerebral cortex, cerebellum, and brain stem of rats. Similarly, the levels of glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT) were declined. In contrast, serotonin (5-HT), lipid peroxidation (MDA), nitrite/nitrate (NO), and tumour necrosis factor (TNF-α) levels were increased after arsenate treatment. The presence of degenerated Purkinje cells in cerebellum was noticed. Results revealed that, post-treatment with TQ suppressed the arsenate-induced neurotoxic effects as it decreased the levels of 5-HT, MAD, NO, TNF-α and increased the levels of NE, DA, GH, GPx, GR, SOD, and CAT, in the cerebral cortex, cerebellum, and brain stem. Likewise, AChE and Na+-K+-ATPase activities were increased after TQ post-treatment. In conclusion, TQ ameliorated the neurotoxic effect of arsenate and suppressed the oxidative stress induced in the nervous system through its antioxidant mechanism.
Keywords:
1 Introduction
Thymoquinone (TQ) is the main constituent of the volatile oil derived from N. sativa seeds. It has different pharmacological properties such as anticonvulsant [Citation1], antitussive [Citation2], and anti-tumour [Citation3] as well as anti-inflammatory and antioxidant activities [Citation4]. TQ crosses the blood brain barrier and exerts neuromodulatory activities. It has a neuroprotective effect and improves brain injuries resulting from Parkinson’s disease [Citation5] and status epilepticus [Citation6]. It is also useful in the treatment of glial tumours by inducing apoptosis of glial tumour cells [Citation7]. Several studies reported the protective role of TQ against neurotoxicty induced by heavy metals and radiation. It reduces the cerebral oxidative injuries induced by lead and ionizing radiation [Citation7]. In addition it has a nephroprotective role against lead [Citation8] and cadmium toxicity [Citation9].
Neurotoxicity is caused by the exposure to certain chemicals that affect the nervous system. It results from the degeneration of the neuronal cells [Citation10]. Symptoms of neurotoxicity may include brain damage, dementia oramnesia, anxiety, depression, limb weakness and blurred vision [Citation11]. Neurotoxicity occurs upon the exposure to natural or synthetic toxic substances, called neurotoxins. Neurotoxins such as: aluminum, mercury, copper, arsenic, lead and manganese are characterized by their abilities to alter the normal activity of the nervous system causing neuronal damage [Citation12,Citation13] .
Arsenic is an environmental contaminant found naturally in ground water [Citation14]. Other less common sources of arsenic exposure are incineration of arsenic preserved wood products, inhalation of indoor air polluted with coal combustion, consumption of tainted foods, ingestion of kitchen dust, and tobacco smoke [Citation15]. It is ranked the first among toxicants posing significant potential threats to human health [Citation16]. Arsenic exposure makes the brain tissue of rat vulnerable to free radical attack resulting in abnormal apoptosis of neural cells [Citation17]. It could pass through the blood-brain barrier, invade the brain parenchyma and induce brain toxicity. Brain toxicity includes, altered cholinergic and monoaminergic signalling. Behavioural deficits including learning, memory, and locomotion also results after arsenic exposure [Citation18].
Therefore, the present study aimed to evaluate the effect of sodium arsenate on different brain areas (cerebral cortex, cerebellum, and brain stem) then to examine the role of TQ in ameliorating neurotoxic effects of arsenate.
2 Material and methods
2.1 Chemicals
Sodium arsenate (E.C. No. 231-547-5) and TQ (EC No. 207-721-1) were purchased from Sigma (St. Louis, MO, USA). TQ was first dissolved in DMSO then was diluted with normal saline to a final DMSO concentration of 0.1%. The solution was then given orally.
All other chemicals and reagents were of analytical grade. Double distilled water was used as the solvent.
2.2 Experimental animals
Fourty Wistar female adult (4 month old) rats weighing 180–200 g were obtained from the Holding Company for Biological Products and Vaccines (VACSERA, Giza, Egypt). Animals were subjected to an adaptation period of 10 days in the animal facility before experiments, they were housed in wire bottomed cages in a room under standard conditions of illumination with a (12–12 h) light-dark cycle at 25 ± 1 °C. They were provided with water and a balanced diet ad libitum. All animals received care in compliance with the Egyptian rules for animal protection.
