Abstract
The present study was designed to estimate the protective or curative potency of an extract from Cocculus hirsutus leaves against CCl4 intoxication via its antioxidant property in rats. Liver enzyme markers (SGOT, SGPT, ALP, LDH, and bilirubin) and oxidative stress markers {lipid peroxidation (LPO), enzymatic antioxidants [superoxide dismutase, catalase and glutathione peroxidase] and non-enzymatic antioxidants [reduced glutathione, vitamin C and E]} were analyzed by spectrophotometry. Histopathological studies on hepatic tissue were also performed by the method of Hematoxylin and Eosin staining. Rats administrated with 30% CCl4 in olive oil intraperitoneally resulted in significant increase in the levels of SGOT, SGPT, ALP, LDH, and bilirubin compared to control rats. Significant elevation of hepatic LPO and depletion of enzymatic and non-enzymatic antioxidants levels were observed in CCl4 induced rats. When CCl4 induced rats were co-treated with C. hirsutus at doses of 250 and 500 mg/kg b·wt, all the altered levels of liver marker enzymes and oxidative stress markers were restored to near control values. Histopathological studies provided direct evidence of the hepatoprotective effect of C. hirsutus. In conclusion, extract from C. hirsutus could protect the liver from CCl4-induced oxidative damage, by scavenging the free radicals generated during the metabolism of CCl4.
1 Introduction
Hepatic-organ is the imperative organ, which regulates a wide range of physiological processes in the body and it is one of the major organs prone to the oxidative damage by the attack of reactive oxygen species [Citation1,Citation2] . Carbon tetrachloride (CCl4) exerted hepatocellular damage has been largely evaluated and employed model for detecting the novel hepato-protective therapeutics. As the reactive oxygen species are the major cause for the deleterious effects in hepatic disorders, various plant extracts were reported for their hepato-protective activities through their antioxidant activities [Citation3,Citation4] . Natural antioxidants are especially considered as robust candidates to confer protection against chemical induced toxicity [Citation5,Citation6] .
Since the emergence of civilization on the earth, herbal formulations have been used to maintain human health and to treat various diseases by the vast majority of the World’s population [Citation7Citation[8]–Citation9] . Many traditional systems of medicine in India use a number of medicinal plants and their formulations to cure hepatic disorders [Citation10]. In addition to these known plants, there are many other plants used by tribal and folk practitioners which are a potent source of effective hepatoprotective agents that remained unexplored.
Antioxidant property has been reported to play a crucial role in the hepatoprotective capacity of many plants such as Spirulina maxima, Bauhinia hookeri, and various medicinal herbs [Citation8,Citation11,Citation12] . Treating liver disorders with plant drugs has been an age old tradition by Ayurveda, an indigenous system of medicine in India. Thus the search for potent natural source has become a prime focus for new drug development in pharmaceutical industry for hepatoprotection.
Cocculus hirsutus (Linn.) Diels (Menispermaceae), described in ayurvedic literature as patalagarudi is a straggling shrub, widely distributed all over India, especially in dry regions. The leaves and roots of this plant are largely employed in the Indian traditional medicine for a variety of diseases including, hepatic obstruction, jaundice, bronchitis, diabetes mellitus, anorexia, gonorrhoea, and leprosy [Citation13]. C. hirsutus is well documented for its anti-inflammatory, analgesic [Citation14], antidiabetic and spermatogenic [Citation15] activities. Considering its varied biological activities and traditional therapeutic use for hepatic disorders, the present study was aimed to evaluate the hepato-protective potential of this plant via its antioxidant property against the deleterious effects of CCl4 induced oxidative damage.
2 Materials and methods
2.1 Chemicals
2,4-dinitro phenyl hydrazine (DNPH), Disodium phenylphosphate, Thiobarbituric acid (TBA), 1,1,2,2-tetraethoxy propane (TEP), epinephrine (Adrenaline), Glutathione reductase, reduced glutathione (GSH), Nicotinamide adenine dinucleotide phosphate reduced (NADPH) and cumene hydroperoxide were purchased from Sigma chemical company, USA. All the other chemicals utilized for this study were of analytical grade.
