Abstract
The purpose of the study is to design, synthesise and assess the antipsychotic activity of a set of the novel (5-(10-(3-N, N-Dimethylamino) propyl)-10H-phenothiazine-3-yl)-1,3,4-thiadiazo-2-yl) Azodye/Schiff base/Chalcone derivatives. The newly synthesised compound structure was characterised by FT-IR, 1H NMR, Mass spectroscopy and elemental analysis. Each compound has been shown an excellent anti-psychotic activity in a haloperidol-induced catalepsy metallic bar test. The results found are firmly similar to docking study. Among the synthesised derivatives, compound 2-Amino-6-(3-hydroxy-4-methylphenyl) pyrimidine-4-yl) (7-chloro-10-(3-(N, N-dimethylamino) propyl)-10H-phenothiazine-3-yl) methanone (GC8) exhibiting high potency of catalepsy induction. Therefore, the derivative of GC8 has been considered that a potent anti-psychotic agent among the synthesised compounds.
1 Introduction
Dopamine receptors are responsible for several functions such as fine motor control, emotion, learning, cognition, pleasure, sensation, motivation, memory and modulation of neuroendocrine behaviour, movements etc., [Citation1]. Some changes in the role of dopaminergic receptor actions are generated many diseases like parkinsonism, psychomotor, schizophrenia, neurodegeneration, drug abuse, delusions and hallucinations etc., [Citation2]. These receptors are mainly divided into D1-5. They belong to the class of G-protein-coupled-receptors [Citation3,Citation4] . Here, D1 and D5 receptors are known as D1 family associates, whereas D2, D3 and D4 receptors are known as D2 family associates [Citation5]. Both families coupled with G-protein and retard the adenylyl cyclase [Citation6,Citation7] . With the knowledge of some evidence state that the possibility of the existence of D6 and D7 dopamine receptors, but such a type of receptor has not been sturdily documented. Generally, these receptors bind to the plasma membrane as a homodimer, heterodimers or higher-order oligomers etc., [Citation8]. It has been targeted for different psychotic illnesses and also be considered in some non-psychotic disorders [Citation9]. Drugs used to treat the psychotic problem are known as antipsychotic agents (or neuroleptic) is majorly classified into two types. Earlier antipsychotic drugs are called as typical or classical antipsychotic agents, whereas; currently available drugs are recognised as a second generation or atypical antipsychotic agents. Both the type of the antipsychotic agent is having a tendency to obstruct receptors in brain's dopamine pathways [Citation10]. Most of the antipsychotic agents are having substantial side effects, such as dysphoria, parkinsonism, tardive dyskinesia, galactorrhea, sedation, irritability, hyperprolactinaemia, sexual functioning disorder and symptoms of ADHD, depression, narcolepsy, anxiety, improved appetite, obesity threat, paranoia, aggression, psychomotor agitation, diabetes mellitus (Type 2), akathisia, extrapyramidal symptoms and menstrual trouble [Citation11]. Therefore, identification of a novel antagonist of dopamine receptor is needed to treat nervous diseases effectively. In recent years, there has been an immense awareness among the scientists toward the design of new drugs, which consumes less time, highly potent and lower cost to prepare an effective drug molecule against various health problems. Rapid and high throughput method of drug discovery is an only way to improve the therapeutic value of drugs in the animal model. Molecular docking is a one among the method to measure the biological activity of the proposed molecule with the targeted receptor rapidly using Molegro Virtual Docker (MVD). With the support of MVD, we found a bunch of novel compounds known as potent dopamine pathway inhibitors and bearing least side effect due to the presence of trusted thiadiazole and phenothiazine nucleus as part of the molecular structure. This study stated that easy way for the synthesis of novel Azo dye/Schiff bases/Chalcone derivatives and their antipsychotic activity by using virtual docking and a metallic bar test. The synthesised compound structure was characterised by FT-IR, 1H NMR, mass spectroscopy and elemental analysis.
2 Materials and method
2.1 Molecular docking
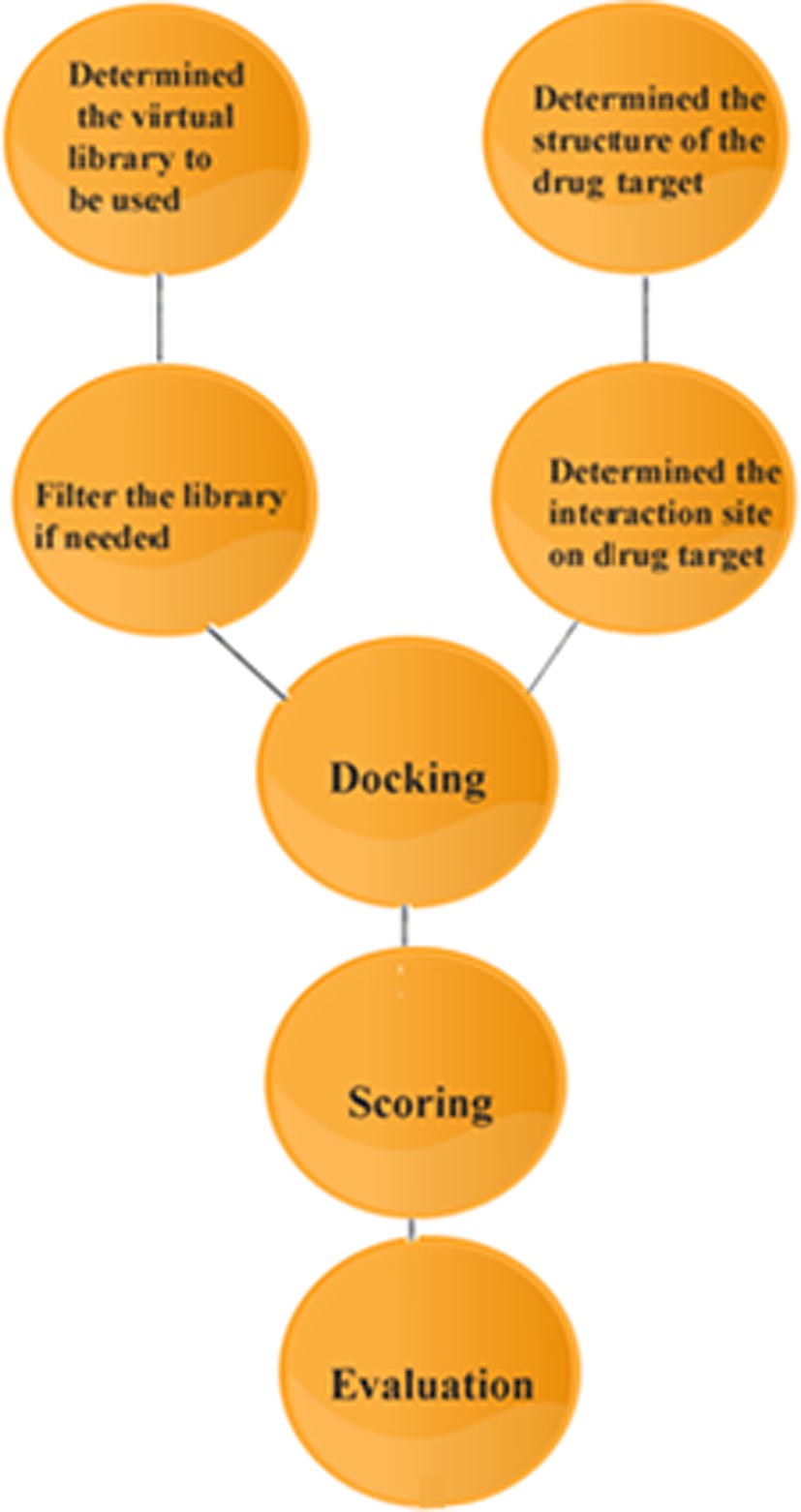
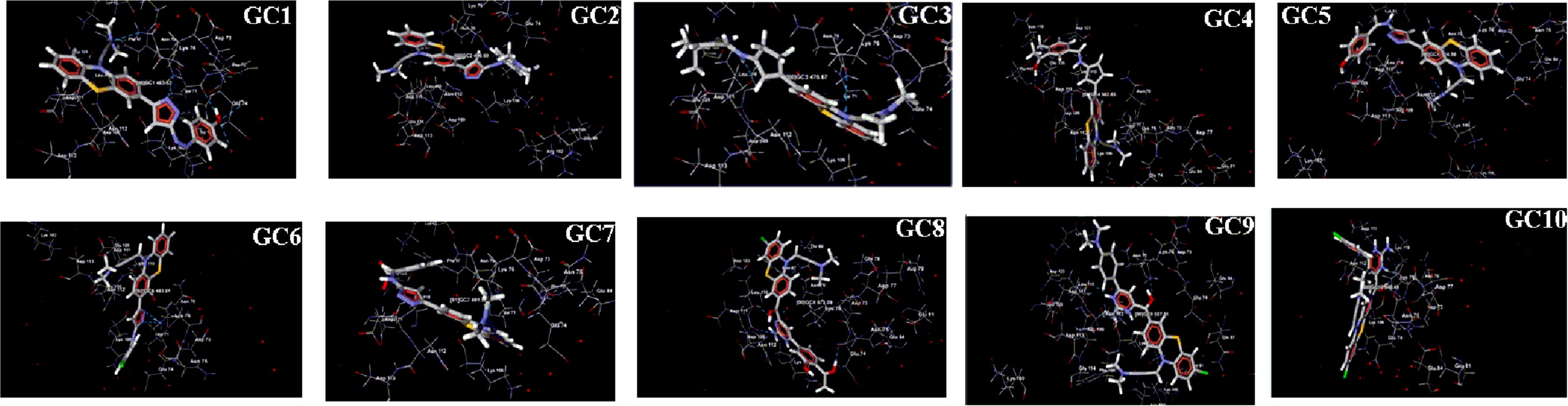
Virtual screening has been playing an important role in drug discovery processes which deal with a quick search of chemical structures likely to have more chemical binding to the drug target (protein or enzyme) from large libraries. MVD is a powerful docking tool used to detect the binding ability lies between the ligand and receptor. Before we start the docking process, the human dopamine D2 receptor template was collected from the protein bank as mentioned in . A setup of 26 different ligands was built in ChemDraw (), and the 2D structure was converted to the 3D structure using molegro virtual software [Citation12]. The best 3D structure of ligand was selected from energy minimization through molecular objective functions and modeller score in MVD [Citation13,Citation14] . The properties of each ligand such as absorption, distribution, metabolism and excretion were also studied. The best conformation was selected and used to predict the strength of the bond between the receptor and ligand. The result reveals that around 10 compounds () out of 26 are capable of making a perfect binding to the active site of the receptor amino acid. It also helped us to find out the order of prioritising molecules to synthesise from the bunch of the molecule based on moledock score, rerank score and hydrogen bond binding energy with DA. The docking study pathway was presented in .