2.3 Experimental protocol
Rats were classified randomly into four groups (n = 10) and treated orally for 21 consecutive days with:
| – | distilled water (control group). | ||||
| – | arsenic as sodium arsenate (20 mg/kg body weight/day) according to Yadav et al. [Citation19] (As group). | ||||
| – | thymoquinone (10 mg/kg body weight/day) according to Gilhotra and Dhingra [Citation20] (TQ group). | ||||
| – | sodium arsenate (20 mg/kg body weight/day) then, after one hour they have received TQ (10 mg/kg body weight/day) (As.TQ). | ||||
At the end of the experiment, rats of all groups were sacrificed by fast decapitation; brains were removed, and dissected. Cerebelli were removed and fixed for histopathological studies. Cerebral cortex, cerebellum and brain stem were stored in −70 °C until the performance of the physiological measurements.
2.4 Histopathological study
Cerebelli were fixed in 10% formalin, dehydrated, cleared in xylene, embedded in paraffin wax, then sectioned, hydrated, stained with hematoxylin and eosin, and mounted in DPX [Citation21].
2.5 Physiological measurments
The tested monoamines were estimated by high performance liquid chromatography (HPLC) according to Pagel et al. [Citation22].
2.5.1 AChE assay
Thiocholine, produced by the action of acetylcholinesterase (E.C. No. 3.1.1.7), forms a yellow color with 5,5′-dithiobis (2-nitrobenzoic acid). The intensity of the produced color, measured at 412 nm, proportionate to the enzyme activity in the sample [Citation23].
2.5.2 Na+/K+ ATPase assay
The enzyme activity was determined by measuring the amount of inorganic phosphate (Pi) liberated from ATP during the incubation of cerebrum, cerebellum and brain stem aliquots. Before, the slices were incubated with Meth (0.05, 0.1, 0.5 and 1 μM) at different times (5 or 15 min). Then, the reaction mixture containing 95 mM NaCl, 15 mM KCl, 1.0 mM ATP (disodium salt), 38 mM Tris–HCl buffer (pH 7.4) was added to aliquot of homogenized slices (50 μg of protein) in a final volume of 0.3 mL. After a 5-min pre-incubation at 37 °C in the presence of 0.1 mM ouabain to specifically inhibit Na+/K+-ATPase (E.C. No. 3.6.3.9), the reaction was initiated by addition of ATP and terminated after 15 min of incubation by addition of 1 mL of color reagent (Ammonium Molybdate 2%, Triton X 5% solubilized in H2SO4 1.8 M). The released inorganic phosphate was measured spectrophotometrically at λ = 405 nm. Na+/K+-ATPase activity was calculated from the difference between amounts of inorganic phosphate found after incubation in the absence and presence of 1.5 M ouabain [Citation24].
2.5.3 Measurment of lipid peroxidation
Lipid peroxidation in brain homogenate were determined according to the method of Ohkawa et al. [Citation25] using 1 mL of trichloroacetic acid 10% and 1 mL of thiobarbituric acid 0.67%, followed by heating in a boiling water bath for 30 min. Thiobarbituric acid reactive substances were determined by the absorbance at 535 nm and expressed as malondialdehyde (MDA) equivalents formed.
2.5.4 Measurement of Nitrite/Nitrate level
The assay of nitrite/nitrate (NO) in brain homogenate was done according to the method of Berkels et al. [Citation26]. In acid medium and in the presence of nitrite the formed nitrous acid diazotises sulphanilamide, which is coupled with N-(1-naphthyl) ethylenediamine. The resulting azo dye has a bright reddish-purple color, which was measured at 540 nm.
2.5.5 Estimation of glutathione
Glutathione (GSH) of brain was determined by the methods of Ellman [Citation27]. The method based on the reduction of Ellman’s reagent (5,5′ dithiobis, 2-nitrobenzoic acid) with GSH to produce a yellow compound. The reduced chromogen directly proportional to GSH concentration and its absorbance were measured at 405 nm.
The enzymatic antioxidants, GPx (E.C. no. 1.1.1.9), GR (E.C. 1.8.1.7), CAT (E.C. no. 1.11.1.6), and SOD (E.C. no. 1.15.1.1) were determined according to the manufacturer instructions and purchased from Cayman chemical, Ann Arbor, Michigan, USA.
TNF-α (E.C. no. 1272/2008) was determined by quantitative ELISA kits purchased from R&D Systems Inc (Minneapolis, USA).
2.6 Statistical analysis
The recorded data were presented as mean ± standard error. One way ANOVA was carried out, and the statistical comparisons among the groups were performed with Duncan’s test using a statistical package program (SPSS version 17.0). P ≤ 0.05 was considered as significant for all statistical analysis.