2.2 Plant material collection and extract preparation
Leaves from Cocculus hirsutus plant were collected in and around Tirumala Tirupathi hills, India and compared with the voucher specimen deposited at the Department of Botany, Sri Venkateswara University, Tirupati, India for its identification. The leaves were shade dried for a week and finely powdered using a blender. Ethanol extract of C. hirsutus (EECH) was prepared by soxhlation process. The plant powder (100 g) was extracted with 500 mL of 70% ethanol for more than 6 h and concentrated by rotary evaporation and vacuum drying. The yield of the plant extract was recorded (5.3%) and stored at −20 °C for further use.
2.3 Animals
Male Wistar rats (200 ± 50 g; Sri Raghavendra animal suppliers, Bangalore, India) were maintained under standard hygienic conditions at 25–28 °C with 12 h light/dark cycle and provided standard pellet diet (Hindustan Lever Ltd., Mumbai, India) and water ad libitum. The animal experiments in the present study were conducted by following the Institute Animal Ethics Committee regulations; approved by the Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India.
2.4 Acute oral toxicity study
This experiment was performed according to the Organization for Economic Cooperation Development (OECD) guidelines. Animals were divided into one control group and five plant extract treated groups (N = 6). After an overnight fast, rats were dosed orally with ethanol extract of Cocculus hirsutus (EECH) in distilled water at doses of 100, 250, 500, 1000, and 2000 mg/kg b. wt. Hourly observations of the animals for any signs of behavioral changes, toxicity and mortality was recorded over a period of 72 h [Citation16].
2.5 Experimental design to access the hepato-protective activity of EECH against CCl4 induced liver injury
Male albino rats were allocated into 4 groups each containing 6 rats. Group I received olive oil only (1 mL/kg body weight, i.p.) as a control, Group II (CCl4 induced) received mL/kg bodyweight of 30% CCl4 in olive oil, i.p. The EECH Extract (250 and 500 mg/kg body weight) was admintrated (orally) once in a day and CCl4 was administered after every 72 h. Treatment period was 10 days [Citation4]. Blood was collected from all the animals through retro-orbital plexus. After collecting the blood, the rats were sacrificed and their livers were excise, rinsed in ice cold normal saline followed by 0.15 M Tris-HCl (pH 7.4), blotted dry and then frozen at −80 °C for further biochemical analyses.
2.6 Estimation of enzyme levels in serum
Serum was separated from the blood samples by allowing the blood to clot at room temperature for 45 min followed by centrifugation (2500 rpm at 30 °C for15 min). Serum transaminases [glutamate pyruvate transaminase (GPT) and glutamate oxaloacetate transaminase (GOT)], alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) was estimated according to standard protocol described previously [Citation17Citation[18]–Citation19] .
2.7 Estimation of bilirubin in serum
Bilirubin content was estimated according to standard protocol by Jain et al. [Citation20].
2.8 Preparation of liver homogenate
Liver homogenates (10%) were prepared by homogenizing the liver samples in 50 mM phosphate buffer (pH 7.0) and centrifuging the homogenates at 4 °C; 10,000×g for 15 min. The supernatant was collected and preserved at −20 °C for further Biochemical analytical purpose.
All the Biochemical parameters were expressed in the units of activity per mg protein. The protein content in each liver homogenate was estimated according to the standard protocol [Citation21].
2.9 Estimation of lipid peroxides
LPO in the liver was determined by the method described earlier [Citation4] by measuring the amount of malondialdehyde (MDA).
2.10 Quantitative analysis of enzymatic antioxidants
The mean activities of superoxide dismutase SOD (units/min/mg protein) [Citation22,Citation23] , catalase (CAT; μmol of H2O2 consumed/min/mg protein) [Citation24] and glutathione peroxidase (GPx; μmol of glutathione oxidized/min/mg protein) was evaluated by the previously described standard methods [Citation25].