Table 1 Structure and name of proposed molecules (ligand).
Table 2 Structure and name of synthesised compounds.
2.2 Chemistry
The raw materials and solvents were purchased from Ranbaxy, Sigma-Alrich, Ranchem companies. The melting points of prepared analogues were recorded in open capillary tube method on an Electrothermal 9100 melting point apparatus and are uncorrected. Functional group of synthesised compound was confirmed by using Fourier transform infrared spectroscopy (FT-IR) between the ranges from 4000 cm−1 to 400 cm−1. The number of proton present in the analogues was recorded on the Bruker 1H NMR spectroscopy from chemical shift (δ) and the molecular mass of the compound was analysed by the Shimadzu mass spectroscopy. The element analysis was performed on Perkin Elmer 2400 CHN elemental analyser.
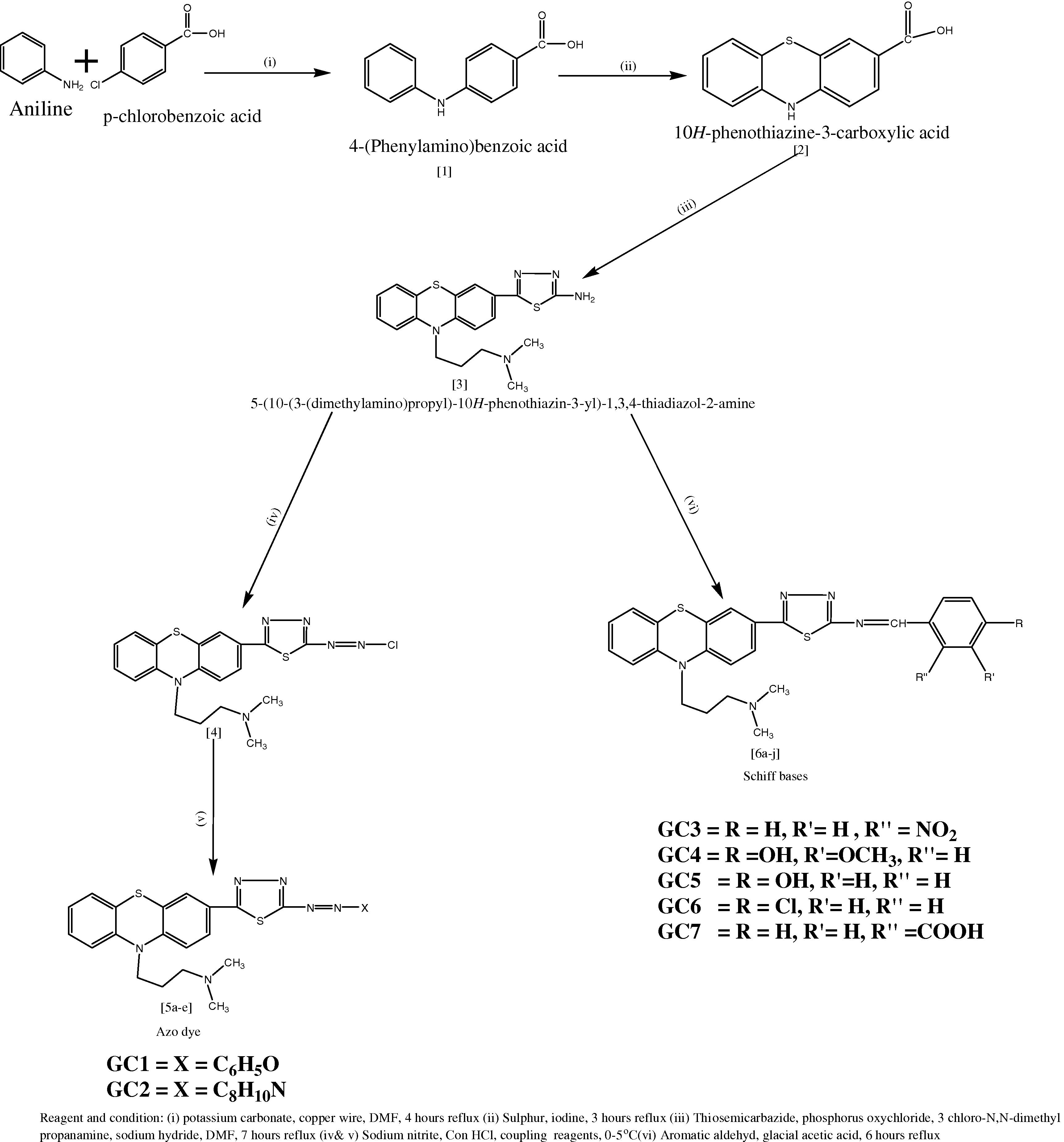
2.2.1 Synthesis of 4-(Phenylamino)benzoic acid (Scheme-I)
Aniline (0.1 mol, 9.3 ml), para chloro benzoic acid (0.1 mol, 15.6 g), potassium carbonate (0.01 mol, 1.38 g) and 0.63 g of copper wire were dissolved in 30 ml of N, N-dimethylformamide (DMF) contained round bottom flask of about 250 ml capacity. The mixture was allowed to agitate for 30 min at 20–25 °C. The flask was fitted with a reflux condenser and heated at 80 °C for 4 h with occasional shaking. The crude 4-(Phenylamino) benzoic acid was filtered, washed with little cold water and crystallized from ethanol.
2.2.2 Synthesis of 10H-Phenothiazine 3-carboxylic acid
An ethanolic solution of 4-(Phenylamino) benzoic acid (0.01 mol, 2.13 g) was added dropwise to a mixture of sulphur (0.01 mol, 0.32 g) and iodine (0.01 mol, 1.26 g). Shake the mixture until it became a solution. Placed the solution in a round bottom flask of about 250 capacities and fitted with the reflux condenser. The mixture was subjected to reflux on a water bath around 3 h with occasional shaking. The crude 10H-Phenothiazine 3-carboxylic acid was separated with a vacuum pump, washed with a small portion of cold water and re-crystallized from ethanol.
