Abstract
Pulmonary fibrosis is a progressive fatal lung disorder with significantly high mortality rates. Bleomycin (BLM) is one of the most commonly used chemotherapeutic agents for treatment of several carcinomas. The most severe adverse effect of BLM is pulmonary toxicity; therefore, BLM has been repeatedly reported to be considered amongst the most widely used agents for induction of experimental pulmonary fibrosis. In the current study, flavocoxid has been investigated for its ability to ameliorate BLM-induced pulmonary fibrosis. BLM was instilled intratracheally and flavocoxid was administered orally (20 mg/kg) for 5 weeks; one week pre- and 5 weeks post BLM instillation. Flavocoxid significantly decreased lung/body weight index, BALF’s lactate dehydrogenase activity, total protein content and total cell count, lymphocyte and neutrophil counts. Flavocoxid significantly decreased lung MDA content, increased lung GSH content, SOD activity, serum total antioxidant capacity and decreased lung NO content. Moreover, flavocoxid reduced lung content of IL-10. In addition, flavocoxid significantly ameliorated histological changes and prevented collagen deposition with paralleled decrease in lung hydroxyproline content. In conclusion; flavocoxid can be proposed to be a potential therapeutic agent for management of pulmonary fibrosis.
Keywords:
1 Introduction
Pulmonary fibrosis is a progressive lethal lung disorder. It is the end stage of a wide range of lung inflammatory conditions. Loss of alveolar structure, accretion of myofibroblasts, remodeling of lung parenchyma and excessive extracellular matrix depositions are the main characteristic features of pulmonary fibrosis [Citation1].
Pulmonary fibrosis is amongst the most common interstitial lung diseases affecting over 5 million individuals worldwide with a mean survival time of about 3 years [Citation2]. In spite of extensive researches, there are no reports about any medication that can significantly ameliorate pulmonary fibrosis [Citation3]. The only current effective approach is lung transplantation. Therefore, the search for novel drugs with significant efficacy and tolerability for the pulmonary fibrosis is inevitable [Citation4].
Pulmonary fibrosis can developed as an adverse toxic effect of anti-neoplastic drugs such as bleomycin (BLM). Apart from this, cigarette smoking and inhaling mineral dusts/asbestos are added factors implicated in its pathogenesis [Citation5].
Increased evidences suggest that pulmonary fibrosis mainly developed post alveolar epithelial injury and abnormal wound healing involving inflammation and T-helper type 2 cytokines, epithelial apoptosis and absence of appropriate re-epithelialization, fibroblast-myofibroblast migration and proliferation, epithelial mesenchymal transformation, and excessive extracellular matrix (ECM) deposition [Citation6].
BLM is a chemotherapeutic antibiotic used for management of lymphomas, testicular cancer, and carcinomas amongst several types of tumors. It has been reported to induce marked functional and biochemical changes promoting pulmonary fibrosis [Citation7]. Bronchial metaplasia, reactive macrophages recruitment, atypical alveolar epithelial cells, fibrinous edema, and interstitial fibrosis are amongst microscopical changes can be induced by BLM [Citation8].
BLM-induced pulmonary fibrosis in rats and mice has been reported to be a useful tool to study mechanisms involved in the progression of human pulmonary fibrosis and the impact of various drugs on its progression. BLM induces reactive oxygen species (ROS) generation, which binds to DNA causing DNA damage, postulated to initiate inflammatory and fibro-proliferative responses. Furthermore, BLM is reported to promote the depletion of endogenous antioxidant defences, exacerbating oxidant mediated tissue injury [Citation9].
Flavocoxid is a mixture of two flavonoids catechin and baicalin [Citation10]. It is marketed as an FDA-regulated medical food, for management of osteoarthritis in the United States. Flavocoxid is the only currently marketed prescribed anti-inflammatory agent that modulates cycloxygenase (COX) enzymes via an anti-peroxidase activity. It also inhibits 5-lipoxygenase (5-LOX)-mediated leukotrines (LT) production. Flavocoxid has a wide range of strong antioxidant activities mainly via down-regulation of inducible inflammatory gene expression and neutralization of ROS, preventing the conversion of arachidonic acid to oxidized lipids [Citation11].
The current study protocol focused on the evaluation the ability of flavocoxid to attenuate BLM-induced pulmonary fibrosis in a rat model and to draw a schematic conclusion about mechanisms involved.
2 Materials and methods
2.1 Experimental animals
Adult male Sprague Dawely rats (170–220 g) were purchased from Merck Research Center, Faculty of medicine, Mansoura University; they were kept under constant environmental and nutritional conditions throughout the experimental period. The research protocol complies with the ethical guidelines of experimental research; “Research Ethics Committee”, Faculty of Pharmacy, Mansoura University, Egypt in accordance with “Guide for the Care and Use of Laboratory Animals”, 1996.
2.2 Drugs and chemicals
Flavocoxid was purchased from Pimus pharmaceutical Inc. (Scottsdale, AZ, USA) and BLM was purchased from Nippon Kayaku Co. (Ltd., Tokyo, Japan). Ehrlich's reagent (p-dimethylaminobenzaldehyde), Chloramine-T, n-Propanol, Perchloric acid and thiopental sodium were purchased from Sigma Aldrich chemical Co. (St. Louis, MO, USA).
2.3 Experimental protocol
2.3.1 Induction of pulmonary fibrosis
Pulmonary fibrosis was induced by intratracheal instillation of BLM (5 mg/kg) as sulfate salt dissolved in 0.1 ml of normal saline [Citation12]. Rats were anesthetized using thiopental sodium (20 mg/kg body weight, I.P.). A midline incision was made in the neck, the trachea was exposed and BLM was instilled. Rats were kept in vertical position and rotated several times to ensure uniform distribution of BLM within the lung tissues. The incision was surgically sutured sodium fusidate 2% was applied topically to the wound.