3 Results
3.1 Physiological observations
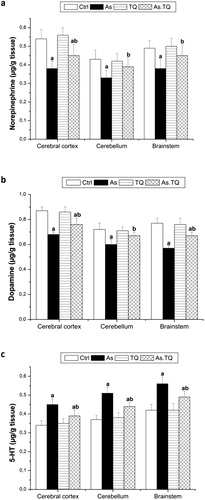
The data recorded in a showed the effect of As and the post-treatment with TQ on the content of NE in the selected brain areas. The level of NE was decreased significantly (P < 0.05) in cerebral cortex, cerebellum and brain stem in As-treated group when compared with control values. After the treatment with TQ, a significant increase in the level of NE in all the tested brain homogenate was observed as compared with As-treated group. Similarly, the concentration of DA declined significantly (P < 0.05) in As-treated group as compared with the control group in all studied brain areas. The greatest decrease was found in brain stem. The treated group with As and TQ showed that the levels of DA restored near to the normal values (b). In addition, the oral administration of As significantly (P < 0.05) elevated 5-HT levels in cerebral cortex, cerebellum and brain stem compared with control group. Meanwhile, the post-tretment with TQ was found to decrease significantly (P < 0.05) the increament in 5-HT content (c).
Fig. 1 Levels of the neurotransmitters, norepinephrine (a), dopamine (b), and serotonin (c) in cerebral cortex, cerebellum, and brain stem after the treatment with As and TQ. a: Significance at (P < 0.05) as compared to control group, b significance at (P < 0.05) as compared to arsenic group.
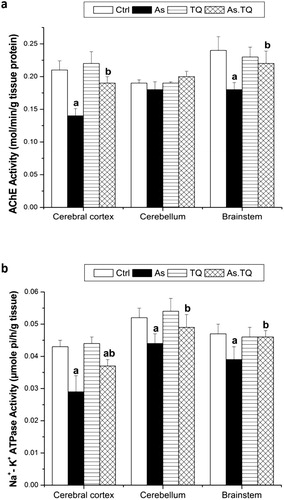
The treated rats with As showed a significant depletion (P < 0.05) in the activity of AChE in cerebral cortex and brain stem when compared with those treated with distilled water as a control. This value was elevated significantly (P < 0.05) after the treatment with TQ (a). Moreover, daily treatment with As for 21 days produced a significant (P < 0.05) depletion in the activity of Na+-K+-ATPase in all examined brain homogenates compared with the control group. Post treatment with TQ increased the activity of Na+-K+-ATPase significantly (P < 0.05) compared with As group (b).
Fig. 2 Acetyl choline esterase activity (a) and Na+-K+ ATPase activity (b) in cerebral cortex, cerebellum, and brain stem after the treatment with As and TQ. a: Significance at (P < 0.05) as compared to control group, b significance at (P < 0.05) as compared to arsenic group.
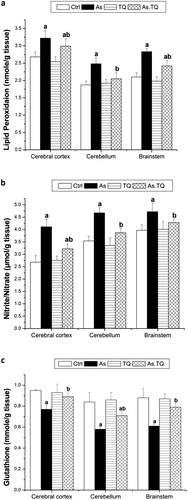
Levels of MDA was elevated significantly (P < 0.05) in As group compared with the control values in all investigated brain areas (a). However, the co-administered group with TQ showed a significant reduction in the level of MDA in the studied brain areas as compared with As group. By the same manner, the As-treated rats showed a significant (P < 0.05) increment in the levels of NO in cerebral cortex, cerebellum and brain stem as compared to the control group, meanwhile, the orally treated group with As and TQ induced a significant decrement in the levels of NO in the experimented brain areas when compared with As group (b). In contrast, levels of GSH were decreased significantly (P < 0.05) in As-group when compared to the control group. The post-treatment with TQ elevated the values of GSH significantly as compared to As treated rats (c).
Fig. 3 Lipid peroxidation level (a), nitrite/nitrate level (b), and Glutathione levels (C) in cerebral cortex, cerebellum, and brain stem after the treatment with As and TQ. a: Significance at (P < 0.05) as compared to control group, b significance at (P < 0.05) as compared to arsenic group.