2.2.3 Synthesis of 5-(10-(3-(N,N-Dimethylamino)propyl-10H-phenothiazine-3-yl)-1,3,4-thiadiazole-2-amine
10H-Phenothiazine 3-carboxylic acid (0.01 mol, 2.43 g) and thiosemicarbazide (0.01 mol, 0.75 g) were dissolved in 60 ml of phosphorus oxychloride with the stirring duration of 10 min. The contents were placed in a distillation flask fitted with the reflux condenser. The flask was heated on a water bath for around 4 h. The reflux was detached from reflux condenser and added dropwise 3-Chloro-N, N-dimethyl propanamine (0.01 mol, 1.21 ml), sodium hydride (0.01 mol, 0.24 g) in DMF. Again, the reaction mixture was warmed for 3 h in a water bath. The hot solution was cooled to room temperature and separated crude product was filtered, washed with a small quantity of water and re-crystallized from ethyl acetate.
2.2.4 General procedure for preparation of varies Azo dye: (GC1-GC2)
5-(10-(3-(N,N-Dimethylamino) propyl-10H-phenothiazine-3-yl)-1,3,4-thiadiazole-2-amine (0.01 mol, 3.83 g), sodium nitrite (0.01 mol, 0.68 g) and Con. HCl (0.1 mol, 3.6 ml) were placed in a 50 ml Erlenmeyer flask, immerse the flask in an ice bath and maintained the temperature below 0–5 °C. Diazotization took place and formation of phenyl diazonium chlorde. Coupling reagents were allowed to interact with phenyl diazonium chloride at 4 °C. The separated azo dye was filtered, washed thoroughly with a small portion of cold water and re-crystallized from ethyl acetate and n-hexane.
2.2.5 General procedure for preparation of various Schiff base: (GC3–GC7)
An equimolar mixture of aromatic aldehydes (0.01 mol) and 5-(10-(3-(N,N-Dimethylamino)propyl-10H-phenothiazine-3-yl)-1,3,4-thiadiazole-2-amine (0.01 mol, 3.83 g) were added gradually to 10 ml of GAA contained two-necked round bottom flask. Then it was refluxed at 80 °C for 6 h with constant stirring. The precipitated solid was filtered, washed with 50 ml of water and re-crystallized from ethanol. The azo dye and Schiff base compounds were synthesised according to the reported procedure [Citation15] ().
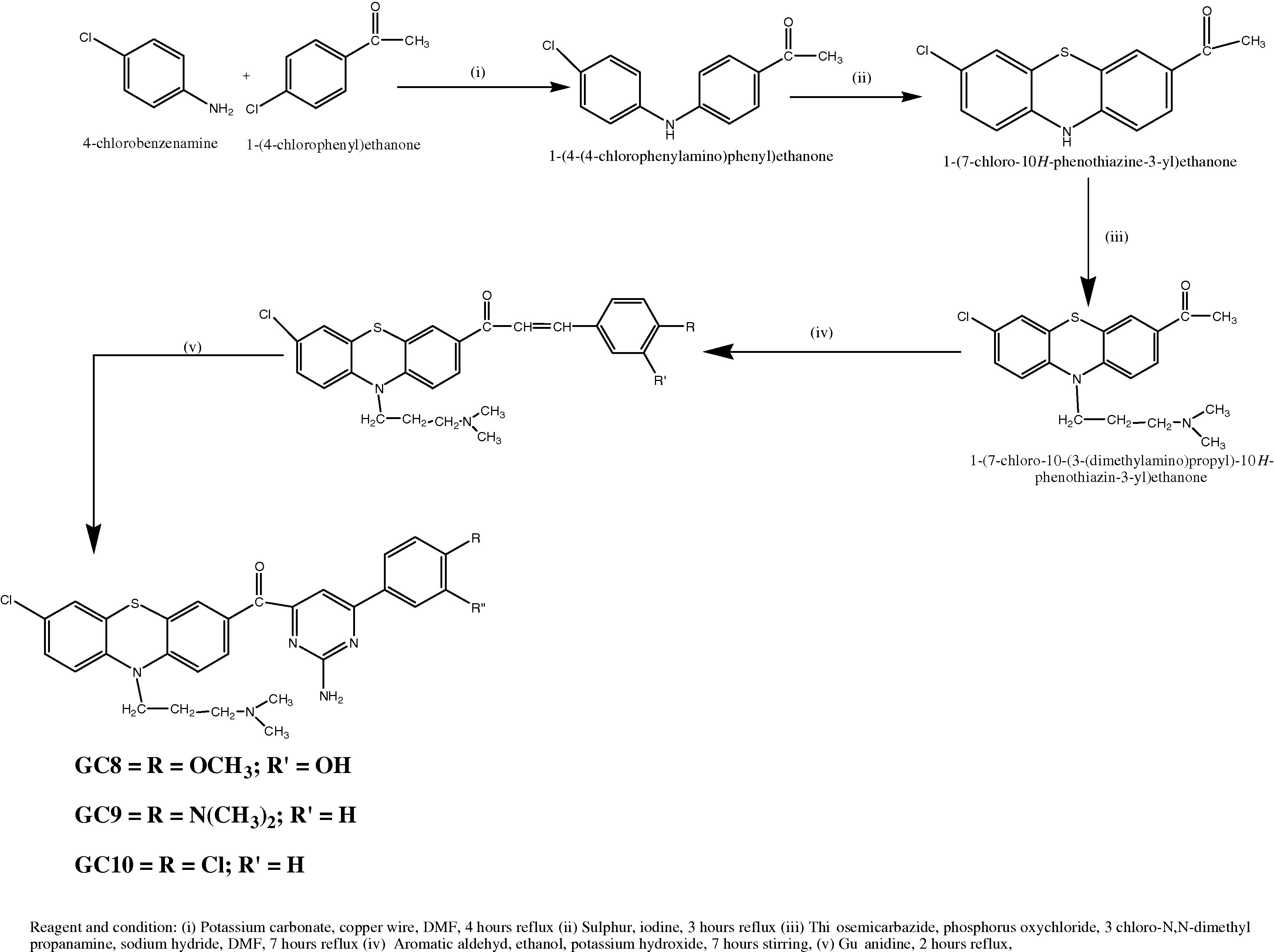
2.2.6 Synthesis of 1-(4-(4-Chlorophenylamino)phenyl)ethanone (Scheme II)
4-Chlorobenzene amine (0.01 mol, 1.27 g), p-chloroacetophenone (0.01 mol, 1.54 g), potassium carbonate (0.01 mol, 1.38 g) and 0.63 g of copper wire were dissolved in 30 ml of DMF contained round bottom flask of about 250 ml capacity. The mixture was stirred for 30 min. The flask was fitted with a reflux condenser and heated at 80 °C for 4 h with occasional shaking. The crude 4-(Phenylamino) benzoic acid was filtered, washed with little cold water and crystallized from ethyl acetate [Citation16].
2.2.7 Synthesis of 1-(7-Chloro-10H-Phenothiazine-3-yl)ethanone
To a solution of 1-(4-(4(Chlorobenzeneamino) phenyl) ethanone (0.01 mol, 2.45 g) in rectified spirit, sulphur (0.01 mol, 0.3 g) and iodine (0.01 mol, 1.26 g) were placed in a two-necked flask fitted with a reflux condenser. The mixture was subjected to reflux on a water bath around 3 h with occasional shaking. The separated 1-(7-Chloro-10H-Phenothiazine-3-yl) ethanone was filtered, washed with distilled water, dried and crystallized from acetone [Citation17].
2.2.8 Synthesis of 1-(7-Chloro-10-(3(N,N-dimethylamino)propyl)-10H-phenothiazine-3-yl) ethanone
A mixture of 1-(7-Chloro-10H-Phenothiazine-3-yl) ethanone (0.01 mol, 2.75 g), 3-Chloro N, N-dimethyl propanamine (0.01 mol, 1.21 ml), sodium hydride (0.01 mol, 0.239 g) in DMF was kept in a reflux condenser and heated on a water bath at 80 °C for 3 h. The resulting solution was cooled and separated 1-(7-Chloro-10-(3(N,N-dimethylamino)propyl)-10H-phenothiazine-3-yl) ethanone and washed with small quantities of cold water and re-crystallized from ethyl acetate [Citation18,Citation19] .