2.3.2 Animals grouping
Rats were randomly allocated to three experimental groups (12 rats/group) as follows: Normal control; 0.1 ml of normal saline was instilled to the trachea as previously described and rats received 0.2 ml of 0.5% carboxymethylcellulose (CMC) orally once daily for 5 weeks, BLM control: BLM was instilled intratracheally (5 mg/kg) and rats received 0.2 ml of 0.5% CMC orally once daily for 5 weeks, flavocoxid treated group: rats were treated with flavocoxid (20 mg/kg, oral) suspended in 0.5% CMC daily for one week prior to BLM instillation and for further 4 weeks post instillation for an overall period of five weeks of flavocoxid administration.
Four weeks post BLM instillation; rats were deeply anesthetized with thiopental sodium. Blood samples were collected via puncture of retro-orbital venous plexus, sera were separated and used immediately for biochemical assessments. Lungs were harvested, rinsed in ice-cold saline and weighed for calculation of lung/body weight index. The left lobes from all the lungs were isolated for preparation of lung homogenate and the right lobes were separated for histopathological examination.
2.3.3 Collection of bronchoalveolar lavage fluid (BALF)
The thoracic cavity was opened and the tracheas were exposed, cannulated and 6 ml sterile 0.9% saline (3 times, 2 ml/time) were slowly infused into the lungs. 50–70% recovery was retrieved after compressing the chest gently several times. BALF was centrifuged at 2000 rpm, 4 °C for 10 min using cooling centrifuge. The sedimented cell pellets were pooled and re-suspended in 500 μl of sterile saline to quantify inflammatory cell contents; total and differential cell counts.
2.4 Assessment of BALF total protein content and lactate dehydrogenase (LDH) activity
Total protein content was assessed according to the method of Smith, Krohn [Citation13] using commercial kit (Thermo Scientific, Rockford, USA) as instructed by manufacturer. Enzymatic LDH activity was assessed using commercial kit (Human diagnostics, Wiesbaden, Germany) according to Henry [Citation14] as instructed by manufacturer.
2.5 Preparation of lung homogenate and biochemical assessment of nitric oxide (NO), malondialdehyde (MDA), reduced glutathione (GSH) contents and superoxide dismutase (SOD) activity
The isolated left pulmonary lobes were rinsed, weighed and homogenized in KCl (1.15%, pH 7.4) to yield 10% w/v tissue homogenate [Citation15]. The homogenate was centrifuged at 2000 rpm, 4 °C for 15 min, and the supernatant was separated and used immediately for assessment oxidative/antioxidative stress biomarkers (MDA, GSH and SOD) as well as nitric oxide using commercially available Biodiagnostic assay kits (Giza, Egypt), as instructed by manufactures according to methods described by Ohkawa, Ohishi [Citation16], Ellman [Citation17], Marklund and Marklund [Citation18] and Montgomery and Dymock [Citation19] respectively.
2.6 Biochemical assessment of serum total antioxidant capacity (TAC)
Serum total antioxidant capacity was determined using commercially available kits (Bio-diagnostic, Giza, Egypt)) according to the supplied manufacturer’s instructions as described by [Citation20].
2.7 Assessment of lung interleukin-10 (IL-10) content
Lung content of IL-10 was quantilified using commercially available enzyme-linked immunosorbent assay (ELISA) kit (Uscn Life Science, INC. USA), according to the supplied manufacturer's instructions.
2.8 Quantification of lung hydroxyproline and collagen content
Lung hydroxyproline content was determined using colorimetric method described by Bergman and Loxley [Citation21]. Lung collagen content calculated by multiplication of hydroxyproline content by 13.5 [Citation22].
2.9 Histopathological examination of hematoxylin-eosin (H&E) and Masson’s Trichrome stained lung specimen
The right upper pulmonary lobe was harvested, rinsed with ice-cold saline and fixed in 10% neutral-buffered formalin, embedded in paraffin wax, sectioned (6 μm) and stained with H&E and Masson’s trichrome. The tissues were examined under a microscope in a random order and the pathologist was blinded to the experimental groups. The structural alterations of tissue were assessed based on the degree hemorrhages, emphysema, alveolar wall thickening, inflammatory lesions and collagen deposition or fibrosis [Citation23]. Alveolitis and fibrosis were semi-quantitatively assessed using H&E staining and Masson staining as described by Szapiel, Elson [Citation24]
For percentage area of fibrosis quantification, Slides were photographed using Olympus® digital camera installed on Olympus® microscope with 1/2 X photo adaptor, using 20 X objective. The images were then analyzed using Intel® Core I3® based computer using VideoTest® Morphology® software (Russia) with a specific built-in routine for area. Five slides from each rat were prepared and five random fields from each slide were analyzed.2.10 Statistical analysis
Results are expressed as mean ± standard error of mean (SEM). Statistical analysis and graphing were carried out using Graphpad software Prism V5 (Graphpad Software Inc., San Diego, CA, USA). Significant differences between groups were determined using one-way analysis of variance (ANOVA) followed by Tukey-Kramers multiple comparisons post hoc test for parametric measures, Kruskal-Wallis test followed by Dunn post hoc test for non-parametric measures and linear regression for construction of standard curves. Statistical significance was accepted at p < .05.
3 Results
3.1 Effect of daily oral flavocoxid on lung/body weight index
As shown in , lung/body weight index significantly increased after BLM instillation compared to normal control. Daily oral flavocoxid (20 mg/kg) for 5 weeks significantly decreased lung/body weight index compared to BLM control.
Table 1 Effect of daily oral flavocoxid on Lung/Body weight index and total and differential cell count.
3.2 Effect of daily oral flavocoxid on total and differential cell counts
Intratracheal instillation of BLM caused significant elevation in total cell, lymphocyte, and neutrophil counts in the BALF compared to normal control. Daily oral flavocoxid for 5 weeks significantly reduced both total cell and neutrophil counts compared to BLM control. There was still a significant elevation in total cell and lymphocyte counts compared to normal control ().
3.3 Effect of daily oral flavocoxid on BALF total protein content and LDH activity
BALF’s total protein content significantly escalated after BLM instillation by compared to normal healthy rats. Daily oral flavocoxid significantly reversed BLM-induced increase in BALF’s total protein content (). Meanwhile, Intratracheal instillation of BLM significantly increased BALF-LDH activity in comparison to normal control group. Daily oral flavocoxid significantly decreased BALF-LDH activity by about 46% in comparison to BLM control ().