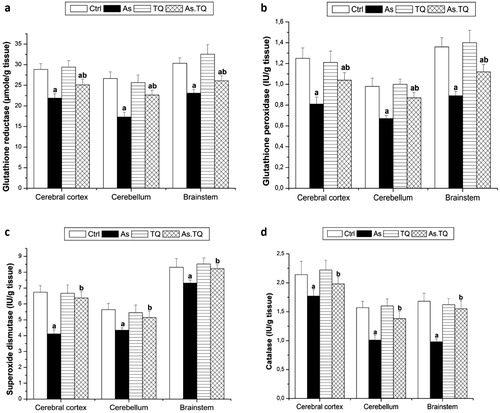
The present study also estimated the enzymatic antioxidant system in the cerebral cortex, cerebellum and brain stem. The data presented in showed that, the exposure to As caused a significant decline (P < 0.05) in the concentration of CAT, SOD, GPx, and GR as compared with the control group, however, the treatment with TQ elevated these values significantly (P < 0.05) in the studied brain areas as compared to As-treated rats.
Fig. 4 Glutathione reductase (a), glutathione peroxidase (b), superoxide dismutase (c), and catalase (d) levels in cerebral cortex, cerebellum, and brain stem after the treatment with As and TQ. a: Significance at (P < 0.05) as compared to control group, b significance at (P < 0.05) as compared to arsenic group.
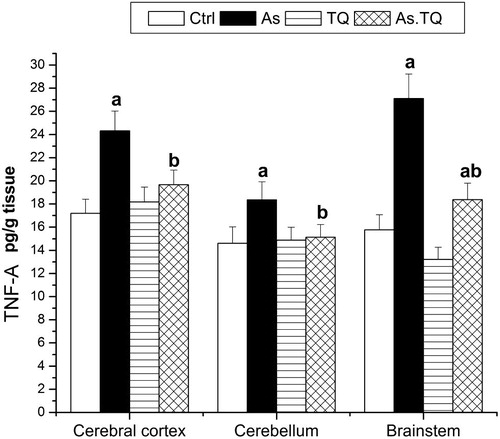
The influence of As and TQ on cytokines was also evaluated. It is clear from that the administration of As for 21 days increased the production of TNF-α significantly in all the studied brain areas when compared with the control values, the maximum elevation was marked in brain stem and cerebral cortex, however, the combined treatment with As and TQ showed a significant decrease (P < 0.05) in the production of TNF-α as compared to As-treated group.
3.2 Histological observations of cerebellum
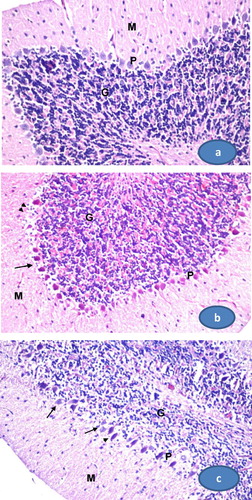
Histological sections of the cerebellum of control rats showed normal architecture with distinct cortical layers: outer molecular, inner granular cell layer, between which is the single layer of large neurons called Purkinje cells. The granular cell layer was very populated with cells (a). Rats treated with arsenate have cerebelli contained shrinked and degenerated Purkinje cells with condensed cytoplasm. Some of them lose their axons. The number of Purkinje cells, granular cells, and molecular cells were decreased (b). The cerebellum of rats post treated with TQ (As+ TQ group) showed both normal and degenerated Purkinje cells (c). The number of molecular cells and granular cells were slightly increased.
Fig. 6 Photomicrographs of rat cerebellum. (a) A section from control group showing the normal histological structure of cerebellum which consists of the outer molecular layer (M), middle Purkinje cell layer (P), and inner granular layer (G). (b) A section from arsenate treated group. The Purkinje cells are degenerated; some cells lose axons and shrinked (arrows). Their numbers are decreased and some areas are depleted from Purkinje cells (arrow heads). (c) A section from cerebellum treated with TQ after arsenate showing normal Purkinje cells (arrows) and other shrinked cells (arrow heads). (400×, H&E stain).