2.2.9 General procedure for preparation of Chalcone derivatives (GC8–GC10)
Dissolve 1-(7-Chloro-10-(3(N,N-Dimethylamino)-10H-phenothiazine-3-yl)ethanone (0.01 mol, 3.60 g) and aromatic aldehyde (0.01 mol) in a 20 ml ethyl alcohol contained 10% potassium hydroxide, stirring with a glass rod for 7 h or until it became a clear solution. Guanidine (0.01 mol, 0.59 g) in N, N-dimethylformamide was added and stirred. Then the mixture was refluxed for 2 h with continuous stirring. The hot solution was cooled to room temperature and the separated compound was filtered, washed with a portion of cold water and re-crystallized from ethyl alcohol and ethyl acetate [Citation20,Citation21] ().
2.3 X-ray crystallography
X-ray crystallography is a tool used to investigate the three-dimensional picture of the atomic and molecular structure of a crystal by using X-ray light, which has wavelengths of 1 angstrom (10−8 cm). The beam of X-ray strikes a crystal and causes the diffraction of light into specific directions, fed into the computer and using a mathematical equation to calculate the position of every atom in the crystallized molecule.
3 Results
3.1 Molecular docking
Each ligand potency has been identified from moldock score (), rerank score () and hydrogen bonding score () after successful interaction with DA. The potent anti-psychotic agents are perfectly docked to a hydrophobic and the hydrophilic center of the target (DA) and were exhibiting excellent score as compared to standard drugs. The key amino-acids responsible for the binding site of DA were found to be Asp 123, Lue110, Asp 111, Asp 109, Asn 112, Thr 66, Asn 70 Lys 76, Glu 74, Arg 79, Asn 75 and Glu 84 residues etc., Each compound had shown a significant affinity towards DA due to the formation of a salt bridge between the ligand and some hydrophobic amino acid residue (Asp111 and Asp123), hydrogen bond formation with hydrophilic amino acid (Asn112, Glu74) and π-π interaction with some hydrophobic amino acid residue (Phe67). Here, compound GC8 and GC2 had exhibited admirable moldock score as compared to other derivatives due to presence of electron releasing groups such as OH, OCH3 and N(CH3)2 etc., The overall decreasing order of dopamine-inhibiting action of the entire synthesised analogues was found to GC8 > GC2 > GC1 > GC7 > GC3 > GC5 > GC4 > GC9 > GC6 > GC10. The interaction of each ligand and DA was shown in .
Table 3 In-silico docking analysis of GC1–GC10 on dopamine D2 receptor (DA) ranking based on MolDock Score.
Table 4 In-silico docking analysis of GC1–GC10 on dopamine D2 receptor (DA) ranking based on Rerank score.
Table 5 In-silico docking analysis of GC1–GC10 on human dopamine D2 receptor (DA) ranking based on H bond.
3.2 X-ray crystallography determination and refinement
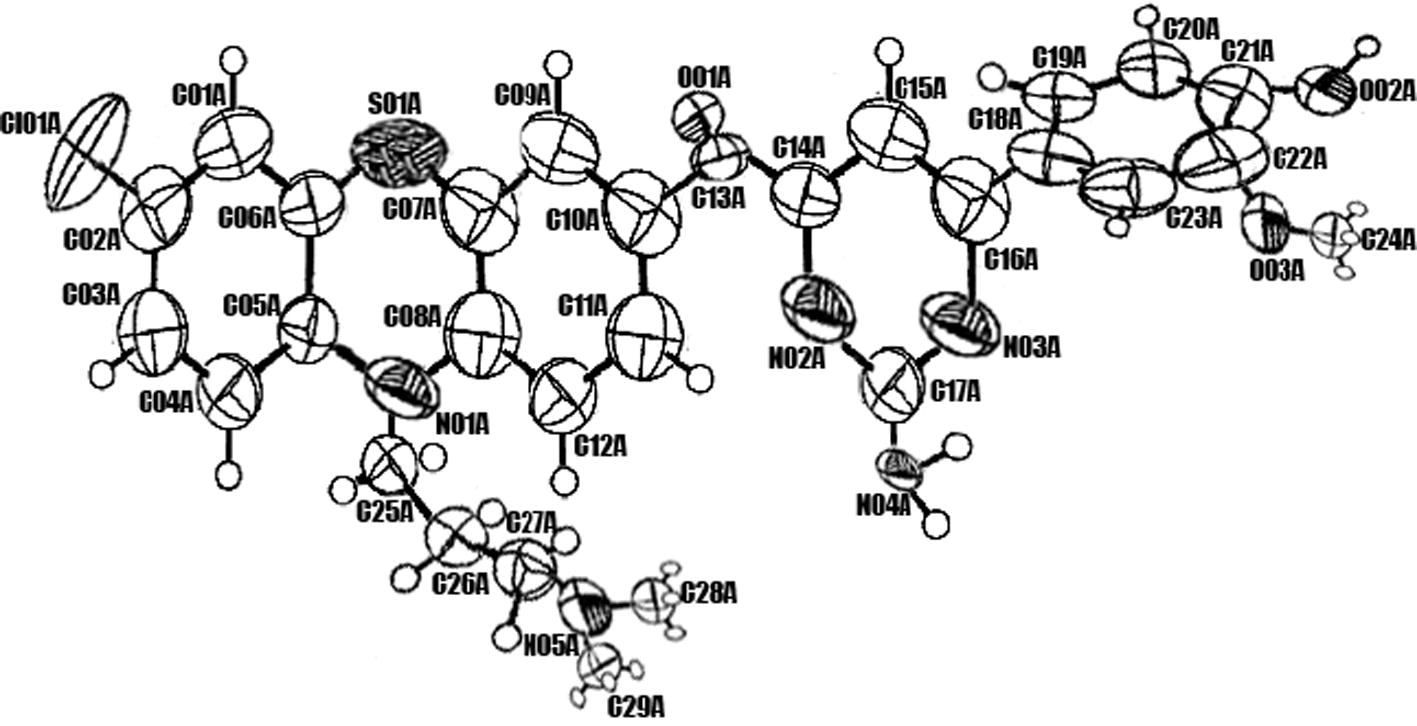
Diffraction data of the compound GC8 were collected from 8031 reflections using X calibur CCD diffractometer equipped with area detector and graphite monochromator (λ = 0.71835). The dimension of the crystal employed for data collection was 0.28 × 0.28 × 0.25 mm at 30% probability with selected bond length and bond angle. The refinement was carried out by full-matrix least squares using SHELXL 97 [Citation22]. The complete detail of data collections, condition and different parameters of refinement process were furnished in . The ORTEP view of the crystal, bond length and bond angle were shown in and .
Table 6 Crystal data and structural refinement for compound GC8.
Table 7 Bond distances and bond angle of GC8 compound.