2.11 Quantitative analysis of non-enzymatic antioxidants
Reduced glutathione (GSH) content was determined by its chromogenic reaction with dithio-bis-2-nitrobenzoic acid (DTNB) [Citation26]. Vitamin C was estimated by following the standard procedure described by Santhrani et al. [Citation27]. Vitamin E was determined according to the protocol of Ramanathan et al. [Citation28].
2.12 Histopathological examination
Liver tissues were embedded in paraffin blocks and thin sectioning was performed according to paraffin slice techniques. The sections were further mounted on to the microscopic slides and stained with Hematoxylin and Eosin stains [Citation29]. These microscopic slides were observed under the light microscope and photographed.
2.13 Statistical analysis
The data was calculated and analyzed by using Statistical Package for Social Sciences (SPSS) software; version 15.0. One-way Analysis of Variance for comparing the difference in the means across the groups was performed. Duncan’s Multiple Range Test (DMRT) is used to identify significantly differing group means. The results presented in Tables are mean and standard error (mean ± SE).
3 Results
3.1 Acute oral toxicity
No mortality, abnormal signs and behavioral changes were observed in rats administered orally up to the dose of 2000 mg/kg b·wt. of EECH, which is the maximum recommended dose of any drug for testing acute toxicity by OECD guidelines [Citation16].
3.2 Serum enzyme parameters
A significant increase in the levels of SGOT, SGPT, ALP and LDH in the CCl4 administered rats (Group II) was observed, when compared to control (Group I) rats. However, a significant (p < .05) decrease in the levels of these enzymes was observed in the rats treated with two different doses of C. hirsutus extract (250 and 500 mg/kg body weight respectively) than in Group II rats ().
Table 1 Effects of ethanol extract from Cocculus hirsutus on serum GOT, GPT, ALP, LDH, and bilirubin levels in control and experimental rats against CCl4 induced toxicity.
3.3 Serum bilirubin
A marked increase in serum bilirubin was noticed in CCl4 exposed rats when compared to Control rats. Significant decrease in the levels of bilirubin was perceived in Group III and Group IV (250 and 500 mg C. hirsutus extract treated) rats on comparing to control (Group I) rats ().
3.4 Hepatic concentration of TBARS
A pronounced increase in the mean levels of TBARS was noticed in Group II (CCl4 treated) rats on comparing to control (Group I) rats. Treatment with two doses of C. hirsutus ethanol extract to Group III and Group IV rats resulted in a significantly (p < .05) low concentrations of TBARS, apparently by hindering hepatic lipid peroxidation ().
Table 2 Effect of ethanol extract of Cocculus hirsutus on hepatic levels of TBARS, enzymatic and non-enzymatic antioxidants in control and experimental rats against CCl4 induced toxicity.
3.5 Hepatic enzymatic and non-enzymatic antioxidants
Hepatic enzymatic and non-enzymatic antioxidants levels in all the four groups of rats are represented in . In comparison to control rats, a marked decline in the levels of both enzymatic and non-enzymatic antioxidants was noticed in the rats administered with CCl4. Treatment with two doses of C. hirsutus extract in Group III and Group IV rats alleviated the CCl4 damage by retrieving the declined levels of both enzymatic and non-enzymatic antioxidants to near control values.