4 Discussion
Arsenic affects many transporter systems including the monoamines, DA, 5-HT and NE. It also induces overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS) in the body resulting in nucleic acid damage of the nerve cells [Citation28]. In the present study, treatment of female rats with 20 mg/kg arsenate for 21 days induced a decline in DA and NE and an elevation in 5-HT in cerebral cortex, cerebellum, and brain stem. This result agrees in part with Mejia et al. [Citation29] who studied the effect of arsenic on discrete brain regions of rats. The study revealed a decrease in norepinephrine levels and an increase in levels of dopamine, serotonin, and their metabolites. In another study, exposure to moderate levels of arsenic (1, 2, and 4 mg/L) for 60 days reduced the levels of NE, DA, and 5-HT in both the cerebrum and cerebellum of 7-weeks old mice. Similarly, mRNA levels of monoamine synthetases (including dopamine β-hydroxylase, tyrosine hydroxylase, and tryptophan hydroxylase) were reduced after arsenic exposure [Citation30]. High levels of arsenic cause insufficiency of dopaminergic and serotonergic signaling in the corpus striatum, hippocampus, and frontal cortex [Citation19,Citation31] . It could be concluded from those previous studies and the present result that the levels of monoamines after arsenic exposure increases or decreases in a dose dependant manner, age, and the exposed brain area.
Oxidative stress may play a role in the metalloid’s neurotoxicity [Citation32]. It is well known that the CNS is armed with an endogenous antioxidant defence mechanism consisting of antioxidant enzymes that produced upon exposure to ROS via a mechanism regulated at the transcriptional level [Citation33,Citation34] . Reduced glutathione is the major thiol present in the brain tissue, which has an essential role in the protection against oxidative injury due to ROS [Citation35]. Reduction of glutathione and glutathione peroxidase reduces the capacity of an organism to defend itself from the damage caused by ROS [Citation36]. Rodriguez et al. [Citation37] reported an alteration in mRNA of several antioxidant genes including superoxide dismutase (SOD) upon arsenic exposure which depend on the dose and the region of the brain (nucleus accumbens, prefrontal cortex, or striatum).
The present study revealed a decrease in GSH, and the antioxidant enzyme system GPx, GR, SOD, and CAT in all brain areas studied of the female rat after arsenate treatment as well as an increase in lipid peroxidation levels. GSH is converted into its oxidized form (GSSG) in the reaction catalyzed by GPx, then it can be reduced back to GSH by GR [Citation8]. Consequently, the decrease of GSH may be due to deficiency of the antioxidant enzyme system GPx and GR that may result from alteration in their genes. In agreement with this study, Chaudhuri et al. [Citation38] found an increase in the level of lipid peroxidation and a decrease in GSH level, superoxide dismutase and glutathione reductase activities in the brain of rat after permissible dose (50 mg/L, the national standard in Bangladesh) of arsenic. Similarly, sodium arsenite in drinking water led to the generation of ROS and subsequent lipid peroxidation in the brains of developing rat pups. In addition, the pups’ levels of the antioxidant GSH as well as the activity of the antioxidant enzyme GPx were decreased after arsenic exposure [Citation36]. This decrease in the antioxidant system indicates a free radical-mediated cellular degeneration.
Thymoqinone (TQ), the active component of Nigella sativa (NS) seeds, has broad and versatile pharmacological effects that include strong antioxidant activity against free radical-generating agents [Citation39]. Treatment of rats with TQ after exposure to arsenate in the present study, decreased the elevated levels of 5-HT, MAD and increased the lowered levels of NE, DA, and GSH. The enzymatic antioxidant system, GPx, GR, SOD, and CAT were also increased in the cerebral cortex, cerebellum, and brain stem. Similarly, Safhi [Citation40] found that oral administration of TQ after treatment of chlorpromazine reduced the levels of lipid peroxidation, increased levels of antioxidant enzymes i.e., reduced glutathione, GPx, GR, CAT, and glutathione-S-transferase in the brain of rat.
Thymoquinone has been proved experimentally to be an anti-inflammatory substance [Citation41]. In this study, it reduced the elevated levels of NO and TNF-α in the cerebral cortex, cerebellum and brain stem of female rats after arsenate treatment. El-Mahmoudy et al. [Citation42] investigated the effect of TQ on NO production by macrophages after lipopolysaccharide stimulation. They found that TQ suppressed NO production by macrophage. It mediates its inhibitory effect on NO production via reduction of iNOS mRNA and protein expression which might be important in ameliorating the inflammatory and autoimmune conditions. Likewise, TQ decreased IL-6, TNF-α, MDA and NO metabolites and increased thiol content, SOD and CAT in the brain of rats treated with lipopolysaccharides [Citation43]. Moreover, Umar et al. [Citation44] found a significant reduction in the levels of pro-inflammatory mediators {IL-1b, IL-6, TNF-α, IFN-c and PGE (2)} and an increase in the level of IL-10 in arthritic rats after TQ treatment.