3.3 Spectral data
3.3.1 Preparation of azo dye and Schiff base derivative: (GC1-GC7) [Citation15]
3.3.1.1 Preparation of 2-Amino-6-(3-hydroxy-4-methylphenyl) pyrimidine-4-yl)(7-chloro-10-(3-(N,N-dimethylamino)propyl)-10H-phenothiazine-3-yl)methanone: (GC8)
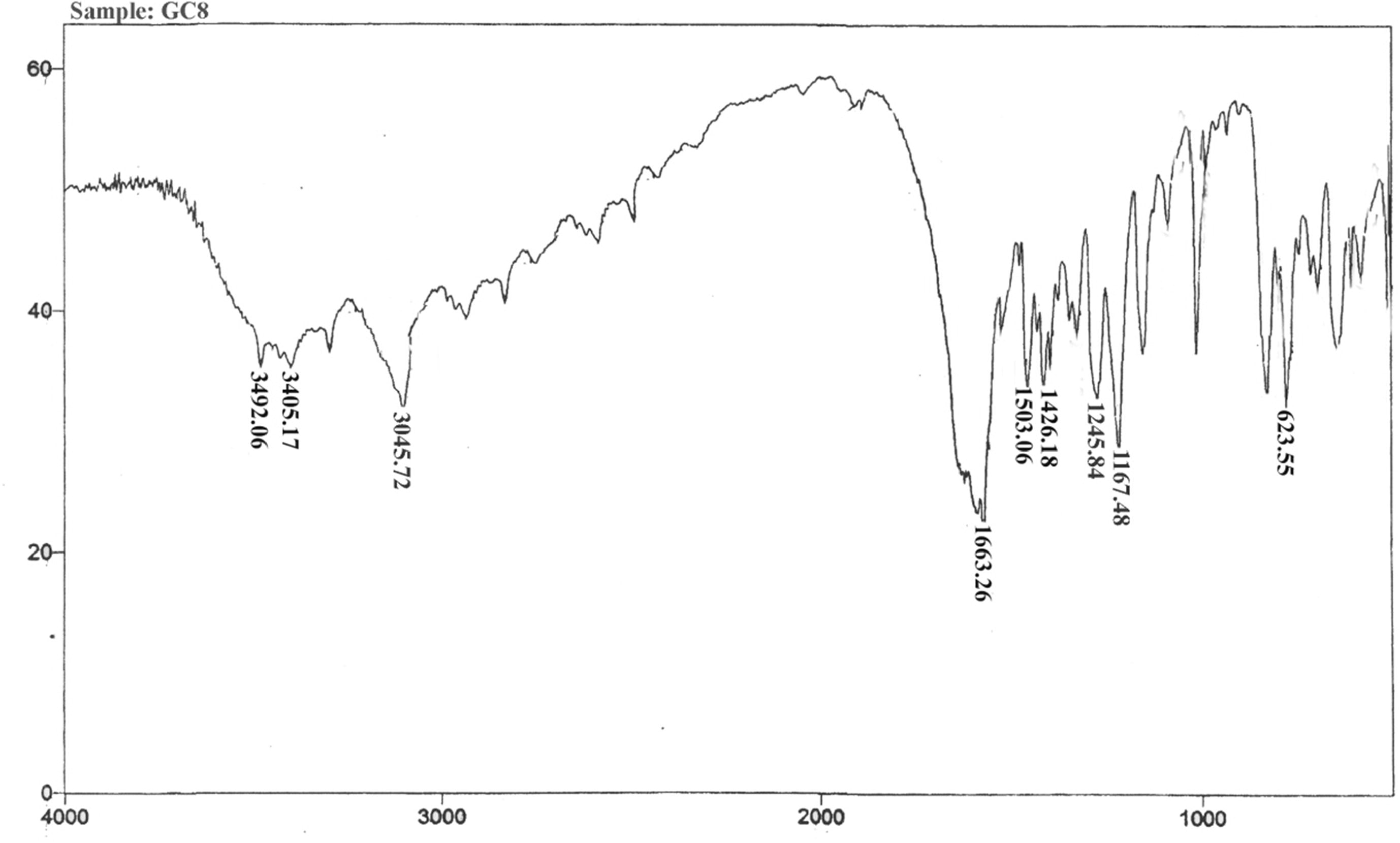
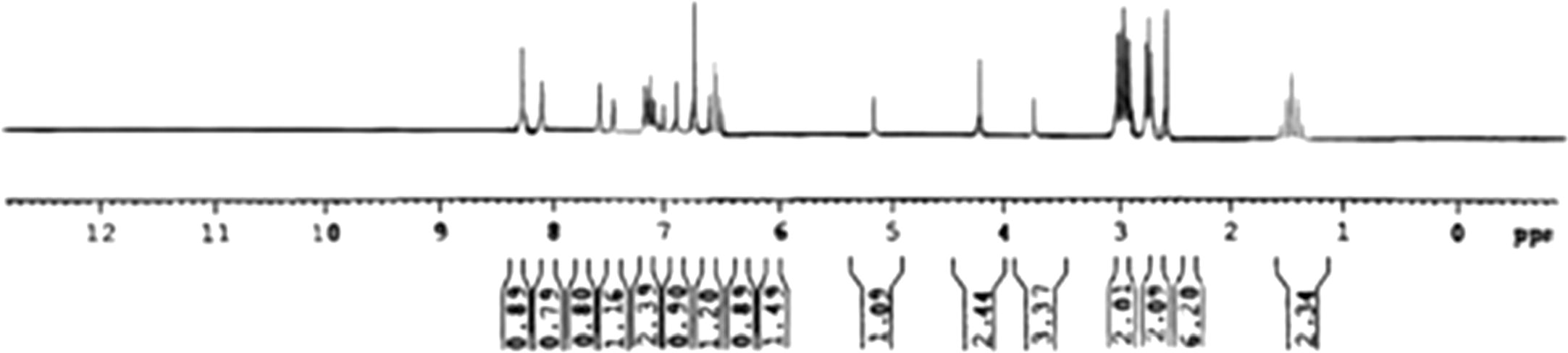
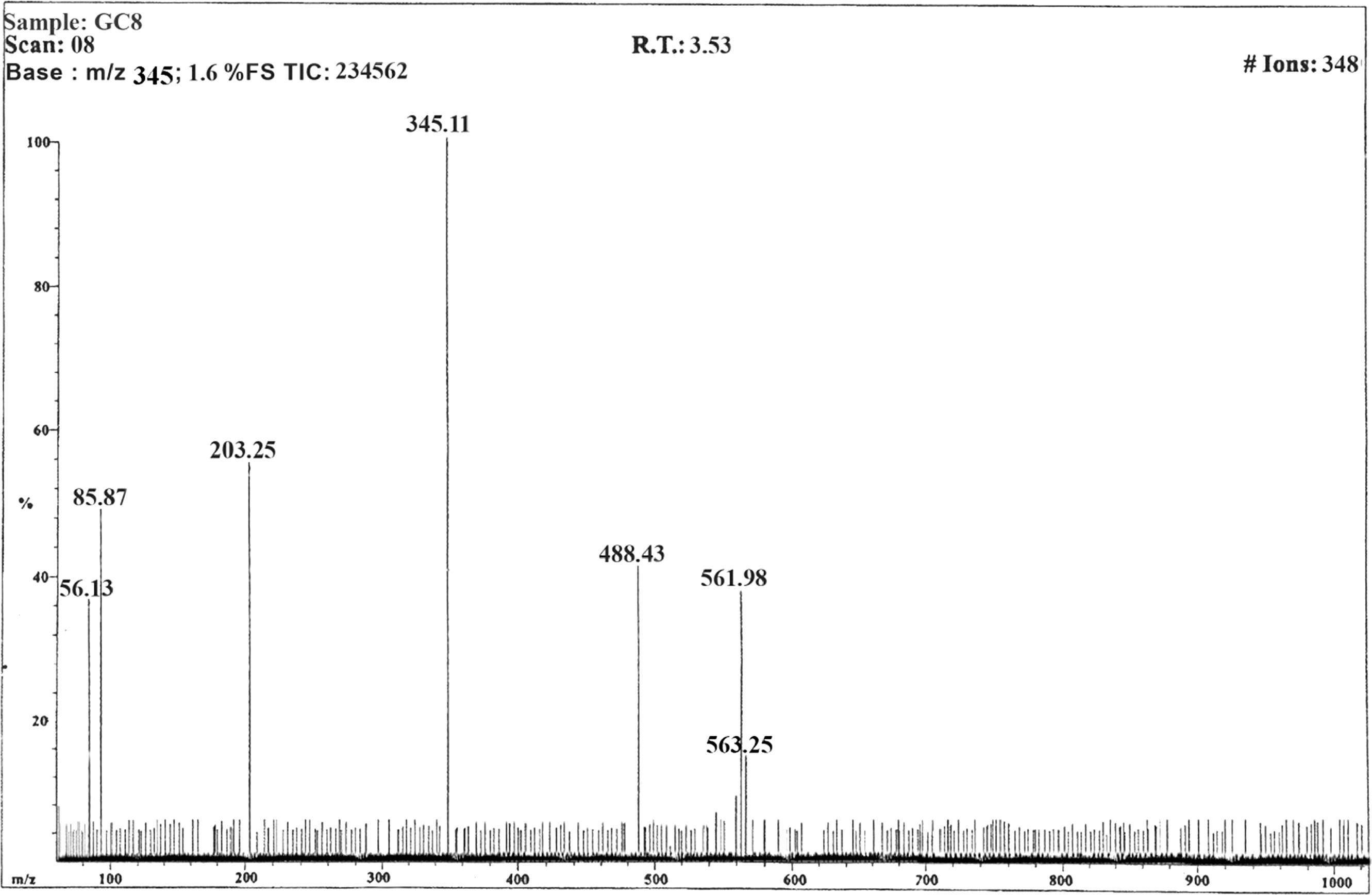
Molecular formula – C29H28ClN5O3S; Yield (86%); M.P – 217 °C; IR (νmax, cm–1): 3045 (Ar-H str), 1426(Ar-C str), 1503 (C=N), 1167(C–N), 1663(C=O), 3405 (OH), 1245 (OCH3), 3492 (NH2), Cl (6 2 3); 1H NMR (CDCl3, δppm, 500 MHz): δ1.30–1.62 (q, 2H, CH2-H, J = 13.6 Hz), δ2.52–2.64(s, 6H, N(CH3)2), δ2.67–2.85 (t, 2H, CH2-H, J = 4.7 Hz), δ2.91–3.18(t, 2H, CH2-H, J = 7.8 Hz), δ3.71–3.78(s, 3H, OCH3-H), δ4.15–4.28(s, 2H, NH2-H), δ5.10–5.27(s, 1H, OH-H) δ6.46–6.63(t, 1H, Ar-H, J = 15.8 Hz), δ6.72–6.79(s, 1H, Ar-H), δ6.85–6.94(s, 1H, Ar-H), δ6.97–7.08(s, 1H, Ar-H), δ7.09–7.22(m, 2H, Ar-H), δ7.43–7.48(s, 1H, Ar-H), δ7.53–7.60(s, 1H, Ar-H), δ8.06–8.15(s, 1H, Ar-H), δ8.20–8.37(s, 1H, Ar-H); Mass(m/z)-561(38) [M+], 563(15)[M++2], 489 (42), 345 (100), 203 (55), 85 (50), 58 (37); Anal. Calcd for C29H28ClN5O3S (561): C, 61.97; H, 5.02; N, 12.46; S, 5.70; O, 8.54; Cl, 6.31. Found: C, 61.82; H, 5.14; N, 12.26%.
