Abstract
The new analogs of benzimidazole fused heterocyclic compounds such as triazinane and oxadiazinanes were synthesised by classical amino methylation with different aryl-N,N′ unsymmetrical thioureas. The antibacterial activity of triazinane and oxadiazinane compounds have been assessed with zone of inhibition by well diffusion method using a panel of selected gram positive and gram negative bacterial strains and which have showed good activity. The synthesised molecules were subjected to molecular docking studies with two proteins, namely topoisomerase II (PDB ID: 1JIJ) and DNA gyrase subunit b (PDB ID: 1KZN). The molecular docking studies are supporting the antibacterial activity exhibiting high inhibition constant and binding energy.
1 Introduction
The organic compounds specifically with N-heterocyclic ring systems exhibit a wide range of biological activities through effective binding to enzyme receptor site. As per the present global scenario, thousands of new heterocyclic compounds either isolated from natural sources or synthesized in the laboratories are added to the literature every year. Many of these compounds have drawn the attention of researchers based on their biological, therapeutic and industrial potential.
Benzimidazoles are found to be useful intermediates for the development of new molecules of biological or pharmaceutical interest. Substituted benzimidazole derivatives have been found to possess Biological activities such as antitumor [Citation1], antimicrobial [Citation2], anthelmintic [Citation3], antibacterial [Citation4], analgesic [Citation5], anti inflammatory [Citation6] etc. In recent times, new techniques have been adopted for the efficient synthesis of novel heterocycles by using heterogeneous, nano-catalysts and photocatalysis that are highly and ecofriendly [Citation7Citation[8]Citation[9]Citation[10]Citation[11]Citation[12]–Citation13] . Triazinane derivatives belonging to nitrogen heterocycles have greatest importance due to their potential applications. These have been identified as commercial products which are used as H2S scavengers in the areas with relatively low concentrations of H2S and it is inexpensive to use. The products of the scavenging reactions are believed to be biodegradable and water soluble [Citation14]. Little work has been published in the area of 1,3,5-triazinane-4-thiones and 1,3,5-triazinane-2-ones showing antimicrobial activity [Citation15]. These are also promising intermediates in the synthesis of tyrosine derivatives, which are well-known compounds used as Biocidal [Citation16] and enantio-differentiating coupling reagents [Citation17]. Heterocyclic structural unit has a significant place among pharmaceutically important synthetic and natural materials [Citation18], showing powerful antiproliferative action [Citation19]. For these reasons, 1,3,5-triazinane derivatives incorporating thiourea unit may be important in many fields [Citation20].