Locomotion is affected by arsenic exposure in rodent models. Early studies demonstrated impaired motor coordination and delayed spontaneous alteration in rats administered with arsenic (36 mg/L) for four months [Citation8]. Low levels of arsenic seem to induce hyperactivity in male mice, while high levels induce hypo-activity [Citation37,Citation45] . In the present study, female rats were hypoactive after arsenate treatment.
Altered motor coordination and locomotion could arise from abnormality in cholinergic functioning. In this study, AChE activity declined in cerebral cortex, cerebellum, and brain stem of female rats treated with arsenate, which may be the reason of the hypo- activity observed in rats. The study of Yadav et al. [Citation46] performed on female rats exposed to 20 mg/kg arsenic showed a reduction in AChE activity and ChAT labeling in the hippocampus and frontal cortex. Exposure to less arsenic (5 mg/kg body weight) also inhibited AChE activity in the brain and was associated with poorer performance in operant learning [Citation47]. Another study demonstrated that AChE activity decreased with increasing arsenic concentrations in male rats after five days of exposure [Citation48]. Administration of TQ after arsenate exposure in the current study, increased the AChE activity in all the brain areas studied, indicating the ameliorative effect of TQ on locomotion and motor coordination. Likewise, TQ improved the muscle coordination and spontaneous locomotor activity of rats pretreated with chlorpromazine [Citation40].
Neurons are also susceptible to arsenic toxicity. In the present study arsenate caused a decrease in neuronal cell number of cerebellum and shrinkage of Purkinje cells with a loss of their axons. It is well known that Purkinje cell regulates and coordinates motor movements. These results are in agreement with other studies of rats [Citation49,Citation50] and mice [Citation51]. Sodium arsenate reduced cerebellar neuron viability and induced DNA degradation and nuclear fragmentation in cultures of rat cerebellar neurons [Citation50]. In cultured mouse neuronal cells, sodium arsenate led to neuronal apoptosis, necrosis, and inhibited neurite growth in a dose-dependent manner [Citation51].
Arsenite like any other metal toxins, such as lead, cadmium and mercury, can affect mitochondrial oxidative enzymes. It is possible that this toxin interferes with energy coupling process by altering the redox states of cytochrome C enzyme. The resultant ROS formed will in turn induce peroxidation of membranes and loss of its ion channels. Other studies also show that the sodium and potassium channels are either depressed or down regulated in this toxicity process [Citation52]. In this study arsenate decreased the activity of Na+-K+ ATPase which indicated changes in electro-activity of the brain of rat.
TQ protects slightly cerebellar neurons from degeneration and increase the activity of Na+-K+ ATPase after arsenate treatment in the present study. In the same manner Ullah et al. [Citation53] revealed an ameliorative effect of tymoquinone from the apoptosis triggered by ethanol in rat during early development. The mechanism involved the down regulation of caspase-3, cytochrome-c, cleaved caspase-9 and upregulation of Bcl-2. Bcl-2 protein family plays an important role in apoptotic signal transduction by regulating mitochondrial function [Citation54]. This finding implied that TQ potentially prevents apoptosis by regulating the mitochondrial path way [Citation53].
5 Conclusion
In general, previously published reports showed that TQ mainly functions through its antioxidant mechanism, and it has been used as a protective agent in multiple toxicity models. As well, this study showed that TQ attenuated the neurotoxic effect and the oxidative stress resulting from the exposure to arsenic through its powerful antioxidant effect.
Acknowledgment
Research was performed in the laboratories of Zoology and Entomology Department, Faculty of science, Helwan University, Egypt.