3.3.1.2 Preparation of 2-Amino-6-(4-methylphenyl) pyrimidine-4-yl)(7-chloro-10-(3-(N,N-dimethylamino)propyl)-10H-phenothiazine-3-yl)methanone: (GC9)
Molecular formula – C30H31ClN6OS; Yield (79%); M.P – 222 °C; IR (νmax, cm–1): 3093 (Ar-H str), 1452(Ar-H ben), 1514 (C=N), 1240(C–N), 1670(C=O), 3426 (NH2), Cl (6 1 2); 1H NMR (CDCl3, δppm, 500 MHz): δ1.27–1.48 (q, 2H, CH2-H, J = 15.1 Hz), δ2.53–2.71(s, 6H, N(CH3)2), δ2.72–2.85 (t, 2H, CH2-H, J = 9.8 Hz), δ2.87–2.95(s, 6H, N(CH3)2), δ3.00–3.22(t, 2H, CH2-H, J = 9.8 Hz), δ4.15–4.29(s, 2H, NH2-H), δ6.42–6.58(m, 3H, Ar-H), δ6.59–6.87(m, 2H, Ar-H), δ6.88–7.00(t, 1H, Ar-H, J = 15.1 Hz), δ7.06–7.21(d, 2H, Ar-H, J = 15.5 Hz), δ7.23–7.31(s, 1H, Ar-H), δ7.37–7.44(s, 1H, Ar-H), δ7.46–7.63(d, 1H, Ar-H, J = 16.8 Hz); Mass (m/z)-558 (40) [M+], 560(15) [M++2], 528(22), 514 (33), 345 (1 0 0), 203 (53), 197 (49), 59 (33); Anal. Calcd for C30H31ClN6OS (5 5 8): C, 64.44; H, 5.59; N, 15.03; S, 5.73; O, 2.86; Cl, 6.35. Found: C, 64.38; H, 5.62; N, 15.10%.
3.3.1.3 Preparation of 2-Amino-6-(4-chlorophenyl) pyrimidine-4-yl)10-(3-(N,N-dimethylamino) propyl)-10H-phenothiazine-3-yl)methanone: (GC10)
Molecular formula – C28H25Cl2N5OS; Yield (71%); M.P – 217 °C; IR (νmax, cm–1): 3187 (Ar-H str), 1473(Ar-H ben), 1514 (C=N), 1257(C–N), 1665(C=O), 3436 (NH2), Cl (7 3 5); 1H NMR (CDCl3, δppm, 500 MHz): δ1.20–1.44 (q, 2H, CH2-H, J = 14.8 Hz), δ2.47–2.62(s, 6H, N(CH3)2), δ2.64–2.82 (t, 2H, CH2-H, J = 10.1 Hz), δ2.83–3.02(t, 2H, CH2-H, J = 10.5 Hz), δ4.12–4.29(s, 2H, NH2-H) δ6.52–6.67(d, 2H, Ar-H, J = 13.2 Hz), δ6.70–6.91(t, 2H, Ar-H, J = 8.1 Hz), δ6.94–7.20(m, 1H, Ar-H), δ7.22–7.34(t, 1H, Ar-H, J = 9.6 Hz), δ7.37–7.52(m, 2H, Ar-H), δ7.54–7.64(m, 2H, Ar-H), δ7.68–7.82(d, 1H, Ar-H, J = 15.1 Hz); Mass (m/z)-549 (56) [M+], 551(19)[M++2], 505 (1 0 0), 476 (31), 317 (61), 203 (42), 59 (29); Anal. Calcd for C28H25Cl2N5OS (5 4 9): C, 61.09; H, 4.58; N, 12.72; S, 5.82; O, 2.91; Cl, 12.88. Found: C, 61.15; H, 4.46; N, 12.80%. FT-IR, 1H NMR and Mass spectroscopy of compound GC8 was shown in Figs. 7Fig. 8–9.
3.3.1.4 Mass fragmentation of compound (GC8)
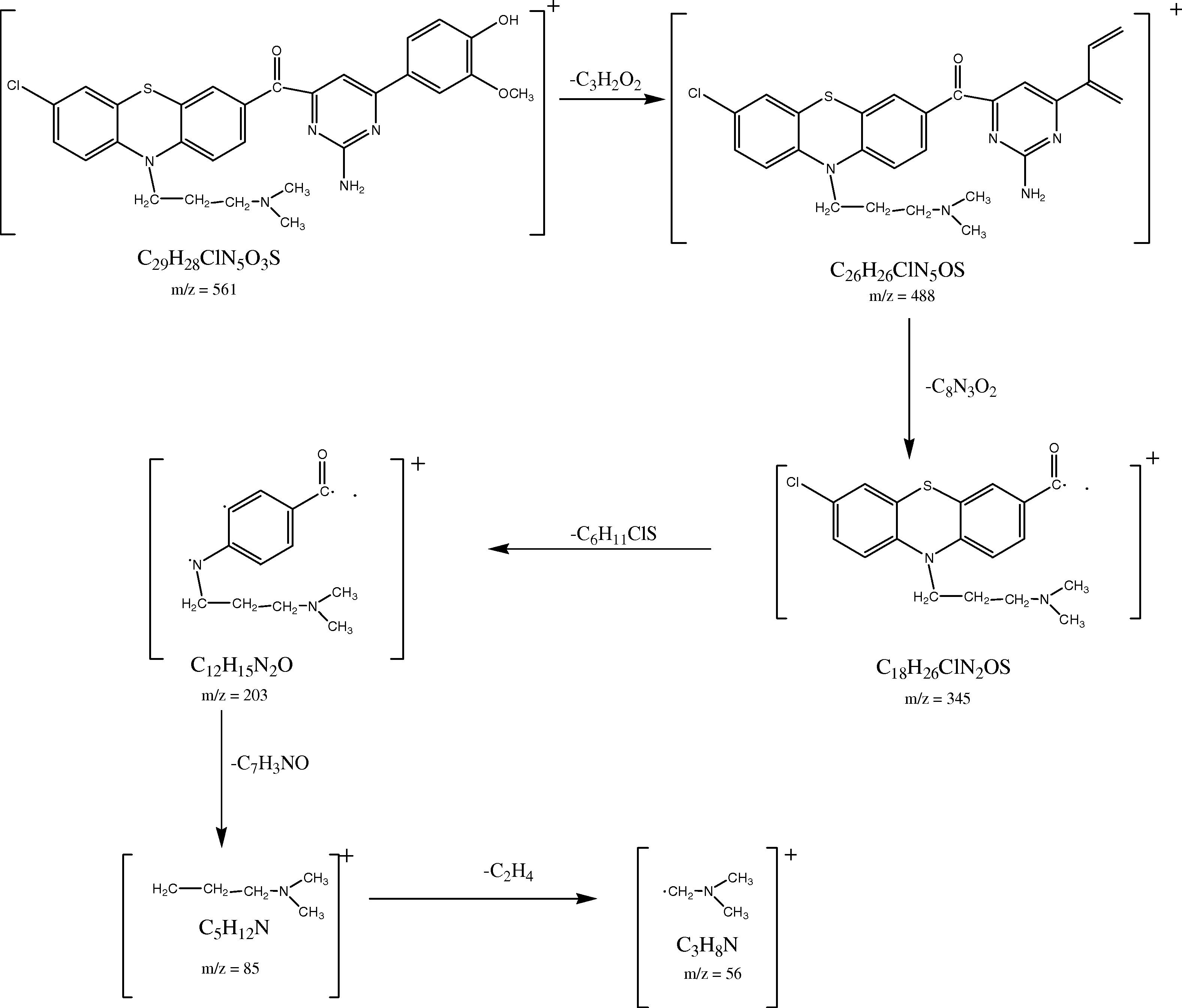
The mass spectral decomposition mode of the prepared 2-Amino-6-(3-hydroxy-4-methyl phenyl) pyrimidine-4-yl) (7-chloro-10-(3-(N,N-dimethylamino) propyl)-10H-phenothiazine-3-yl)methanone (GC8) has been investigated by electrospray ionization mass spectrometry (ESI-MS). The mass spectrum of compound GC8 showed the molecular ion (M+)/(molecular isotope ion (M++2) peaks (m/z) at 561/563 corresponding to the molecular formula of C29H28ClN5O3S (). The molecular ion of m/z 561 has been fragmented and gives a peak at 488 m/z. The peak at m/z 488 underwent fragmentation and produced a daughter ion at m/z 345.It further loss of the certain group of atoms such as C6H11ClS, C7H3NO and C2H4 to give a peak at m/z 203, 85 and 56 respectively. The ESI mass spectral fragmentation pathway of compound GC8 was discussed with typical example and other chalcone derivatives displayed similar mass spectral fragmentation pattern.