2 Experimental section
2.1 Materials and methods
Melting points of the synthesized compounds were determined in open capillary tubes and were uncorrected. Reaction Progress was observed by TLC plates, Bruker 300 MHz instrument was used to record 1H NMR spectra in DMSO/CDCl3 using TMS as internal standard. Chemical shifts (δ) are expressed in ppm. Perkin Elmer BX series FT-IR was to record IR spectra, Elemental analysis were performed on a PerkinElmer 240 CHN analyzer ().
Scheme 1 Synthesis of 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-methyl-3-phenyl-1,3,5-triazinane-2-thiones/oxadiazinane-2-thiones.
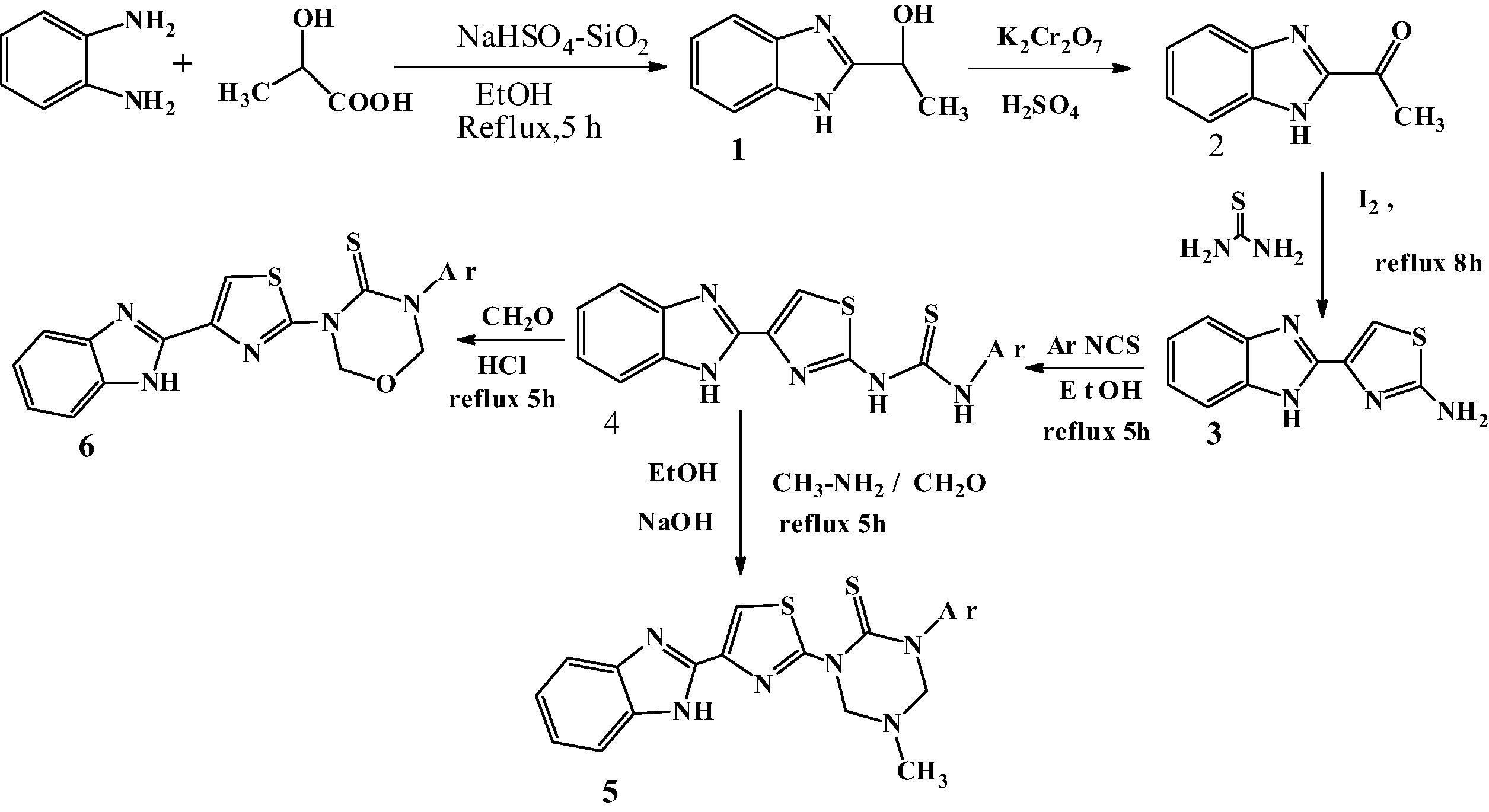
2.1.1 Synthesis of 4-(1H-Benz[d]imidazol-2yl)-1,3-thiazol-2-amine (3)
A solution of 2-acetyl benzimidazole (0.01 mmol) in isopropanol (20 ml) was added to the mixture of thio urea (0.01 mmol) and iodine (0.12 mmol) taken in isopropanol (20 ml). The reaction mixture was heated under reflux for 3 h. After completion of the reaction, (monitored by TLC) the solvent was removed in vacuo. The solid separated was washed with aq. Sodium bicarbonate solution, dried and recrystallized from ethanol to give the product (3) in a pure state (Figs. 1 and 2).
2.1.2 Synthesis of 4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-3-phenyl thiourea (4)
A mixture of compound (3) (0.01 mmol) and sodium hydride (0.5 g, 20 mmol) in Ethanol (80 ml) was heated under reflux for 30 min and cooled. Phenylisothiocynate (0.01 mmol) was added and refluxing continued for a further 4 h. The solvent was evaporated off and the residue dissolved in DCM (50 ml) was washed with dilute HCl. The organic phase was dried (MgSO4) and the solvent was evaporated off to give the desired compound (4). The progress of the reaction was monitored by TLC and recrystallized from ethanol. Compounds 4(b–e) were prepared by similar procedure with minor changes in reaction conditions. The structures of the compounds (4) have been confirmed on the basis of analytical and spectral IR, 1H NMR and Mass data.
2.1.2.1 4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-3-phenyl thiourea (4a)
Yield 72%, m.p 162–164 °C. IR (KBr) λmax in (cm−1) 3368 (NH), 1590 (C=N), 1231 (C=S); 1H NMR (DMSO-d6, 300 MHz, δ ppm) 8.28–8.40 (m, 4H, Ar H), 7.49–7.68 (m, 2H, Ar H), 6.87–7.13 (m, 3H, Ar H), 11.28 (s, 1H, NH), 6.52 (S, 1H, CH Ar); MS, m/z (%), 352 (M+) Anal. Calcd. For C17H13N5S2: C, 58.10; H, 3.73; N, 19.93%; Found: C, 57.78; H, 3.58; N, 19.85%.
2.1.2.2 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-3-P-tolyl thiourea (4b)
Yield 70%, m.p 165–167 °C. IR (KBr) λmax in (cm−1) 3371 (NH), 1586 (C=N), 1248 (C=S), 1H NMR (DMSO-d6, 300 MHz, δ ppm), 8.25–8.38 (m, 4H, Ar H), 7.46–7.65 (m, 2H, Ar H), 6.85–7.11 (m, 2H, Ar H), 10.45 (s, 1H, NH), 6.60 (s, 1H, CH, Ar), 2.27 (s, 3H, CH3); MS, m/z (%), 365 (M+) Anal. Calcd. For C18H15N5S2: C, 59.15; H, 4.14; N, 19.16%; Found: C, 58.78; H, 4.02; N, 18.78%.