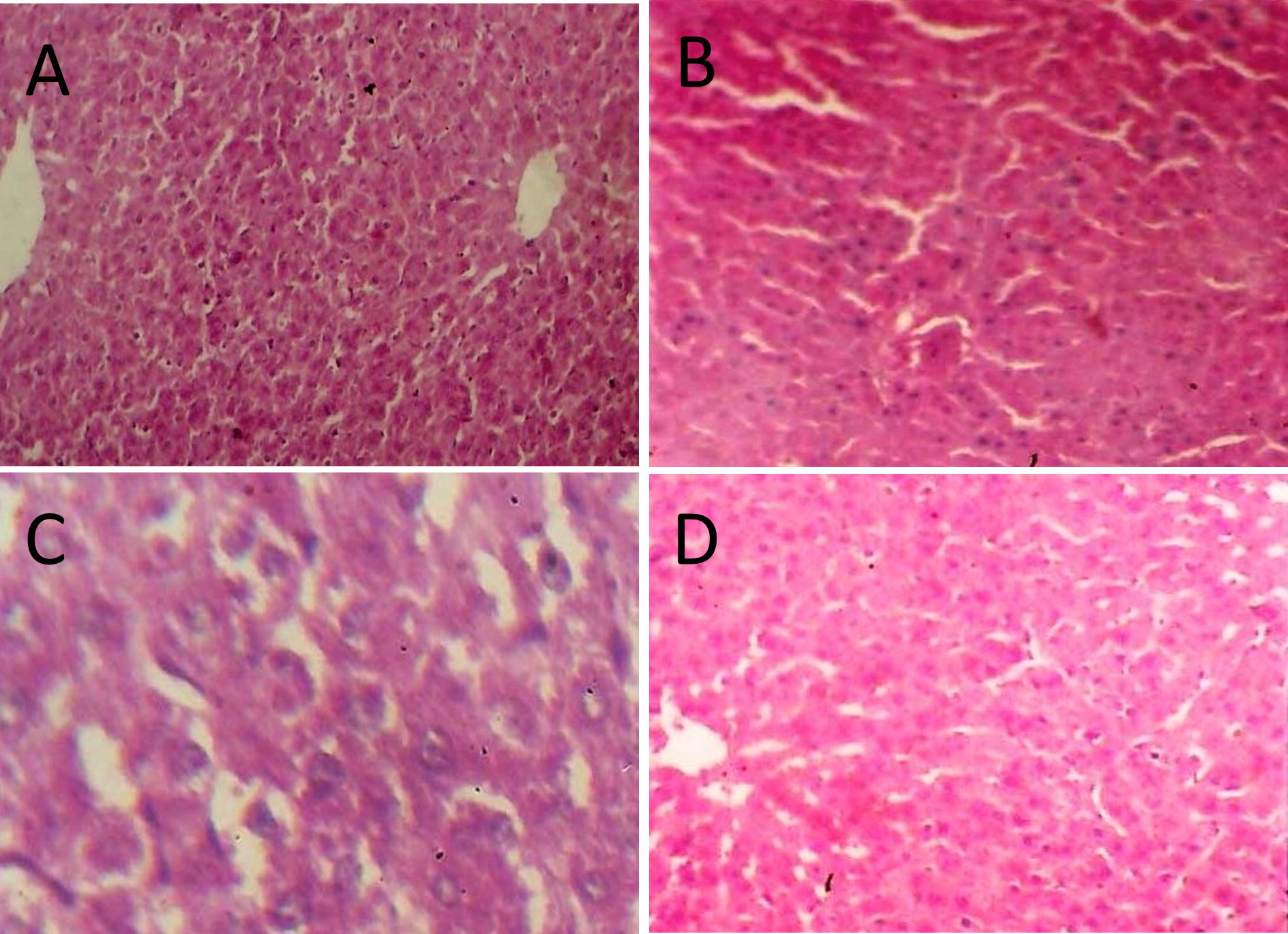
3.6 Histopathological examination
represents the histopathological examination of hepatic tissue of all the four groups of rats. Extensive damage to the histoarchitecture of hepatic tissue (the disruption of the lattice nature of the hepatocyte, damaged cell membranes, disintegrated central vein and damaged hepatic sinusoids; B) of the rats exposed to CCl4 was noticed compared to the histoarchitecture of hepatocytes in control rats (A). Minimum disturbance of the hepatic cellular structure was noticed in both the C. hirsutus extract treated groups (C and D), when compared to CCl4 alone treated rats.