References
- H.HosseinzadehS.ParvardehAnticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in micePhytomedicine1120045664
- H.HosseinzadehM.EskandariT.ZiaeeAntitussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigsPharmacol Online22008480484
- S.AttoubO.SperandioH.RazaK.ArafatS.Al-SalamM.A.Al Sultanet al.Thymoquinone as an anticancer agent: evidence from inhibition of cancer cells viability and invasion in vitro and tumor growth in vivoFundam Clinl Pharmacol272013557569
- C.C.WooA.P.KumarG.SethiK.H.B.TanThymoquinone: potential cure for inflammatory disorders and cancerBiochem Pharmacol832012443451
- S.S.EbrahimiS.OryanE.IzadpanahK.HassanzadehThymoquinone exerts neuroprotective effect in animal model of Parkinson's diseaseToxicol Lett2762017108114
- Y.Y.ShaoB.LiY.M.HuangQ.LuoY.M.XieY.H.ChenThymoquinone attenuates brain injury via an anti-oxidative pathway in a status epilepticus rat modelTransl Neurosci82017914
- I.ElmaciM.A.AltinozThymoquinone: an edible redox-active quinone for the pharmacotherapy of neurodegenerative conditions and glial brain tumorsBiomed Pharmacother832016635640
- A.MabroukCheikh H.BenThymoquinone ameliorates lead-induced suppression of the antioxidant system in rat kidneysLibyan J Med11201631018
- M.ErbogaM.KanterC.AktasU.SenerZ.Fidanol ErbogaY.Bozdemir Donmezet al.Thymoquinone ameliorates cadmium-induced nephrotoxicity, apoptosis, and oxidative stress in rats is based on its anti-apoptotic and anti-oxidant propertiesBiol Trace Elem Res1702016165172
- V.V.TroshinPathogenesis and classification of chronic encephalopathy due neurotoxic chemicalsMed Tr Prom Ekol720092126
- K.AsadaK.ToyotaT.NishimuraJ.IkedaK.HoriAccumulation and mobility of zinc in soil amended with different levels of pig-manure compostJ Environ Sci Health B452010285292
- E.A.BelyaevaT.V.SokolovaL.V.EmelyanovaI.O.ZakharovaMitochondrial electron transport chain in heavy metal-induced neurotoxicity: effects of cadmium, mercury, and copperSci World J2012136063
- H.R.PohlN.RoneyH.G.AbadinMetal ions affecting the neurological systemMet Ions Life Sci82011247262
- P.B.TchounwouB.WilsonA.IshagueImportant considerations in the development of public health advisories for arsenic and arsenic containing compounds in drinking waterRev Environ Health141999211229
- S.KapajH.PetersonK.LiberP.BhattacharyaHuman health effects from chronic arsenic poisoning—a reviewJ Environ Sci Health41200623992428
- M.F.Hugheset al.Arsenic exposure and toxicology: a historical perspectiveToxicol Sci1232011305332
- V.M.RodriguezL.CarrizalesM.S.MendozaO.R.FajardoM.GiordanoEffects of sodium arsenite exposure on development and behavior in the ratNeurotoxicol Teratol242002743750
- C.R.TylerA.M.AllanThe effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: a reviewCurr Environ Health Report12014132147
- R.S.Yadavet al.Attenuation of arsenic neurotoxicity by curcumin in ratsToxicol Appl Pharmacol2402009367376
- N.GilhotraD.DhingraThymoquinone produced antianxiety-like effects in mice through modulation of GABA and NO levelsPharmacological Rep632011660669
- F.DelafieldHaematoxylin and Eosin for General Staining. Staining of the Animal Tissues Practical and Theoretical1984Oxford University PressLondon
- P.PagelJ.BlomeH.U.WolfHigh-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s diseaseJ Chromatogr B7462000297304
- V.GorunI.ProinovV.BaltescuG.BalabanO.BarzuModified Ellman procedure for assay of cholinesterase in crude enzymatic preparationAnal Biochem861978324326
- L.A.MuszbekHighly sensitive method for the measurement of the ATP-ase activityAnal Biochem771997286288
- H.OhkawaN.OhishiK.YagiAssay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal Biochem951979351358
- R.BerkelsS.Purol-SchnabelR.RoesenMeasurement of nitric oxide by reconversion of nitrate/nitrite to noMethods Mol Biol279200418
- G.L.EllmanTissue sulfhydryl groupsArch Biochem Biophys8219597077
- D.MishraS.J.FloraDifferential oxidative stress and DNA damage in rat brain regions and blood following chronic arsenic exposureToxicol Ind Health242008247256
- J.J.MejiaF.Diaz-BarrigaJ.CalderonC.RiosM.E.Jimenez-CapdevilleEffects of lead-arsenic combined exposure on central monoaminergic systemsNeurotoxicol Teratol191997489497