2.1.2.3 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-3-(4-methoxy phenyl)thiourea (4c)
Yield 68%, m.p 166–168 °C. IR (KBr) λmax in (cm−1) 3365 (NH), 1565 (C=N), 1252 (C=S), 1085 (OCH3), 1H NMR (DMSO-d6, 300 MHz, δ ppm), 10.48 (s, 1H, NH), 6.66 (S, 1H, CH Ar), 3.52 (s, 3H, CH3), 8.22–8.35 (m, 4H, Ar H), 7.43–7.60 (m, 2H, Ar H), 6.85–7.18 (m, 2H, Ar H), MS, m/z (%), 381 (M+); Anal. Calcd. For C18H15N5OS2: C, 56.67; H, 3.96; N, 18.36 (%); Found: C, 56.12; H, 3.45; N, 18.22 (%).
2.1.2.4 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-3-(4-nitrophenyl)thiourea (4d)
Yield 64% m.p 164–166 °C. IR (KBr) λmax in (cm−1) 3373 (NH), 1565 (C=N), 1520 (NO2); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 8.26–8.36 (m, 4H, Ar H), 7.45–7.60 (m, 2H, Ar H), 6.857.23 (m, 2H, Ar H), 10.92 (s, 1H, NH), 6.62 (s, 1H, CH Ar), MS, m/z (%), 396 (M+); Anal. Calcd. For C17H12N6O2S2: C, 51.50; H, 3.06; N, 21.20%; Found: C, 50.88; H, 2.82; N, 20.75%.
2.1.2.5 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-3-(4-chloro phenyl)thiourea (4e)
Yield 65% m.p 168–169 °C. IR (KBr) λmax in (cm−1) 3366 (NH), 1562 (C=N), 780 (C–Cl); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 8.22–8.33 (m, 4H, Ar H), 7.42–7.63 (m, 2H, Ar H), 6.80–7.25 (m, 2H, Ar H), 11.17 (s, 1H, NH), 6.62 (s, 1H, CH Ar); MS, m/z (%), 387 (M+); Anal. Calcd. For C17H12ClN5S2: C, 52.91; H, 3.13; N, 18.15%; Found: C, 52.21; H, 2.78; N, 17.84%.
2.1.3 Synthesis of 4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl-5-methyl-3-phenyl-1,3,5-triazinane-2-thione (5)
A mixture of compound (4) in (1 mmol), 30% formaldehyde (2 mmol) methyl amine (1 mmol) and (0.01 mol) NaOH was taken in ethanol (30 ml) and refluxed for about 5–6 h. The progress of the reaction was monitored by TLC. After completion of the reaction, it was cooled and the product was filtered. The crude product was passed through silica gel by column and the product was eluted from 60% ethyl acetate and hexane. These compounds were purified by crystallization from suitable solvents. The structures of the compounds (5) have been confirmed on the basis of analytical and spectral IR, 1H NMR and Mass data. Compounds 5(b–e) were prepared by similar procedure with minor changes in reaction conditions.
2.1.3.1 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-methyl-3-phenyl-1,3,5-triazinane-2-thione (5a)
Yield 74% m.p 175–178 °C. IR (KBr) λmax in (cm−1) 3355 (NH), 1563 (C=N), 1263 (C=S); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 11.20 (s, 1H, NH), 6.38 (s, 1H, CH Ar), 4.64 (s, 4H, CH2), 3.30 (s, 3H, CH3), 7.71–7.86 (m, 4H, Ar H), 7.14–7.37 (m, 5H, Ar H), 6.78–6.94 (m, 2H, Ar H), MS, m/z (%), 407 (M+); Anal. Calcd. For C20H18N6S2: C, 59.10; H, 4.46; N, 20.67%; Found: C, 58.78; H, 4.04; N, 19.98%.
2.1.3.2 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-methyl-3-p-tolyl-1,3,5-triazinane-2-thione (5b)
Yield 71% m.p 177–180 °C. IR (KBr) λmax in (cm−1) 3360 (NH), 3086 (C–H), 3011 (C–H), 1569 (C=N), 1265 (C=S); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 7.78–7.89 (m, 4H, Ar H), 7.16–7.36 (m, 5H, Ar H), 6.72–6.90 (m, 2H, Ar H), 11.42 (s, 1H, NH), 6.56 (s, 1H, CH Ar), 4.54 (s, 4H, CH2), 2.25 (s, 3H, CH3); MS, m/z (%), 420 (M+); Anal. Calcd. for C21H20N6S2: C, 59.97; H, 4.79; N, 19.98%; Found: C, 59.78; H, 4.64; N, 19.78%.
2.1.3.3 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)3-(4-methoxy phenyl)-5-methyl-1,3,5-triazinane-2-thione (5c)
Yield 68% m.p 178–180 °C. IR (KBr) λmax in (cm−1) 3363 (NH), 3080 (C–H), 1563 (C=N), 1275 (C=S); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 7.68–7.79 (m, 4H, Ar H), 7.12–7.26 (m, 5H, Ar H), 6.70–6.88 (m, 2H, Ar H), 11.48 (s, 1H, NH), 6.53 (s, 1H, CH Ar), 4.52 (s, 4H, CH2), 2.27 (s, 3H, CH3), 3.55 (s, 3H, CH3); MS, m/z (%), 437 (M+); Anal. Calcd. For C21H20N6OS2: C, 57.78; H, 4.62; N, 19.25 (%); Found: C, 56.98; H, 4.54; N, 18.78 (%).