Fig. 1 Light microscopic photographs of liver histology in control and experimental rats. (A) Control mice showing normal histoarchitecture of hepatocytes, (B) A liver section of rat received CCl4 showing hepatocellular damage, (C) A liver section of rat received CCl4 + 250 mg/kg b·wt. EECH showing minimal damage to liver cells, and (D) A liver section of rat received CCl4 + 500 mg/kg b. wt. EECH showing almost normal architecture of liver. Hematoxilin and Eosin stain; Original magnification ×10.
4 Discussion
Hepatic injuries caused by CCl4 are the specific symptoms of xenobiotic-induced hepatotoxicity and regularly used models for the screening of hepatoprotective drugs [Citation30]. The major causes of CCl4 induced hepatic damage is generation of free radicals causing lipid peroxidation and contaminant decrease in antioxidant enzymes [Citation30]. As free radicals play a vital role in CCl4-induced hepatotoxicity, it seems reasonable that compounds that counteract such radicals may have hepatoprotective effect. Several natural products have been reported to protect against CCl4-induced hepatotoxicity [Citation31Citation[32]Citation[33]–Citation34] .
Spotlight of the present study was on investigating the role of C. hirsutus against the hepatotoxicity of CCl4 and to understand the possible mode of action in hepatoprotection. Direct evidence of CCl4 causing liver damage was observed by the manifestation of alterations in various hepatic parameters {elevated MDA concentration, depleted levels of non-enzymatic antioxidants (GSH, Vitamin C and E), reduced levels of antioxidant enzymes (SOD, CAT, GPx)} in CCl4 alone administered (Group II) rats in comparison to control rats. The increase in levels of SGOT, SGPT, ALP and LDH in Group II rats also suggests the hepatotoxicity in these rats. To elucidate the hepatoprotective activity of ethanol extract of C. hirsutus, this plant extract was administered in the concentrations of 250 and 500 mg/kg body weight to the respective group of rats. This concentration of the extract was chosen on the basis of earlier related studies suggestion that this dose would be effective [Citation14,Citation35] .
Serum transaminases, ALP, and LDH are well chosen specific markers of hepatic damage [Citation36]. CCl4 induced damage of hepatocytes can leads to effect on their transport function and membrane permeability, leading to these enzymes leakage from the cells and releasing them into the blood stream [Citation37]. In the present study, rats induced with CCl4 showed significant elevation of all these enzymes demonstrating serious damage of hepatocytes. Hyper bilirubinaemia which is the indicator of severe hepatic damage [Citation38], was noticed in rats treated with CCl4. Treatment with ethanol extract of C. hirsutus, restored all the altered levels of liver marker enzymes and bilirubin in a dose dependent manner demonstrating its curative potential to maintain the normal functional status of the hepatic tissue.
The level of TBARS is related to lipid peroxidation and the lipid peroxide levels in liver were found to be considerably elevated in CCl4-challenged rats [Citation39]. These free radicals trigger cell damage through two mechanisms namely covalent binding to cellular macromolecules and lipid peroxidation which affect the ionic permeability of the membrane preventing the disintegration and solubilization of membrane structure. The reduced TBARS formation after treatment with the EECH may be attributed to the antioxidant activity of the plant by scavenging the CCl3-radical generated due to the metabolic transformation of CCl4 in the liver. The antioxidant enzymes SOD, catalase and GPx constitute primary defense mechanisms against reactive oxygen species (ROS).
The antioxidant enzymes SOD, CAT and GPx constitute a mutually supportive group of defense against ROS. The decrease in the levels of these antioxidant enzymes in liver of CCl4-treated rats may be due to the elevated lipid peroxidation or inactivation of the enzymes by cross-linking with malondialdehyde; this will cause massive accumulation of free radicals, which could further stimulate lipid peroxidation [Citation40]. Administration of EECH significantly increased the activities of SOD, CAT, and GPx in the rats induced with CCl4; this suggests administration of C. hirsutus ethanol extract appears to have brought about a remarkable improvement in the activity of these antioxidant enzymes in CCl4 intoxicated rats. Similar improvement of antioxidant enzymes by supplementation of phytochemicals and extracts such as polyphenols from Bauhinia hookeri [Citation41] and Juniperus phoenicea [Citation42] extract against CCl4 induced oxidative stress was recently reported.