4 Biological evaluation
4.1 Catalepsy test
Catalepsy is defined as the inability of an animal to correct its abnormal posture after a particular period of time [Citation23]. It can accurately predict by haloperidol-induced catalepsy metallic bar test [Citation24]. To test the catalepsy, Wistar rats of either sex with an average weight of 200–225 g were selected, grouped (standard, test and control) and placed into different cages. Before starting the experiment protocol has been approved by the institutional animal ethics committee at GIET School of pharmacy, Rajahmundry, India (GSP/PY/04/2015). Each group maintained six animals under standard lab conditions. Haloperidol was administered 1 mg/kg, IP route to induce catalepsy in standard group of animals. Synthesised compounds (7.5 mg/kg and 15 mg/kg) were also given simultaneously to test group of animals. The animal front paws had been placed on a metallic bar, which elevated at 10 cm above the base of the cage. If the animal maintained abnormal posture more than 30 s called as catalepsy. The response observed at the various intervals like 0, 30, 60, 120, 180 and 240 min etc.,
4.2 Catalepsy score
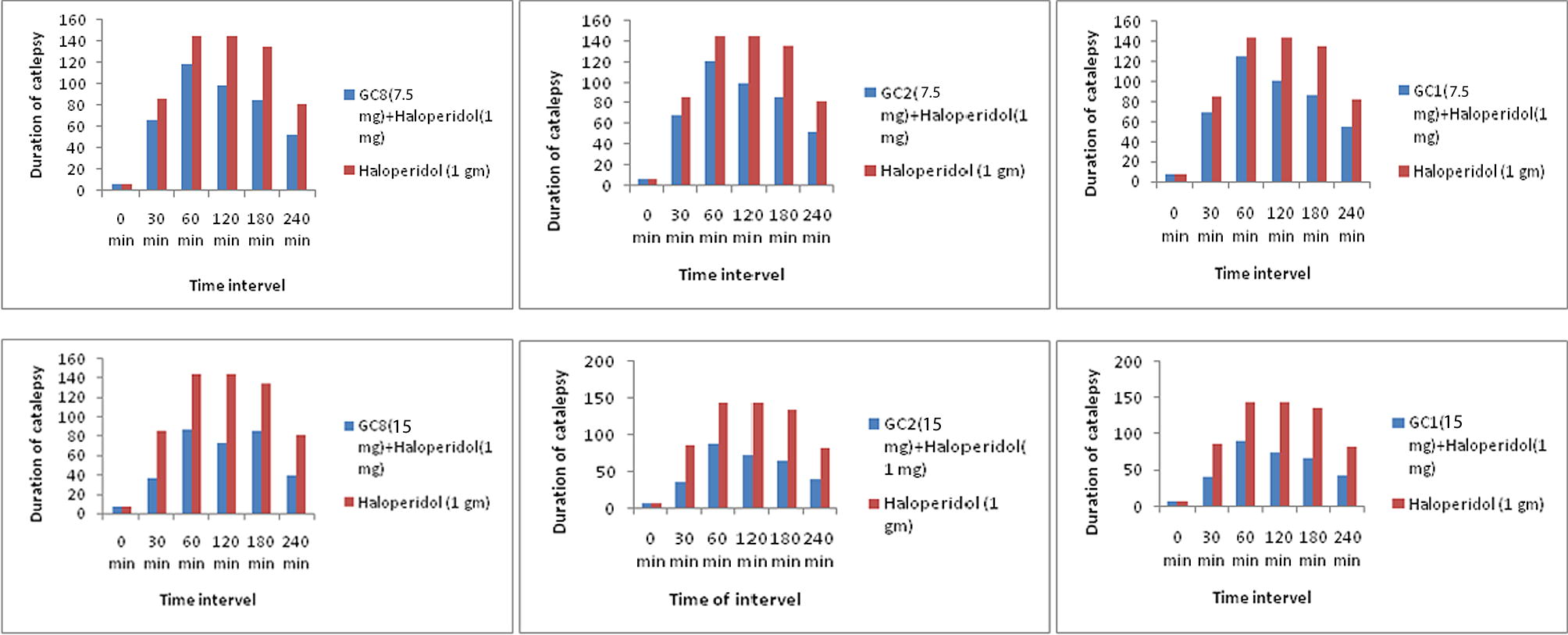
The cataleptic score of test, standard and control batch of Wister rats had taken at the interval of 30, 60, 120, 180 and 240 min. The score is significantly increased with the standard and test compounds in between 60 min and 120 min and the highest catalepsy score was achieved after 120 min of administration of standard and test compounds. This evidence proves that our test compounds were the severely block the dopaminergic neurotransmission in the striatum effectively to show anti-psychotic activity. Among the synthesised analogues, compound 2-Amino-6-(3-hydroxy-4-methylphenyl) pyrimidin-4-yl) (7-chloro-10-(3-(N, N-dimethylamino) propyl)-10H-phenothiazin-3-yl) methanone (GC8) induced the highest catalepsy period at the various time intervals such as 60, 120, 180 and 240 min. Compound GC8 (7.5 mg/kg and 15 mg/kg) significantly reduces the level of normal dopamine and generated catalepsy as similar to that of haloperidol treated animals with the percentage of 17.3%,41.6%,37.0%,35.8% and 39.5%, 57.1%, 54.0%, 51.8% respectively at various time interval such as 60, 120, 180 and 240 min. Effects of behavioural assessment in haloperidol/synthesised compound administered rat by metallic bar test were shown in and .
Table 8 Effect of anti-psychotic activity of synthesised compounds in Wistar rats.