Fig. 1 Effect of daily oral flavocoxid on BALF total protein content and lactate dehydrogenase (LDH) activity. Data are expressed as mean ± SEM, n = 12. Data were statically analyzed using One-Way ANOVA followed by Tukey- Kramer multiple comparisons test (p < .05). *,$Significantly different from normal and BLM controls respectively.

3.4 Effect of daily oral flavocoxid on lung oxidant/antioxidant biochemical parameters and total antioxidant capacity
Intratracheal instillation of BLM significantly increased lung MDA content with concomitant significant decline in lung SOD activity, GSH content and serum TAC in comparison to normal control. Flavocoxid significantly opposed BLM-induced alterations, lung MDA content significantly decreased while, lung GSH content, SOD activity and serum TAC significantly increased in comparison to BLM control ().
Fig. 2 Effect of daily oral flavocoxid on lung oxidant/antioxidant biochemical parameters (malondialdehyde (MDA) content, superoxide dismutase (SOD) activity, reduced glutathione (GSH) content), serum total antioxidant capacity (TAC) and lung nitric oxide (NO) content. Data are expressed as mean ± SEM, n = 12. Data were statically analyzed using One-Way ANOVA followed by Tukey-Kramer multiple comparisons test (p < .05). *,$Significantly different from normal and BLM controls respectively.
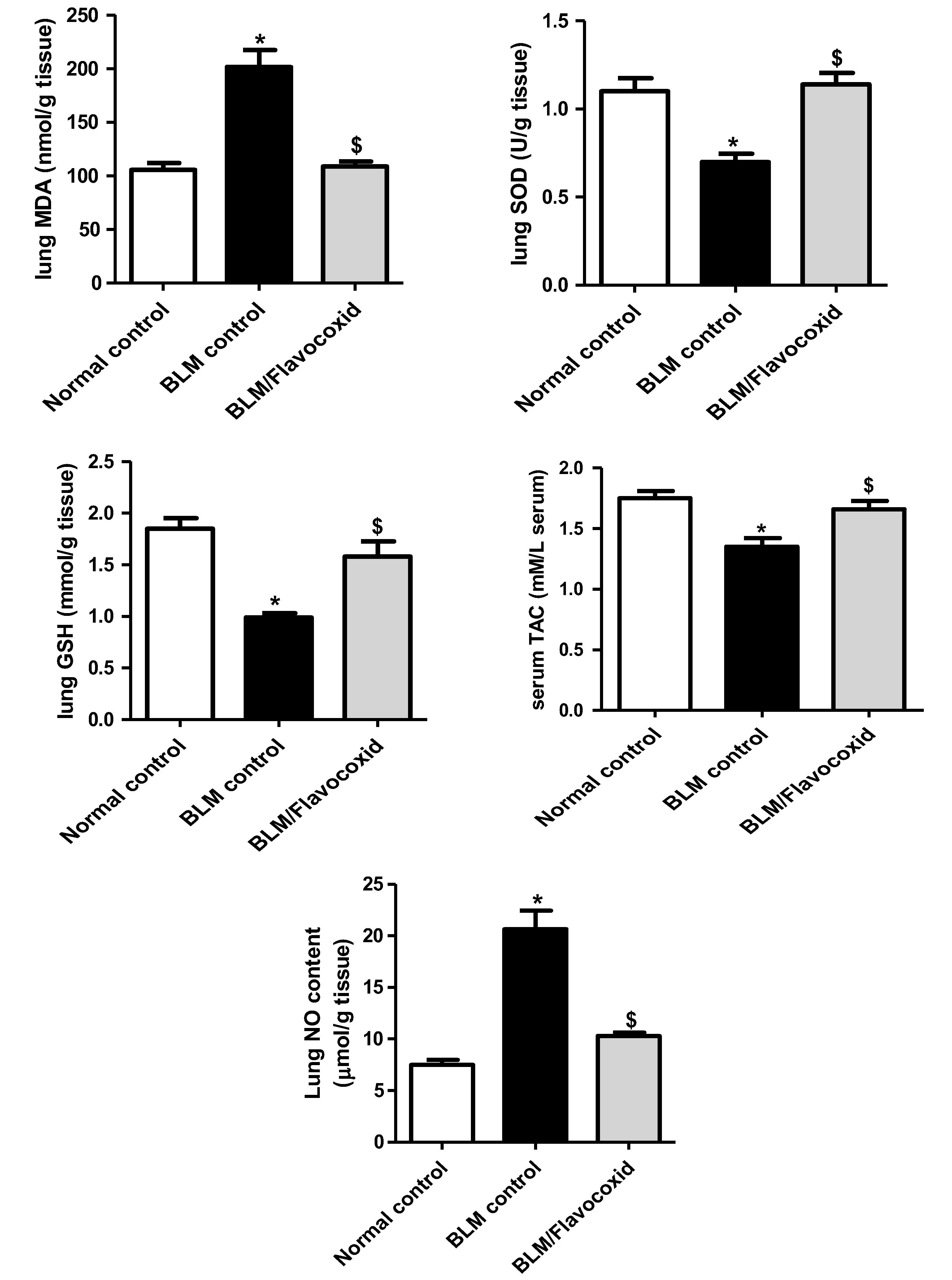
3.5 Effect of daily oral flavocoxid on lung nitric oxide (NO) content
Lung NO content significantly elevated in BLM control group in comparison to normal control group. Flavocoxid significantly reduced lung NO content compared to BLM control ().