2.1.3.4 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)5-methyl-3-(4-nitro phenyl)-1,3,5-triazinane-2-thione (5d)
Yield 66% m.p 175–177 °C. IR (KBr) λmax in (cm−1) 3358 (NH), 3076 (C–H), 1525 (–NO2) 1569 (C=N), 1275 (C=S), 695 (C–S). 1H NMR (DMSO-d6, 300 MHz, δ ppm), 7.65–7.76 (m, 4H, Ar H), 7.18–7.29 (m, 5H, Ar H), 6.72–6.89 (m, 2H, Ar H), 11.05 (S, 1H, NH), 6.64 (S, 1H, CH Ar), 4.51 (s, 4H, CH2), 2.25 (s, 3H, CH3); MS, m/z (%), 452 (M+); Anal. Calcd. For C20H17N702S2: C, 53.20; H, 3.79; N, 21.73 (%); Found: C, 52.78; H, 3.51; N, 20.85 (%).
2.1.3.5 1-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)3-(4-chloro phenyl)-5-methyl-1,3,5-triazinane-2-thione (5e)
Yield 65% m.p 179–181 °C. IR (KBr) λmax in (cm−1) 3371 (NH), 3084 (C–H), 1569 (C=N), 1270 (C=S), 783 (C–Cl) 690 (C–S). 1H NMR (DMSO-d6, 300 MHz, δ ppm), 7.68–7.79 (m, 4H, Ar H), 7.16–7.28 (m, 5H, Ar H), 6.70–6.85 (m, 2H, Ar H), 10.52 (s, 1H, NH), 6.62 (s, 1H, CH Ar); MS, m/z (%), 442 (M+), 443 (M++). Anal. Calcd. For C20H17ClN6S2: C, 54.47; H, 3.89; N, 19.06%; Found: C, 53.86; H, 3.78; N, 18.85%.
2.1.4 Synthesis of 3-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-substituted phenyl-1,3,5-oxadiazinane-4-thione (6)
A mixture of compound (4) in (1 mmol), 30% formaldehyde (2 mmol) was taken in ethanol (30 ml) and added 1 ml concentrated HCl and refluxed for about 5–6 h at 100–110 °C. The progress of the reaction was monitored by TLC. After completion of the reaction, it was cooled and neutralized with 10% NaOH. The product was passed through silica gel by column and it was eluted from 60% ethyl acetate and hexane. These compounds were purified by recrystallisation from suitable solvents. The structures of these compounds have been confirmed on the basis of analysis and spectral (IR, 1H NMR and Mass) data. Compounds 6(b–e) were prepared by similar procedure with minor changes in reaction conditions.
2.1.4.1 3-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-phenyl-1,3,5-oxadiazinane-4-thione (6a)
Yield 70% m.p 175–177 °C. IR (KBr) λmax in (cm−1) 3368 (NH), 1569 (C=N), 1260 (C=S), 1H NMR (DMSO-d6, 300 MHz, δ ppm), 11.20 (s, 1H, NH), 7.14–7.32 (m, 4H, Ar H), 6.65–6.85 (m, 5H, Ar H), 5.52 (s, 4H, CH2); MS m/z (%), 394 (M+) Anal. Calcd. For C19H15N5OS2: C, 58.00; H, 3.84; N, 17.80%; Found: C, 57.86; H, 3.78; N, 17.25%.
2.1.4.2 3-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-p-tolyl-1,3,5-oxadiazinane-4-thione (6b)
Yield 65% m.p 175–178 °C. IR (KBr) λmax in (cm−1) 3362 (NH), 1569 (C=N), 1265 (C=S); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 11.25 (s, 1H, NH), 7.18–7.36 (m, 4H, Ar H), 6.69–6.88 (m, 5H, Ar H), 5.55 (s, 4H, CH2), 2.25 (s, 3H, CH3); MS, m/z (%), 407 (M+); Anal. Calcd. For C20H17N5OS2: C, 58.90; H, 4.20; N, 17.19 (%); Found: C, 57.86; H, 4.12; N, 17.02 (%).
2.1.4.3 3-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-(4methoxyphenyl)-1,3,5-oxadiazinane-4-thione (6c)
Yield 68% m.p 174–176 °C. IR (KBr) λmax in (cm−1) 3368 (NH), 1569 (C=N), 1260 (C=S); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 7.18–7.36 (m, 4H, Ar H), 6.69–6.88 (m, 5H, Ar H), 11.14 (s, 1H, NH), 5.53 (s, 4H, CH2), 3.77 (s, 3H, OCH3); MS, m/z (%), 423 (M+); Anal. Calcd. For C20H17N5OS2: C, 58.90; H, 4.20; N, 17.19 (%); Found: C, 57.86; H, 4.12; N, 17.02 (%).
2.1.4.4 3-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-(4-nitro phenyl)-1,3,5-oxadiazinane-4-thione (6d)
Yield 66% m.p 176–178 °C. IR (KBr) λmax in (cm−1) 3353 (NH), 1566 (C=N), 1523 (NO2), 1262 (C=S), 1H NMR (DMSO-d6, 300 MHz, δ ppm), 11.22 (s, 1H, NH), 7.20–7.36 (m, 4H, Ar H), 6.70–6.85 (m, 5H, Ar H), 5.53 (s, 4H, CH2); MS, m/z (%), 423 (M+); Anal. Calcd. For C19H14N6O3S2: C, 52.04; H, 3.20; N, 19.18 (%); Found: C, 51.86; H, 3.02; N, 18.82 (%).