5 Discussion
The route of prepared analogues was depicted in the synthetic scheme (Figs. & ) outlines the preparation part of the synthesised analogues. The Azodye compounds were prepared by performing an interaction between 5-(10-(3-(N,N-Dimethylamino)propyl-10H-phenothiazine-3-yl)-1,3,4-thiadiazole-2-amine, sodium nitrite, Con. HCl and the different coupling reagents. The Schiff base derivatives were prepared by condensation of 5-(10-(3-(N,N-Dimethylamino) propyl-10H-phenothiazine-3-yl)-1,3,4-thiadiazole-2-amine and different aromatic aldehydes. The derivatives of Chalcone were prepared from Claisen-Schmidt condensation reaction between 1-(7-Chloro-10-(3-(N,N-dimethylamino)-10H-Phenothiazine-3-yl) ethanone, different aldehyde and quanidine. The FT-IR and 1H NMR spectrum of synthetic analogues were shown peaks due to different groups present in the analogues. In FT-IR spectrum, strong bands at the region of 704, 735, 623, 612, 1502, 1306, 1210, 1245, 3457, 3364, 3414, 3405, 1725, 2854, 3436, 3492, 3426, 1663, 1670, 1665 cm−1 could be attributed to the chloro, nitro, methoxy, hydroxyl, carboxylic, amino and carbonyl group respectively. The aryl ring was raised stretching peak in between 3187–3016 cm−1 and 1473–1403 cm−1. A strong absorption peak at 1662–1649 cm−1 is due to the presence of the azomethine group (–C=NH). The number of proton present in the synthetic compounds was identified by 1H NMR spectroscopy from the chemical shift. The spectra showed a quintet at δ 1.20–1.90 ppm corresponding to methylene proton (CH2), a triplet at δ 2.52–3.47 ppm corresponding to methylene proton (CH2), a singlet at δ 2.24–2.71 ppm corresponding to N,N-dimethyl amine proton (N-(CH3)2); a singlet at δ 3.64–3.70 ppm corresponding to a methoxy proton (OCH3); a singlet at δ 4.11–4.28 ppm corresponding to amine proton (NH2); a singlet at δ 4.95–5.27 ppm corresponding to hydroxyl proton (OH)); a singlet at δ 6.72–8.37 ppm corresponding to aromatic protons(Ar-H); a doublet at δ 6.42–8.62 ppm corresponding to aromatic protons (Ar-H); a triplet at δ 6.46–8.23 ppm corresponding to aromatic protons (Ar-H); a multiplet at δ 6.25–7.65 ppm corresponding to aromatic protons (Ar-H) and a singlet at δ 7.94–8.71 ppm corresponding to azomethine proton (C=NH), a singlet at δ 10.95–11.05 ppm corresponding to a carboxylic acid proton (COOH). The synthesised compound molecular mass was confirmed by the Shimadzu mass spectrometer. The result of the antipsychotic activity of synthetic compounds was depicted in . It indicates catalepsy time of experimental animals after administration of haloperidol/synthesised compounds at the interval of 30, 60, 120, 180 and 240 min. Compound with electron donating groups on aryl ring showed remarkable antipsychotic activity over the unsubstituted and electron withdrawing group oriented compound.
6 Conclusion
The study stated that easy way of synthesis of novel Azodye/Schiff base/Chalcone derivatives and act as a powerful template for making a potent antipsychotic agent through molecular docking and haloperidol-induced catalepsy metallic bar test. Most of the compounds were perfectly docked to a hydrophobic and hydrophilic centre of the human dopamine D2 receptor with the support of key amino-acids like Asp 123, Leu110, Asp 111, Asp 109, Asn 112, Thr 66, Asn 70 Lys 76, Glu 74, Arg 79, Asn 75 and Glu 84 etc., these amino acid residues are allowing the molecules to bind firmly with human dopamine D2 receptor by forming a salt bridge, hydrogen bond and π-π interaction with the ligand. Therefore, the binding energy of each ligand lies between the ranges of 09.19985–0.533093 kcal/moles (). In addition to that each compound had been shown an excellent anti-psychotic activity in a haloperidol-induced catalepsy metallic bar test. The results found are firmly similar to docking study. Among the various analogues, compound GC8 and GC2 were bound more effectively to the receptor through electron donating groups such as OH, OCH3 and N(CH3)2 present in the part of the molecular structure and offered excellent antipsychotic activity. Therefore, there is a need for further study of the above-mentioned compounds for the development of the novel atypical antipsychotic agent.
Supplementary data 1
Download MS Word (1.6 MB)Supplementary data 2
Download MS Word (247 KB)Supplementary data 3
Download MS Word (266.5 KB)Acknowledgments
All authors wish to express the gratitude to GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, India, for providing research facilities. We are also thankful to Dr N. Murugeshan, Indian Institute of Technology, Chennai, Tamil Nadu, India, for providing a spectral data of synthesised compounds time to time.
References
- Y.QianM.ChenH.ForssbergHeijtz R.DiazGenetic variation in dopamine-related gene expression influences motor skill learning in miceGenes Brain Behav122013604614
- J.SongJ.KimDegeneration of dopaminergic neurons due to metabolic alterations and parkinson’s diseaseFront Aging Neurosci82016111
- S.J.BradleyA.B.TobinDesign of next-generation g protein–coupled receptor drugs: linking novel pharmacology and in vivo animal modelsAnnu Rev Pharmacol Toxicol562016535559
- E.V.GurevichR.R.GainetdinovV.V.GurevichG protein-coupled receptor kinases as regulators of dopamine receptor functionsPharmacol Res1112016116
- M.F.LanzenkaL.P.LegakisS.S.NegusOpposing effects of dopamine D1- and D2-like agonists on intracranial self-stimulation in male ratsExp Clin Psychopharmacol242016 193-05
- D.I.ChoC.MinK.S.JungS.Y.CheongM.ZhengS.J.Cheonget al.The N-terminal region of the dopamine D2 receptor, a rhodopsin-like GPCR, regulates correct integration into the plasma membrane and endocytic routesBr J Pharmacol1662012659675
- J.K.DreyerThree mechanisms by which striatal denervation causes breakdown of dopamine signalingJ Neuro Sci3420141244412456
- X.Y.MengM.MezeiM.CuiComputational approaches for modeling gpcr dimerizationCurr Pharm Biotechnol152014 996-06
- S.KumarS.K.WahiR.SinghSynthesis and preliminary pharmacological evaluation of 2-[4-(aryl substituted) piperazin-1-yl]-N-phenylacetamides: potential antipsychoticsTrop J Pharm Res102011265272
- A.JaszczyszynK.GsiorowskiP.SwiatekW.MalinkaK.Cieoelik-BoczulaJ.Petruset al.Chemical structure of phenothiazines and their biological activityPharmacol Rep6420121623
- Y.M.MaoM.D.ZhangAugmentation with antidepressants in schizophrenia treatment: benefit or riskNeuropsychiatr Dis Treat112015701713
- S.P.SinghC.R.DebS.U.AhmedY.SaratchandraB.K.KonwarMolecular docking simulation analysis of the interaction of dietary flavonols with heat shock protein 90J Biomed Res3020166774
- O.KorbB.T.TenF.R.Victor Paul RajM.KeilT.E.ExnerAre predefined decoy sets of ligand poses able to quantify scoring function accuracyJ Comput Aided Mol Des262012185197
- G.CenJ.M.HeroldD.KireevAssessment of free energy predictors for ligand binding to a methyllysine histone code readerJ Comput Chem332012659666
- C.GopiV.G.SastryM.D.DhanarajuSynthesis, spectroscopic characterization, X-ray crystallography, structural activity relationship and antimicrobial activity of some novel 4- (5-(10-(3-N,N-dimethylamino) propyl)-10H-phenothiazine-3-yl) -1,3,4-thiadiazole-2yl) Azo dye/Schiff base derivativesFuture J Pharma Sci2017 xx: 1-11
- R.KumarM.SinghD.N.PrasadO.M.SilakariS.SharmaSynthesis and chemical characterization of 9-anilinoacridinesChem Sci Trans22013246250
- B.SatyanarayanaP.MuralikrishnaD.R.KumarD.RamachandranPreparation and biological evaluation of phenothiazine derivativesJ Chem Pharma Res52013262266
- M.PoojaA.K.SuroorV.SurajpalA.OzairSynthesis, characterization and antimicrobial activity of new thiadiazole derivativeBull Korean Chem Soc31201023452350
- B.Morak-MlodawskaK.PlutaM.ZimeckiM.JelenJ.ArtymM.KociebaSynthesis and selected immunological properties of 10-substituted 1,8-diazaphenothiazinesMed Chem Res24201514081418
- N.KumarS.DrabuK.ShaliniSynthesis and pharmacological screening of 4,6-substituted di-(phenyl) pyrimidin-2-amineArab J Chem102017S87780
- R.KantD.KumarD.AgarwaR.D.GuptaR.TilakS.K.AwasthiA.AgarwalSynthesis of newer 1,2,3-triazole linked chalcone and flavone hybrid compounds and evaluation of their antimicrobial and cytotoxic activitiesEur J Med Chem11320163449
- B.GanapayyaA.JayaramaR.SankolliV.R.HathwarS.M.DharmaprakashSynthesis, growth, and characterization of a new NLO material3-(2,3-dimethoxyphenyl)-1-(pyridin-2-yl)prop-2-en-1-oneJ Mol Struct10072012175178
- N.BhattacharjeeR.PaulA.GiriA.BorahChronic exposure of homocysteine in mice contributes to dopamine loss by enhancing oxidative stress in nigrostriatum and produces behavioral phenotypes of Parkinson’s diseaseBiochem Biophys Rep620164753
- B.S.NishchalS.RaiM.N.PrabhuS.D.UllalS.RajeswariH.N.GopalakrishnaEffect of Tribulus terrestris on Haloperidol-induced Catalepsy in MiceIndian J Pharm Sci762014564567
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ejbas.2017.10.003.