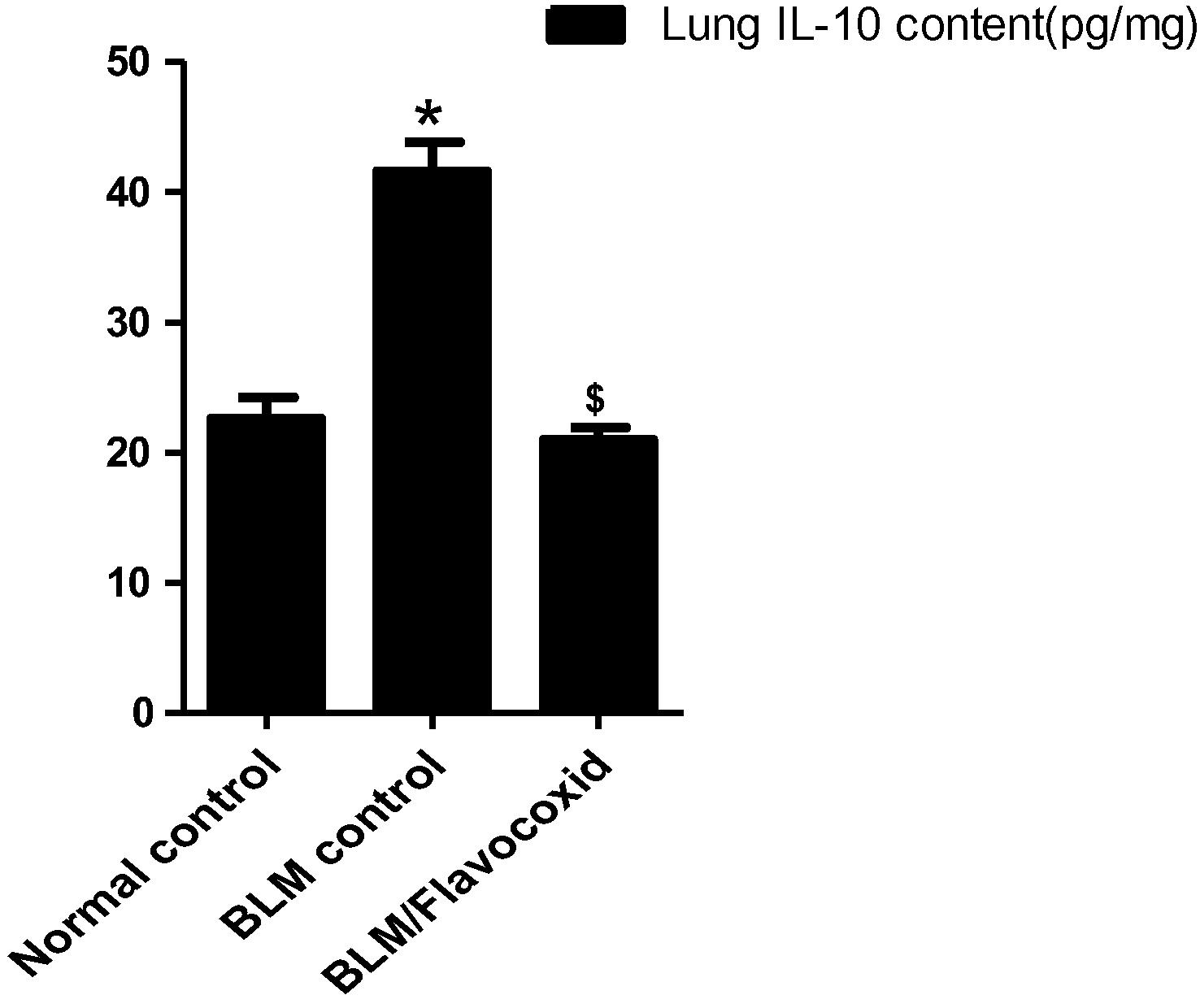
3.6 Effect of daily oral flavocoxid on lung Interleukin-10 (IL-10) content
BLM control group revealed a significant increase in lung IL-10 content in comparison to normal control group. Daily oral flavocoxid significantly reduced lung IL-10 content compared to BLM group ().
Fig. 3 Effect of daily oral flavocoxid on lung n Interleukin-10 (IL-10) content. Data are expressed as mean ± SEM, n = 12. Data were statically analyzed using One-Way ANOVA followed by Tukey-Kramer multiple comparisons test (p < .05). *,$Significantly different from normal and BLM controls respectively.
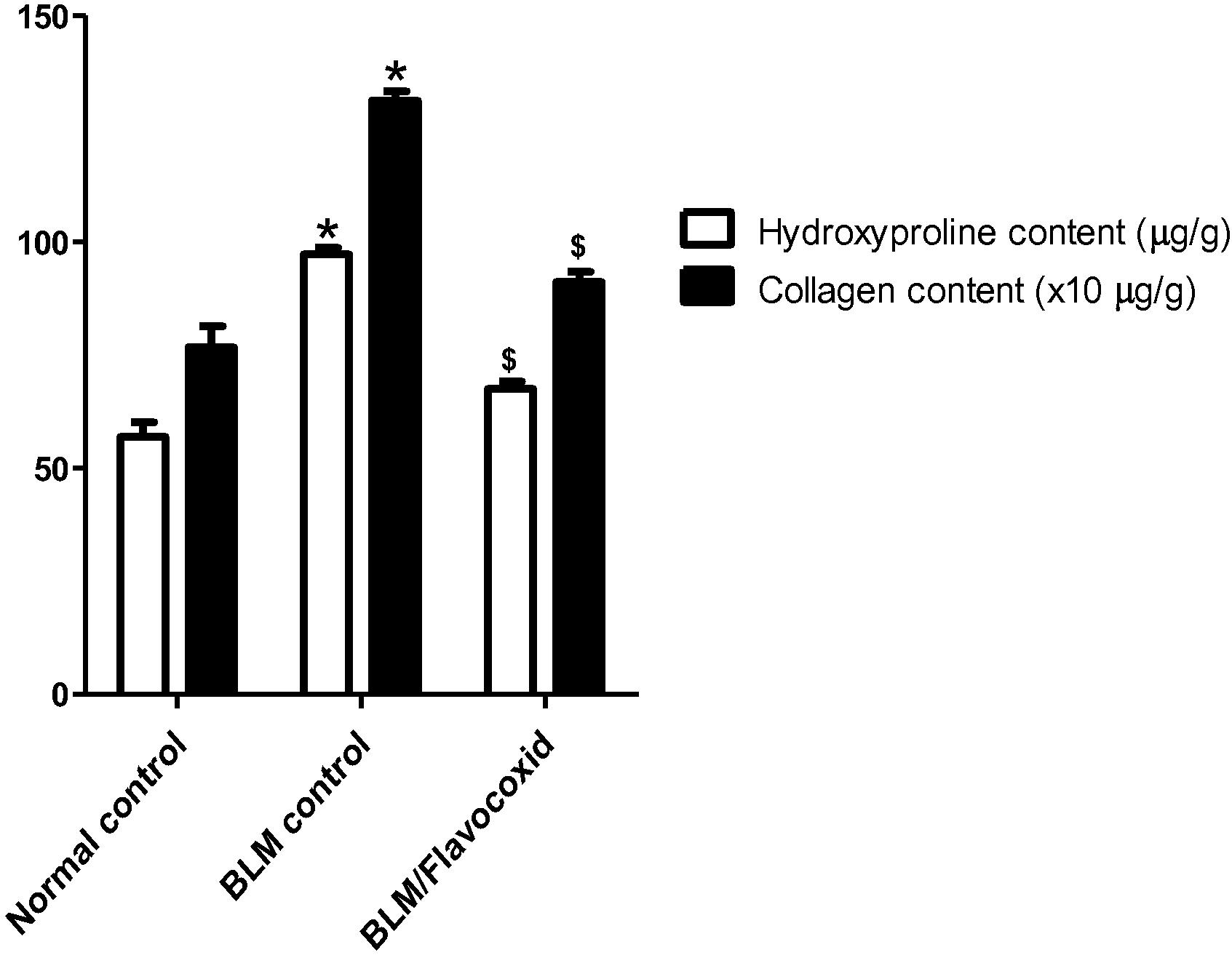
3.7 Effect of daily oral flavocoxid on lung hydroxyproline and collagen contents
Significant increase in lung hydroxyproline and collagen contents was observed following intratracheal BLM instillation when compared to the normal control group. On the other hand, daily oral flavocoxid significantly decreased lung hydroxyproline and collagen contents compared to BLM control ().