2.1.4.5 3-(4-(1H-benzo[d]imidazol-2-yl)thiazol-2-yl)-5-(4-chlorophenyl)-1,3,5-oxadiazinane-4-thione (6e)
Yield 65% m.p 175–178 °C. IR (KBr) λmax in (cm−1) 3358 (NH), 1563 (C=N), 1260 (C=S), 780 (C–Cl); 1H NMR (DMSO-d6, 300 MHz, δ ppm), 11.26 (s, 1H, NH), 7.18–7.36 (m, 4H, Ar H), 6.72–6.86 (m, 5H, Ar H), 5.55 (s, 4H, CH2); MS, m/z (%), 423 (M+); Anal. Calcd. For C19H14N6ClOS2: C, 53.34; H, 3.30; N, 16.35 (%); Found: C, 52.85; H, 3.22; N, 16.25 (%).
3 Anti bacterial activity
The antibacterial activity of synthesized compounds was evaluated against six bacteria by using the well diffusion method. Gentamycin was employed as standard drug to compare the results. Strains of Bacillus subtilis MTCC 441, Bacillus cereus ATCC 9372, Staphylococcus aureus ATCC 96, E. coli ATCC 8739, Klebsiella pneumoniae MTCC 109, Salmonella typhi ATCC 4420 were taken from the department of microbiology, Kakatiya University Warangal. The bacterial cultures were developed by selective nutrient broth at 37 °C and stored at 4 °C for further use. Nutrient broth was used for the preparation of inoculums of the bacteria and nutrient agar was used for the screening method [Citation21] and their results were tabulated in .
Table 1 Antibacterial activity of compounds 5 and 6 (a–e).
3.1 Molecular docking