Glutathione, Vitamin C and E protect cells against CCl4 induced toxicity by preventing the formation of electrophiles, oxidation of proteins sulfhydryl groups and by scavenging free radicals [Citation43]. The key protective mechanism of glutathione against oxidative stress is acting as a co-factor for several detoxifying enzymes and also regenerate Vitamins C and E back to their active forms [Citation38]. In the present study, rats administered with two doses of EECH leaves had observed significantly higher levels of GSH, vitamin C and E when compared with CCl4 induced group and also near to the values obtained in normal rats. These results suggests that C. hirsutus extract appears to be a potent hepatoprotective agent against CCl4 induced oxidative damage by maintaining the GSH, vitamin C and E levels. In the present study, replenishment of GSH, Vitamins C and E by the supplementation of plant extract to confer protection against CCl4-induced liver damage, goes in accordance with Lavanya et al. (2012) [Citation4] and Manubolu et al. (2014) [Citation22] reports.
To provide direct evidence on protective effect of EECH against CCl4 induced toxicity, histopathological changes in liver tissues were evaluated. Marked disruption of the cellular structure of liver was noted in rats challenged with CCl4. Minimal disruption of the hepatocellular structure was noted in two doses of EECH treated groups of rats; these results provided supportive evidence for biochemical analysis (SGOT, SGPT, SALP, and LDH activities and MDA levels approximated to the levels in normal rats).
In conclusion, the results of this study indicated that EECH has a potent hepatoprotective activity on carbon tetrachloride induced hepatocellular destruction in rats. From the present experiments it was elucidated that the hepatoprotective nature of EECH may be due to its antioxidative and free radical scavenging properties. It also reveals that C. hirsutus is a robust medicinal herb for developing as a phytomedicine against hepatic disorders and further studies are essential in the direction of isolating the active principle of the plant which acts as an effective antihepatotoxic agent.
Authors’ contribution
GL: Designed the research, conducted the research, analyzed the data, drafted and revised the manuscript. MM: helped with portions of conducting research and revised the manuscript.
PK and PRP: revised the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
The authors declare that there are no conflicts of interest. None of the authors had any financial or personal interests in any company or organization sponsoring the research currently or at the time of research was done.
References
- S.WuY.YueH.TianZ.LiX.LiW.Heet al.Carthamus red from Carthamus tinctorius L. exerts antioxidant and hepatoprotective effect against CCl4-induced liver damage in rats via the Nrf2 pathwayJ Ethnopharmacol14822012570578
- S.LiH.-Y.TanN.WangZ.-J.ZhangL.LaoC.-W.Wonget al.The role of oxidative stress and antioxidants in liver diseasesInt J Mol Sci161120152608726124
- N.ChengN.RenH.GaoX.LeiJ.ZhengW.CaoAntioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in miceFood Chem Toxicol552013234240
- G.LavanyaS.P.VoravuthikunchaiN.H.TowatanaAcetone extract from Rhodomyrtus tomentosa: a potent natural antioxidantEvidence Based Complementary Altern Med201220128
- G.LavanyaR.SivajyothiM.ManjunathP.ParthasarathyFate of biomolecules during carbon tetrachloride induced oxidative stress and protective nature of Ammannia baccifera Linn.: a natural antioxidantInt J Green Pharm342009300305