Fig. 4 Effect of daily oral flavocoxid on lung hydroxyproline and collagen content. Data are expressed as mean ± SEM, n = 12. Data were statically analyzed using One-Way ANOVA followed by Tukey-Kramer multiple comparisons test (p < .05). *,$Significantly different from normal and BLM controls respectively.
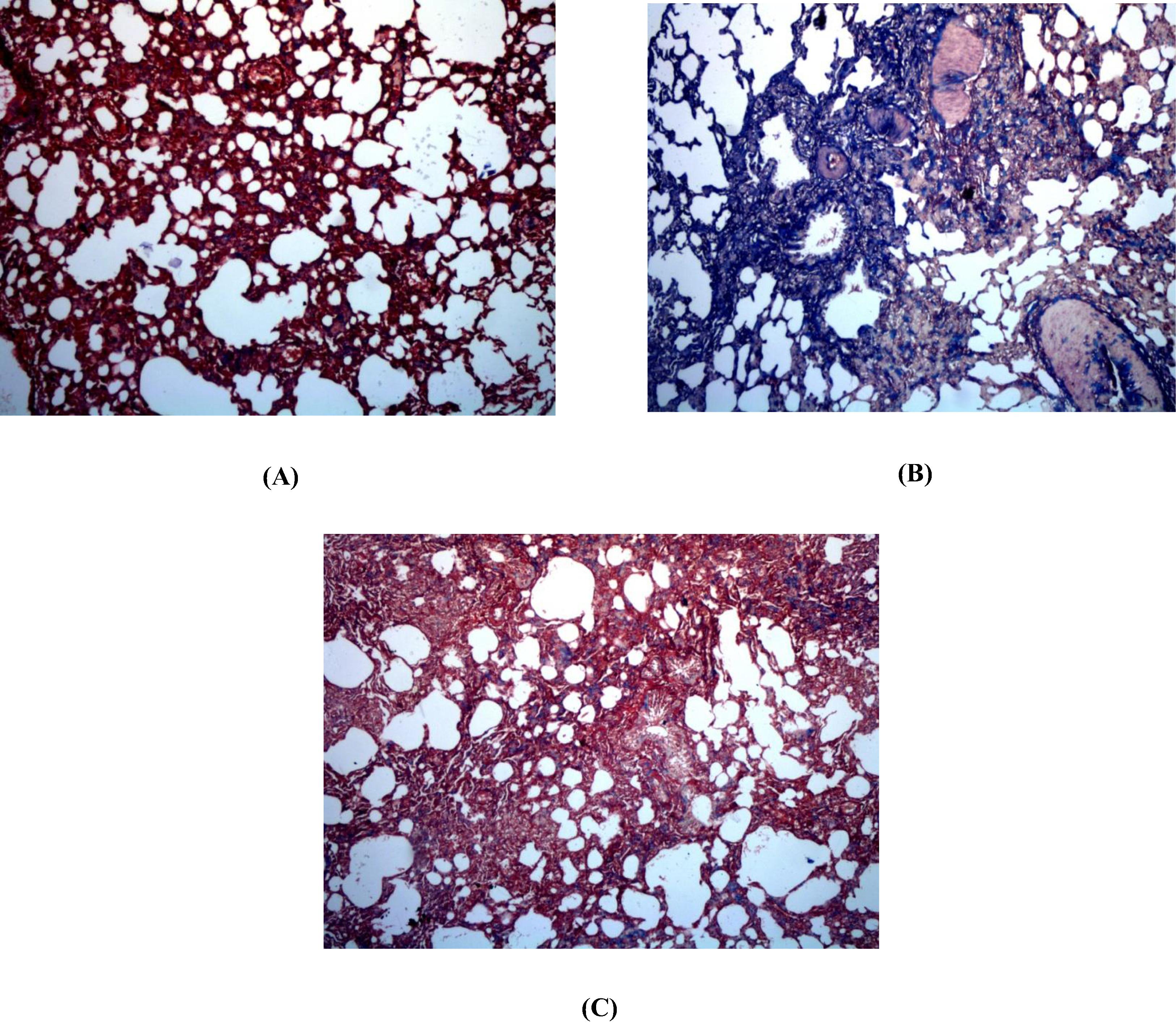
3.8 Histopathological examination of lung specimen stained with H&E and Masson’s Trichrome
Normal control group revealed ideal lung architecture (A). On the other hand, BLM control examination revealed distorted architecture of lung tissue; moderate to severe hemorrhage, emphysema, areas of increased thickening of alveolar septa, leukocytic infiltration in alveolar walls, and fibroblasia were detected (B & C) compared to normal control group. Flavocoxid significantly ameliorated inflammatory lesions developed by BLM. Pulmonary histoarchitectural changes in animals treated with flavocoxid were only mild to moderate degree of septal thickening with few inflammatory cells infiltration. Emphysematous changes and alveolar hemorrhages were significantly reduced (D). Inflammatory lesion scores of BLM control group was significantly high compared to normal group. Szapiel scores and percentage area of fibrosis of flavocoxid treated group significantly decreased compared to BLM control ().