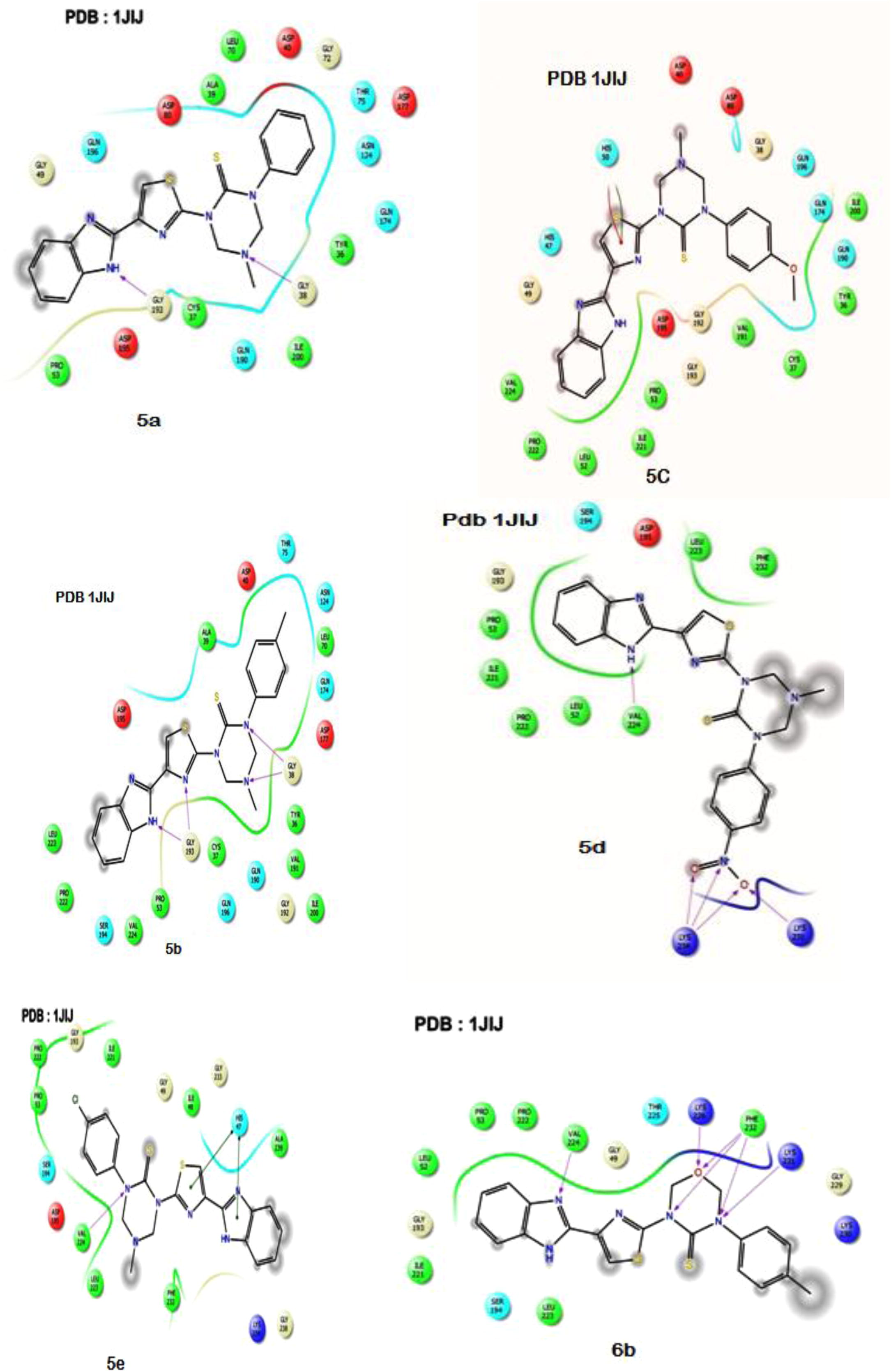
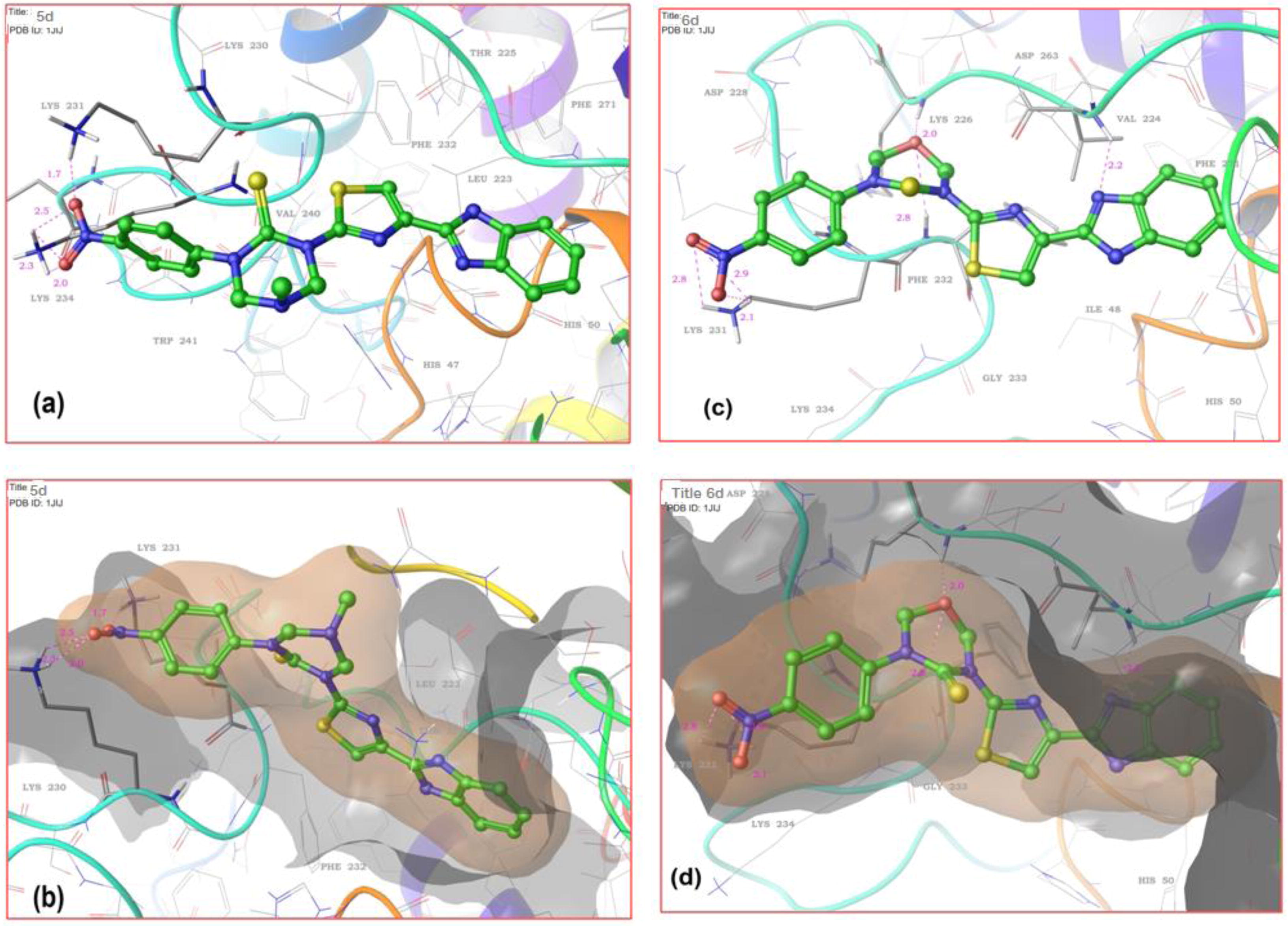
Auto Dock 4.2 (MGL tools-1.5.6) was used to perform all docking simulations. A set of new triazinane–benzimidazole derivatives were subjected to docking with topoisomerase II (PDB ID: 1JIJ) and DNA gyrase subunit b (PDB ID:1KZN). From the Protein Data Bank (RCSB) (http://www.rcsb.org/pdb). The DNA gyrase subunit b and crystal structure of target enzyme topoisomerase II was retrieved. To carry out in silico studies, the 2D structures of the synthesized ligands 5(a–e), 6(a–e) were drawn and converted to energy minimized 3D structures in the pdb file format using Marvin Sketch (Chem Axon). By removing the hetero atoms, water molecule and cofactors, the target protein file was prepared by leaving the associated residue with protein by using Auto Dock 4.2 (MGL tools-1.5.6). Preparation of target protein file Auto Dock 4.2 (MGL tools-1.5.6) tool has been done, which involves the assign of Gasteiger charges for all the atoms of molecules converting into AD4 type. Docking simulations for the compounds 5(a–e), 6(a–e) were performed against the active site of topoisomerase II and DNA gyrase subunit b, finally Maestro elements tutorial 1.8 was used to visualize docking results tabulated in .