- X.LiuF.PiaoY.LiProtective effect of taurine on the decreased biogenic amine neurotransmitter levels in the brain of mice exposed to arsenicAdv Exp Med Biol7762013277287
- R.S.Yadavet al.Neuroprotective effect of curcumin in arsenic-induced neurotoxicity in ratsNeurotoxicol312010533539
- A.RaiS.MauryaP.KhareA.SrivastavaS.BandyopadhyayCharacterization of developmental neurotoxicity of As, Cd, and Pb mixture: synergistic action of metal mixture in glial and neuronal functionsToxicol Sci1182010586601
- H.MotohashiM.YamamotoNrf2-Keap1 defines a physiologically important stress response mechanismTrends Mol Med102004549557
- K.ItohK.I.TongM.YamamotoMolecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophilesFree Radic Biol Med36200412081213
- W.WangN.BallatoriEndogenous glutathione conjugates: occurrence and biological functionsPharmacol Rev501998335356
- S.XiL.GuoW.SunY.JinG.SunPrenatal and early life arsenic exposure induced oxidative damage and altered activities and mRNA expressions of neurotransmitter metabolic enzymes in offspring rat brainJ Biochem Mol Toxicol242010368378
- V.M.Rodriguezet al.Chronic exposure to low levels of inorganic arsenic causes alterations in locomotor activity and in the expression of dopaminergic and antioxidant systems in the albino ratNeurotoxicol Teratol322010640647
- A.N.ChaudhuriS.BasuS.ChattopadhyayGupta S.DasEffect of high arsenic content in drinking water on rat brainIndian J Biochem Biophys3619995154
- P.J.HoughtonR.ZarkaB.de las HerasJ.R.HoultFixed oil of nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidationPlanta Med61119953336
- M.M.SafhiNeuromodulatory effects of thymoquinone in extenuating oxidative stress in chlorpromazine treated ratsActa Pol Pharm732016529535
- N.ChehlG.ChipitsynaQ.GongC.J.YeoH.A.ArafatAnti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cellsHPB (Oxford)112009373381
- A.El-MahmoudyH.MatsuyamaM.A.BorganY.ShimizuM.G.El-SayedN.Minamotoet al.Thymoquinone suppresses expression of inducible nitric oxide synthase in rat macrophagesInt Immunopharmacol2200216031611
- R.BargiF.AsgharzadehF.BeheshtiM.HosseiniH.R.SadeghniaM.KhazaeiThe effects of thymoquinone on hippocampal cytokine level, brain oxidative stress status and memory deficits induced by lipopolysaccharide in ratsCytokine962017173184
- S.UmarJ.ZarganK.UmarS.AhmadC.K.KatiyarH.A.KhanModulation of the oxidative stress and inflammatory cytokine response by thymoquinone in the collagen induced arthritis in Wistar ratsChem Biol Interact19720124046
- U.Bardullaset al.Chronic low-level arsenic exposure causes gender-specific alterations in locomotor activity, dopaminergic systems, and thioredoxin expression in miceToxicol Appl Pharmacol2392009169177
- R.S.Yadavet al.Neuroprotective efficacy of curcumin in arsenic induced cholinergic dysfunctions in ratsNeurotoxicol322011760768
- T.N.NagarajaT.DesirajuEffects on operant learning and brain acetylcholine esterase activity in rats following chronic inorganic arsenic intakeHum Exp Toxicol131994353356
- A.K.PatlollaP.B.TchounwouSerum acetyl cholinesterase as a biomarker of arsenic induced neurotoxicity in sprague-dawley ratsInt J Environ Res Public Health2200580e3
- J.H.LuoZ.Q.QiuW.Q.ShuY.Y.ZhangL.ZhangJ.A.ChenEffects of arsenic exposure from drinking water on spatial memory, ultra structures and NMDAR gene expression of hippocampus in ratsToxicol Lett1842009121125
- U.NamgungZ.XiaArsenic induced apoptosis in rat cerebellar neurons via activation of JNK3 and p38 MAP kinasesToxicol Appl Pharmacol1742001130e8
- K.H.AungR.KuriharaS.Nakashimaet al.Inhibition of neurite outgrowth and alteration of cytoskeletal gene expression by sodium arseniteNeurotoxicol342012226e35
- D.ShayaM.KreirR.A.RobbinsS.WongJ.HammonA.Brüggemannet al.Voltage-gated sodium channel (NaV) protein dissection creates a set of functional pore-only proteinsProc Natl Acad Sci USA10820111231312318
- I.UllahN.UllahM.I.NaseerH.Y.LeeM.O.KimNeuroprotection with metformin and TQ against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neuronsBMC Neuroscience13201211
- Y.ShyA structural view of mitochondria-mediated apoptosisNat Struct Biol82001394401