Fig. 5 Effect of daily oral flavocoxid on lung histopathological changes using H&E staining (400×). (A) Normal control, intact lung architecture with absence of any evidence of inflammation, edema, hemorrhage, emphysema or fibrosis (B) BLM control, revealing moderate hemorrhages, emphysema, areas of increased thickening of alveolar septa, leukocytic infiltration in alveolar walls, and fibroblasia (C) Flavocoxid, revealing mild to moderate degree of septal thickening with few inflammatory cells infiltration, emphysematous changes and alveolar hemorrhage.
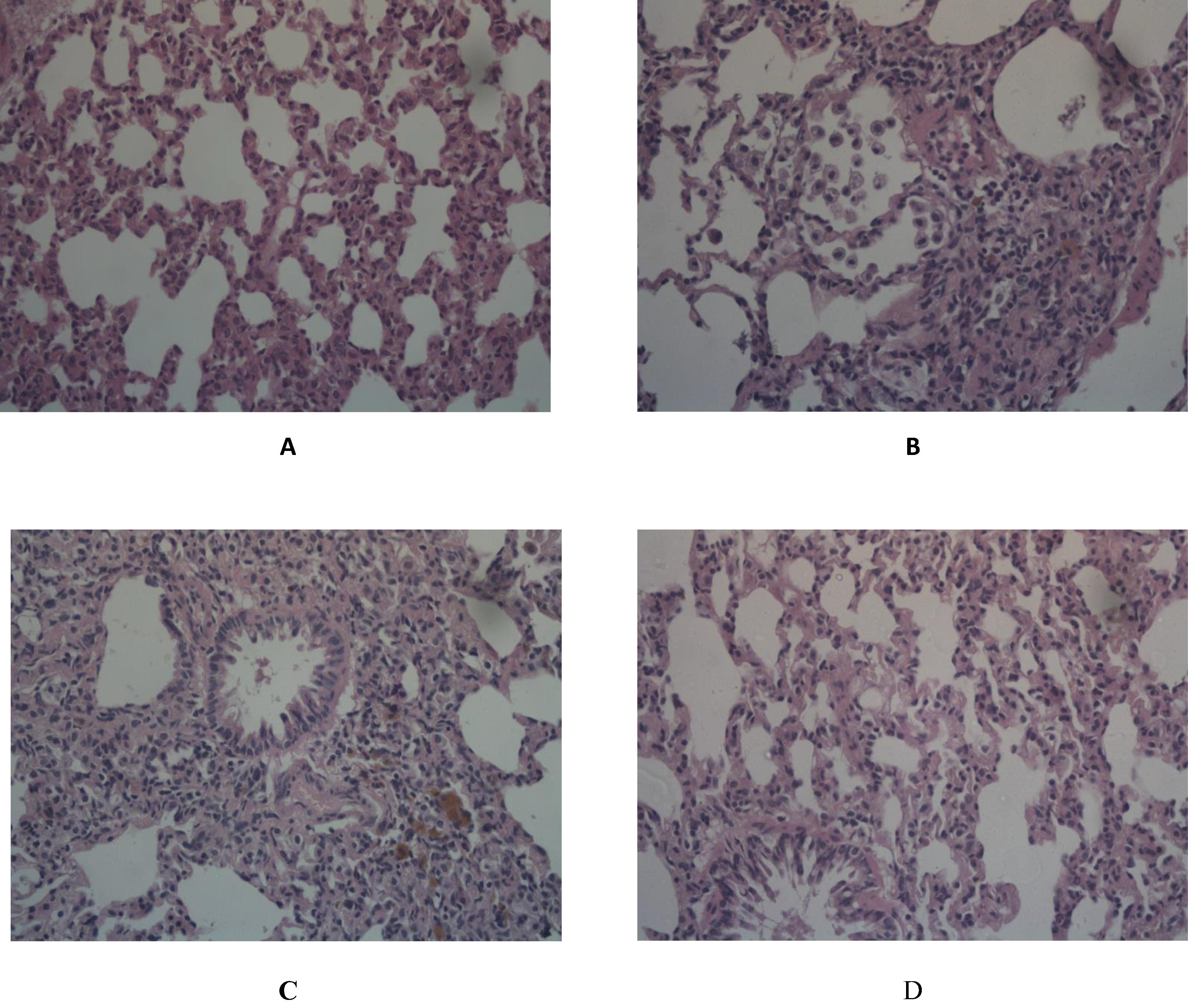
Table 2 Effect of daily oral Flavocoxid on lung inflammatory lesion and fibrosis.
BLM control group displayed increased grade of collagen deposition, compressed alveoli and large fibrotic areas, compared to normal control group. However, collagen accumulation significantly decreased after flavocoxid administration compared to BLM control group (). The Szapiel score of BLM control group was significantly higher when compared to normal control group. While flavocoxid treated group showed marked decrease in fibrosis scores and percentage area of fibrosis compared to the BLM control () ().
Fig. 6 Effect of daily oral flavocoxid on lung histopathological changes using Masson's Trichrome staining (100×). (A) Normal control, revealing absence of blue staining denoting absence of collagen deposition in interstitium as well as alveolar septae. (B) BLM control, revealing bluish discoloration of collagen deposition at interstitial space and peribronchial area. (C) Flavocoxid, revealing mild bluish discoloration of collagen deposition at interstitial space and peribronchial area.