Table 2 Molecular docking reports for compounds 3 and 4 (a–e) against protein 1JIJ and 1KZN.
4 Results and discussion
In the present study, it has been discussed that the synthesis of various benzimidazole fused triazinane 4-thione heterocyclic moieties by cyclic condensation based on classical Mannich amino methylation of N,N′ unsymmetrical thio ureas (4) with 30% HCHO and methyl amine in ethanol. An aliphatic amine has been yielded in the condensation between methyl amine and formaldehyde, it cyclizes immediately to produce corresponding 1,3,5-triazinane since imine is unstable. With respect to biological activity, benzimidazole fused heterocyclic compounds such as triazinane derivatives are of very important than the cyclic compounds. Here the efforts are more focused on the synthesis of compound (1) by using NaHSO4–SiO2 catalyst and high yield of derived product is achieved by refluxing in ethanol. NaHSO4–SiO2 is a heterogeneous and eco friendly catalyst [Citation22].
The chemical structure of title compounds 5 and 6 were established on the basis of spectroscopic analysis. The IR spectrum showed absorption bands in the range from 3355 to 3373 cm−1 for (NH) functions, 1562 to 1590 cm−1 for (C=N) function and C=S absorptions in the range from 1231 to 1277 cm−1. The 1H NMR spectra of test compounds revealed the presence of singlet signals at 6.38 ppm for thiazole protons (CH), 11.20 ppm for NH proton, 4.64 ppm for CH2 (triazinane) and 5.53 ppm CH2 (oxadiazinane) respectively in addition to the aromatic protons as a multiplet (H) in the range 6.78–8.40 ppm. The mass spectrum showed a corresponding molecular ion peak at m/z with respect to their molecular weights ().
Table 3 Substituents for compounds 5 and 6.
4.1 Biological study and structure activity relationship
The newly synthesized title compounds 5a–e and 6a–e were evaluated for their antibacterial activity against Bacillus subtilis, Bacillus cereus, Staphylococcus aureus, E. coli, Klebsiella pneumoniae, and Salmonella typhi. The antibacterial activity of the tested compounds was assessed by zone of inhibition using well diffusion method. Gentamycin was used as standard drug for comparison. Structure activity relationship (SAR) studies for the antibacterial activity of compounds 5a–e and 6a–e mentioned in . The results of antibacterial screening () reveal that the compounds 5a–e and 6a–e displayed a better activity as compared to the standard drug. The electronic property of the compounds has close relationship to their biological activity. The substitution of the electron withdrawing groups such as chloro (5e and 6e), and nitro (5d and 6d) on the benzene ring exhibited more activity, whereas the introduction of electron donating groups such as methyl (5b and 6b) and methoxy (5c and 6c) groups on the benzene ring did not exhibit much activity. Compound 5a and 6a showed least activity because it has no substituent on the benzene ring. This evidence confirmed that suitable functional groups on benzene ring were necessary for better antibacterial activities in drug design [Citation23]. Hence these results implied that electron withdrawing groups play important roles in the antibacterial activities of these tested compounds.
Molecular docking based on the fact that Vosaroxin is a benzimidazole derivative which is an antibacterial drug and it is proved to exert its action by the inhibition of topoisomerase II, in clinical trails. On the basis of drug action, it has been performed docking simulation of the newly synthesized benzimidazole derivatives 5a–e and 6a–e binding the active site of the topoisomerase II crystal structure (PDB ID: 1JIJ) and DNA gyrase subunit b (PDB ID: 1KZN). The docking result of the tested compounds 5d and 6d showed high binding energy with topoisomerase II and DNA gyrase subunit b. Compound 5d and 6d showed very good binding energy with −9.05, −9.10 kcal/mol respectively for topoisomerase II. Compound 5d and 6d showed very good binding energy with −10.04, −9.37 respectively for DNA gyrase subunit b. The binding energies, inhibition constants and residues involved in H-bonding are tabulated in .
5 Conclusions
The research study reported efficient synthesis of new analogs of triazinane and oxadiazinane by classical amino methylation with different aryl-N,N′ unsymmetrical thioureas. All compounds were characterized by standard spectroscopic techniques and evaluation of the antibacterial activity of all new compounds was carried out and proved significant to moderate activity also the compounds showed good inhibitory activity against molecular docking.
Acknowledgements
The authors gratefully acknowledge the Department of Chemistry, Kakatiya University, Warangal for constant support during this research work.