- M.ManjunathG.LavanyaR.SivajyothiO.Vijaya Sarathi ReddyAntioxidant and radical scavenging activity of Actiniopteris radiata (Sw.) linkAsian J Exp Sci25120117380
- B.E.MyagmarE.ShinnoT.IchibaY.AniyaAntioxidant activity of medicinal herb Rhodococcum vitis-idaea on galactosamine-induced liver injury in ratsPhytomedicine1152004416423
- G.D.ChaudharyP.KambojI.SinghA.N.KaliaHerbs as liver savers-a reviewIndian J Nat Prod Resour142010397408
- K.PathakotiL.GoodlaM.ManuboluT.TencomnaoMetabolic alterations and the protective effect of punicalagin against glutamate-induced oxidative toxicity in HT22 cellsNeurotox Res312017521531
- Z.A.ZakariaM.S.RofieeM.N.SomchitA.ZurainiM.R.SulaimanL.K.Tehet al.Hepatoprotective activity of dried- and fermented-processed virgin coconut oilEvidence Based Complementary Altern Med201120118
- P.V.Torres-DuranR.Miranda-ZamoraM.C.Paredes-CarbajalD.MascherJ.Ble-CastilloJ.C.Diaz-Zagoyaet al.Studies on the preventive effect of Spirulina maxima on fatty liver development induced by carbon tetrachloride, in the ratJ Ethnopharmacol6421999141147
- E.Al-SayedO.Martiskainenet al.Hepatoprotective and antioxidant effect of Bauhinia hookeri extract against carbon tetrachloride-induced hepatotoxicity in mice and characterization of its bioactive compounds by HPLC-PDA-ESI-MS/MSBiomed Res Int2014201414 245171
- S.ThakareH.JainS.PatilU.UpadhyayHepatoprotective effect of Cocculus hirsutus on bile duct ligation-induced liver fibrosis in Albino wistar ratsBangladesh J Pharmacol42009126130
- G.SarvankumarK.RajeshS.SengottuveluG.ChiranjeevD.HareeshEvaluation of analgesic and anti-inflamatory activity of methanolic extract of Cocculus hirsutus leavesInt Res J Pharm2122011230234
- B.SangameswaranB.JayakarAnti-diabetic and spermatogenic activity of Cocculus hirsutus (L) DielsAfr J Biotechnol1610200712121216
- OECD. Guidelines for testing of chemicals. Revised draft guideline 423: Acute oral toxicity; 2000.
- N.AnusuyaK.RajuS.ManianHepatoprotective and toxicological assessment of an ethnomedicinal plant Euphorbia fusiformis Buch.–Ham.ex D.DonJ Ethnopharmacol12722010463467
- N.SushmaT.DevasenaAqueous extract of Trigonella foenum graecum (fenugreek) prevents cypermethrin-induced hepatotoxicity and nephrotoxicityHum Exp Toxicol2942010311319
- G.LavanyaM.ManjunathR.SivajyothiP.R.ParthasarathySafety evaluation of the ethanol extract of Ammannia baccifera (Lythraceae): assessment of acute and subacute toxicityJ Pharm Res311201026342637
- A.JainM.SoniL.DebA.JainS.P.RoutV.B.Guptaet al.Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica Roxb. leavesJ Ethnopharmacol115120086166
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the folin phenol reagentJ Biol Chem19311951265275
- M.ManuboluL.GoodlaS.RavillaJ.ThanasekaranP.DuttaK.Malmlofet al.Protective effect of Actiniopteris radiata (Sw.) Link. against CCl4 induced oxidative stress in albino ratsJ Ethnopharmacol15332014744752
- U.N.TripathiD.ChandraThe plant extracts of Momordica charantia and Trigonella foenum-graecum have anti-oxidant and anti-hyperglycemic properties for cardiac tissue during diabetes mellitusOxid Med Cell Longev252009290296
- H.EbaidS.A.BashandyI.M.AlhazzaA.RadyS.El-ShehryFolic acid and melatonin ameliorate carbon tetrachloride-induced hepatic injury, oxidative stress and inflammation in ratsNutr Metab101201317437075
- S.Kumar MishraP.SinghS.K.RathProtective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in miceMalar Res Treat2013201310