4 Discussion
The current study sheds light on the pulmonary protective potential of flavocoxid against BLM-induced pulmonary fibrosis. In the current study, a well characterized rodent model involving single intratracheal instillation of BLM was adapted. BLM instillation significantly impaired biochemical dynamics, histopathological architecture with marked impairment of physiological pulmonary functions.
BLM infusion is believed to induce oxidative stress and inflammation which propel each other with concomitant cessation of the injury/repair process promoting pulmonary fibrosis [Citation15]. BLM is believed to derive ROS generation [Citation25] which target bio-macromolecules such as DNA, protein, and lipid, driving lipid peroxidation which contribute to biochemical, histopathological and physiological dysfunctions [Citation26].
The fact that oxidative stress plays a crucial role in the pathological development of pulmonary fibrosis has been referred to Daniil, Papageorgiou [Citation27]. Generation of ROS and reactive nitrogen species has been reported to be implicated, at least, in part in the pathogenesis of fibrosis [Citation28,Citation29] . Markers of oxidative stress have been identified in the lungs of pulmonary fibrosis patients and aberrant antioxidant activity exacerbated pulmonary fibrosis in animal models [Citation25]. In association, overproduction of NO has been demonstrated to play a key role in induction of pulmonary fibrosis in animal models and humans [Citation30].
In the current study, it was observed that intratracheal BLM instillation significantly compromised oxidants/antioxidants hemostasis as manifested by the significant elevation of lung MDA content and the concomitant reduction in GSH content, SOD activity and serum TAC. In context, BLM increased lung NO content. Similar observations made by previous researches gave credence to the current notions [Citation23,Citation31] .
Flavocoxid administration conferred significant protection against BLM-induced compromise in oxidants/antioxidants hemostasis and decreased lung NO level content, confirming antioxidant impact of flavocoxid.
The improvement in oxidants/antioxidants hemostasis following flavocoxid administration is in agreement with other reported data demonstrating apparent decrease in in vitro MDA production from lipopolysaccharide-stimulated macrophages after incubation with flavocoxid [Citation32]. Furthermore, flavocoxid ameliorated gentamicin induced damage in the kidneys, mainly through inducing restoration of the activities of GSH and SOD and blunting gentamicin-induced MDA production [Citation33].
Concerning the other propellers of BLM-induced pulmonary damage, the current study revealed that intratracheal instillation of BLM caused chronic lung inflammation confirmed by biochemical assessments and histopathological evaluations. All these inflammatory signs were improved upon flavocoxid administration. Moreover, the absence of neutrophils from the BALF of rats post flavocoxid treatment could be attributed to the mechanism of action of flavocoxid which is dependent in part on inhibition of neutrophils infiltration and subsequent augmentation of inflammatory reaction and oxidative stress. It will be highlighted in the discussion section.
Flavocoxid contains both baicalin and catechin, which inhibit in vitro the peroxidase activities of COX-1 and COX-2, and 5-LOX [Citation11]. In vitro, flavocoxid has been reported to affect gene expression and protein levels of inflammatory markers, such as TNF-α from immune inflammatory cells [Citation32]. In vivo studies have confirmed the strong anti-inflammatory activity of flavocoxid [Citation34].
Interleukin 10 (IL-10) is a well-documented anti-inflammatory cytokine produced by many activated immune cell types [Citation35]. IL-10 has been shown to suppress the production of inflammatory mediators and to down-regulate co-stimulatory molecules critical for activation of T cells [Citation36].
Previous studies have reported increased plasma levels of IL-10 in different pathological situations including: sepsis, hemorrhagic shock, multiple trauma, meningococcemia, mycobacterial infections, and parasitic infections [Citation35,Citation37] . In addition, IL-10 levels have been reported to be significantly increased in several experimental models of pulmonary inflammation and fibrosis [Citation38,Citation39] . (Doughty, Carcillo [Citation40] accounted for increased IL-10 level to be a compensatory anti-inflammatory response syndrome hypothesized to be increased and commensurate with the pro-inflammatory response, by either plasma IL-6 or NO levels.
Flavocoxid administration significantly decreased lung IL-10 content, proposing down-regulation of IL-10 to be additional possible pathway implicated in beneficial anti-inflammatory effects of flavocoxid as seen with the results.
In context to all of the aforementioned alteration, histological changes manifested in alterations in the lung architecture and excessive collagen deposition was confirmed following intratracheal BLM instillation in the current study. Excessive collagen deposition is a vital phenomenon in pulmonary fibrosis. The current study reveals increased lung hydroxyproline content following BLM instillation implying increased accumulated collagen and ECM, which was associated with elevation in semi-quantitative scoring of fibrosis. These histological alterations were significantly corrected upon flavocoxid administration. flavocoxid significantly mitigated BLM mediated hydroxyproline and collagen depositions.
In conclusion; flavocoxid confers significant ameliorative influence against BLM-induced pulmonary fibrosis. Combined antioxidant, anti-inflammatory and anti-fibrotic effects are believed to be implicated in the observed efficacy.