References
- S.A.GalalA.S.AbdelsamieM.L.RodriguezS.M.KerwinH.I.El DiwaniSynthesis and studying the antitumor activity of novel 5-(2-methylbenzimidazol-5-yl)-1,3,4-oxadiazole-2(3H)-thionesEur J Chem120106772
- P.T.NguyenJ.D.BaldeckJ.OlssonR.E.MarquisAntimicrobial actions of benzimidazoles against oral streptococciOral Microbiol Immunol200593100
- L.TownsendD.WiseThe synthesis and chemistry of certain anthelmintic benzimidazolesParasitol Today61990107112
- K.HasanD.RizaS.NihatS.GunalSynthesis of some benzimidazole derivatives and their antibacterial and antifungal activitiesAsian J Chem21200961816189
- S.DixitP.Kumar SharmaN.KaushikSynthesis of novel benzimidazole derivatives: as potent analgesic agentMed Chem Res222013900904
- N.C.DesaiA.M.DodiyaN.R.ShihoryA search of novel antimicrobial based on benzimidazole and 2-pyridone heterocyclesMed Chem Res21201225792586
- Y.ShiraishiY.SuganoS.TanakaT.HiraiOne-pot synthesis of benzimidazoles by simultaneous photocatalytic and catalytic reactions on Pt@TiO2 nanoparticlesAngew Chem Int Ed49201016561660
- M.GoudarziM.BazarganipourM.Salavati-NiasariSynthesis, characterization and degradation of organic dye over CO3O4 nanoparticles prepared from new binuclear complex precursorsRSC Adv420144651746520
- M.GoudarziD.GhanbariM.Salavati-NiasariPhoto-catalyst thallium sulfide: synthesis and optical characterization different morphologies of Tl2S nanostructuresJ Mater Sci Mater Electron26201587988806
- M.GoudarziM.Mousavi-KamazaniM.Salavati-NiasariZinc oxide nanoparticles: solvent-free synthesis, characterization and application as heterogeneous nanocatalyst for photodegradation of dye from aqueous phaseJ Mater Sci Mater Electron28201784238428
- M.GoudarziM.Salavati-NiasariControllable synthesis of new Tl2S2O3 nanostructures via hydrothermal process; characterization and investigation photocatalytic activity for degradation of some anionic dyesJ Mol Liq2192016851857
- M.GoudarziD.GhanbariM.Salavati-NiasariA.AhmadiSynthesis and characterization of Al(OH)3, Al2O3 nanoparticles and polymeric nanocompositesJ Clust Sci2720152538
- M.GoudarziM.Salavati-NiasariM.MotaghedifardS.M.Hosseinpour-MashkanSemiconductive Tl2O3 nanoparticles: facile synthesis in liquid phase, characterization and its applications as photocatalytic substrate and electrochemical sensorJ Mol Liq2192016720727
- J.M.BakkeJ.B.BuhaugHydrogen sulfide scavenging by 1,3,5-triazinanes: comparison of the rates of reactionInd Eng Chem Res43200419621965
- A.SolankeeK.KapadiaA.CiricM.SokovicI.DoytchinovaA.GeronikakiSynthesis of some new S-triazine based chalcones and their derivatives as potent antimicrobial agentsEur J Med Chem452010510518
- K.M.El-MahdyR.M.Abdel-RahmanA convenient methods for synthetic isomeric structures of pyrimido-1,2,4-triazine derivatives as biocidal agentsActa Chim Slov582011755764
- H.SugimotoY.YamaneS.InoueEnantiomeric discrimination by novel optically active isocyanurates having peripheral amino acid unitsTetrahedron Asymmetry11200020672075
- T.HanJ.ChoC.OhSynthesis and biological evaluation of 1β-methylcarbapenems having cyclic thiourea moieties and their related compoundsEur J Med Chem412006825832
- I.M.FigueiredoL.V.SantosW.F.da CostaJ.E.de CarvalhoC.C.da SilvaJ.L.Sacomanet al.Synthesis and antiproliferative activity of novel limonene derivatives with a substituted thiourea moietyJ Braz Chem Soc172006954960
- R.R.KhairullinaA.R.GeniyatovaA.G.IbragimovU.M.DzhemilevSynthesis of 5-alkyl-1,3,5-triazinan-2-ones and 5-alkyl-1,3,5-tri-azinane-2-thiones using Cu- and Sm-containing catalystsRuss J Org Chem492013904908
- B.V.RamanA.S.RamkishoreM.U.MaheswariT.RadhakrisnanAntibacterial activities of some folk medicinal plants of Eastern GhatsJ Pure Appl Microbiol32009187194
- K.GullapelliM.ThupuraniG.BrahmeshwariSynthesis and anti bacterial activity of 2-(4-aminophenyl)benzimidazole based pyrimidine derivativesInt J Pharm Biol Sci52014682690
- G.-F.ZhaJ.LengN.DarshiniT.ShubhavathiH.K.VivekA.M.Asiriet al.Synthesis, SAR and molecular docking studies of benzo[d]thiazole-hydrazones as potential antibacterial and antifungal agentsBioorg Med Chem Lett27201731483155