- M.MessarahW.AmamraA.BoumendjelL.BarkatI.BouaslaC.Abdennouret al.Ameliorating effects of curcumin and vitamin E on diazinon-induced oxidative damage in rat liver and erythrocytesToxicol Ind Health1820122012
- T.SanthraniE.MaheswariG.R.SaraswathyAmelioration of carbamazepine induced oxidative stress and hematotoxicity by vitamin CSpatula DD232012173180
- S.RamanathanA.KuppusamyV.M.NallasamyP.PerumalAntitumor effects and antioxidant role of Scutia myrtina in N-Nitroso-diethylamine (NDEA) induced hepatocellular carcinoma in ratsAsian J Pharm Biol Res1220117178
- J.J.DhanasekaranM.GanapathyHepatoprotective effect of Cassia auriculata L. leaf extract on carbon tetrachloride intoxicated liver damage in Wister Albino ratsAsian J Biochem612011104112
- S.ChenL.ZouL.LiT.WuThe protective effect of glycyrrhetinic acid on carbon tetrachloride-induced chronic liver fibrosis in mice via upregulation of Nrf2PLoS One81201314
- H.Z.HuoB.WangY.K.LiangY.Y.BaoY.GuHepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in ratsInt J Mol Sci1210201165296543
- M.RudnickiM.M.SilveiraT.V.PereiraM.R.OliveiraF.H.ReginattoF.Dal-Pizzolet al.Protective effects of Passiflora alata extract pretreatment on carbon tetrachloride induced oxidative damage in ratsFood Chem Toxicol4542007656661
- R.BhattacharjeeP.C.SilProtein isolate from the herb, Phyllanthus niruri L. (Euphorbiaceae), plays hepatoprotective role against carbon tetrachloride induced liver damage via its antioxidant propertiesFood Chem Toxicol4552007817826
- N.M.FahmyE.Al-SayedM.M.Abdel-DaimM.KaronenA.N.SingabProtective effect of Terminalia muelleri against carbon tetrachloride-induced hepato and nephro-toxicity in mice and characterization of its bioactive constituentsPharm Biol5422016303313
- S.BadoleN.PatelS.BodhankarB.JainS.BhardwajAntihyperglycemic activity of aqueous extract of leaves of Cocculus hirsutus (L.) Diels in alloxan-induced diabetic miceIndian J Pharmacol381200649
- J.OzerM.RatnerM.ShawW.BaileyS.SchomakerThe current state of serum biomarkers of hepatotoxicityToxicology24532008194205
- S.C.JeongS.M.KimY.T.JeongC.H.SongHepatoprotective effect of water extract from Chrysanthemum indicum L. flowerChin Med81201317498546
- M.T.OlaleyeA.C.AkinmoladunA.A.OgunboyeA.A.AkindahunsiAntioxidant activity and hepatoprotective property of leaf extracts of Boerhaavia diffusa Linn against acetaminophen-induced liver damage in ratsFood Chem Toxicol488–9201022002205
- O.TarcinD.G.YavuzB.OzbenA.TelliA.V.OguncM.Yukselet al.Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjectsJ Clin Endocrinol Metab9410200940234030
- G.D.HungP.C.LiH.S.LeeH.M.ChangC.T.ChienK.L.LeeGreen tea extract supplementation ameliorates CCl4-induced hepatic oxidative stress, fibrosis, and acute-phase protein expression in ratJ Formos Med Assoc111102012550559
- E.Al-SayedM.M.Abdel-DaimO.E.KilanyM.KaronenJ.SinkkonenProtective role of polyphenols from Bauhinia hookeri against carbon tetrachloride-induced hepato- and nephrotoxicity in miceRen Fail377201511981207
- A.LaouarF.KlibetE.BourogaaA.BenamaraA.BoumendjelA.Chefrouret al.Potential antioxidant properties and hepatoprotective effects of Juniperus phoenicea berries against CCl4 induced hepatic damage in ratsAsian Pac J Trop Med1032017263269
- J.M.VeigasR.ShrivasthavaB.NeelwarneEfficient amelioration of carbon tetrachloride induced toxicity in isolated rat hepatocytes by Syzygium cumini Skeels extractToxicol In Vitro226200814401446