Conflict of interest
Authors declare no conflict of interest amongst each other or any other parties
References
- T.J.GrossG.W.HunninghakeIdiopathic pulmonary fibrosisNew Engl J Med34572001517525
- V.CottinChanging the idiopathic pulmonary fibrosis treatment approach and improving patient outcomesEur Respirat Rev: an official journal of the European Respiratory Society.211242012161167
- K.M.AntoniouG.A.MargaritopoulosN.M.SiafakasPharmacological treatment of idiopathic pulmonary fibrosis: from the past to the futureEur Respirat Rev: an official journal of the European Respiratory Society.221292013281291
- S.KayhanA.GuzelL.DuranS.TutuncuA.GuzelM.Gunaydinet al.Effects of leflunomide on inflamation and fibrosis in bleomycine induced pulmonary fibrosis in wistar albino ratsJ Thorac Dis552013641649
- F.H.GreenOverview of pulmonary fibrosisChest1226 Suppl2002334S339S
- R.J.McAnultyG.J.LaurentPathogenesis of lung fibrosis and potential new therapeutic strategiesExp Nephrol32199596107
- K.YamauchiY.KasuyaF.KurodaK.TanakaJ.TsuyusakiS.Ishizakiet al.Attenuation of lung inflammation and fibrosis in CD69-deficient mice after intratracheal bleomycinRespir Res1212011131
- S.I.HagiwaraY.IshiiS.KitamuraAerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in miceAm J Respir Crit Care Med16212000225231
- L.AtzoriF.ChuaS.E.DunsmoreD.WillisM.BarbarisiR.J.McAnultyet al.Attenuation of bleomycin induced pulmonary fibrosis in mice using the heme oxygenase inhibitor Zn-deuteroporphyrin IX-2,4-bisethylene glycolThorax5932004217223
- A.BittoF.SquadritoN.IrreraG.PizzinoG.PallioA.Mecchioet al.Flavocoxid, a nutraceutical approach to blunt inflammatory conditionsMediators Inflamm20142014790851
- B.P.BurnettA.BittoD.AltavillaF.SquadritoR.M.LevyL.PillaiFlavocoxid inhibits phospholipase A2, peroxidase moieties of the cyclooxygenases (COX), and 5-lipoxygenase, modifies COX-2 gene expression, and acts as an antioxidantMediators Inflamm20112011
- C.JinY.JiaC.JinH.DongP.ChengJ.Zhouet al.Therapeutic effect of Halofuginone on ITP mice by regulating the differentiation of Th cell subsetsInt Immunopharmacol1822014213216
- P.K.SmithR.I.KrohnG.T.HermansonA.K.MalliaF.H.GartnerM.D.Provenzanoet al.Measurement of protein using bicinchoninic acidAnal Biochem150119857685
- Henry R. Colorimetric determination of lactic dehydrogenase. Clinical chemistry: principles and techniques Edited by RJ Henry 2nd ed Harper and Row, Hagerstown, Md. 1974:819–31.
- M.H.DabaK.E.El-TahirM.N.Al-ArifiO.A.GubaraDrug-induced pulmonary fibrosisSaudi Med J2562004700706
- H.OhkawaN.OhishiK.YagiAssay for lipid peroxides in animal tissues by thiobarbituric acid reactionAnal Biochem9521979351358
- G.L.EllmanTissue sulfhydryl groupsArch Biochem Biophys82119597077
- S.MarklundG.MarklundInvolvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutaseEur J Biochem4731974469474
- H.MontgomeryJ.F.DymockThe rapid determination of nitrate in fresh and saline watersAnalyst1034871962374378
- D.KoracevicG.KoracevicV.DjordjevicS.AndrejevicV.CosicMethod for the measurement of antioxidant activity in human fluidsJ Clin Pathol5452001356361
- I.BergmanR.LoxleyTwo improved and simplified methods for the spectrophotometric determination of hydroxyprolineAnal Chem3512196319611965
- C.R.KlimentJ.M.EnglertL.P.CrumT.D.OuryA novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animalInt J Clin Exp Pathol442011349355
- R.VermaL.KushwahD.GohelM.PatelT.MarvaniaS.BalakrishnanEvaluating the ameliorative potential of quercetin against the bleomycin-induced pulmonary fibrosis in wistar ratsPulm Med20132013921724
- S.V.SzapielN.A.ElsonJ.D.FulmerG.W.HunninghakeR.G.CrystalBleomycin-induced interstitial pulmonary disease in the nude, athymic mouseAm Rev Respir Dis12041979893899
- P.ChitraG.SaiprasadR.ManikandanG.SudhandiranBerberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-κB dependant TGF-β activation: a biphasic experimental studyToxicol Lett21922013178193
- X.LiangQ.TianZ.WeiF.LiuJ.ChenY.Zhaoet al.Effect of Feining on bleomycin-induced pulmonary injuries in ratsJ Ethnopharmacol13432011971976
- Z.D.DaniilE.PapageorgiouA.KoutsokeraK.KostikasT.KiropoulosA.I.Papaioannouet al.Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosisPulm Pharmacol Ther21120082631
- B.FubiniA.HubbardReactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosisFree Radical Biol Med3412200315071516
- E.BargagliC.OlivieriD.BennettA.PrasseJ.Muller-QuernheimP.RottoliOxidative stress in the pathogenesis of diffuse lung diseases: a reviewRespir Med1039200912451256
- S.KalayarasanN.SriramG.SudhandiranDiallyl sulfide attenuates bleomycin-induced pulmonary fibrosis: critical role of iNOS, NF-κB, TNF-α and IL-1βLife Sci8223200811421153
- S.SogutH.OzyurtF.ArmutcuL.KartM.IrazO.Akyolet al.Erdosteine prevents bleomycin-induced pulmonary fibrosis in ratsEur J Pharmacol4942–32004213220
- D.AltavillaF.SquadritoA.BittoF.PolitoB.P.BurnettV.Di Stefanoet al.Flavocoxid, a dual inhibitor of cyclooxygenase and 5-lipoxygenase, blunts pro-inflammatory phenotype activation in endotoxin-stimulated macrophagesBr J Pharmacol1578200914101418
- D.H.El-KashefA.E.El-KenawiG.M.SuddekH.A.SalemFlavocoxid attenuates gentamicin-induced nephrotoxicity in ratsNaunyn Schmiedebergs Arch Pharmacol38812201513051315
- D.AltavillaL.MinutoliF.PolitoN.IrreraS.ArenaC.Magnoet al.Effects of flavocoxid, a dual inhibitor of COX and 5-lipoxygenase enzymes, on benign prostatic hyperplasiaBr J Pharmacol1671201295108
- M.GoldmanT.VeluInterleukin-10 and its implications for immunopathologyAdv Nephrol Necker Hosp2419957990
- J.Gomez-JimenezM.C.MartinR.SauriR.M.SeguraF.EstebanJ.C.Ruizet al.Interleukin-10 and the monocyte/macrophage-induced inflammatory response in septic shockJ Infect Dis17121995472475
- R.M.SherryJ.I.CueJ.K.GoddardJ.B.ParramoreJ.T.DipiroInterleukin-10 is associated with the development of sepsis in trauma patientsJ Trauma4041996613617
- E.J.ParkJ.RohS.N.KimM.S.KangY.A.HanY.Kimet al.A single intratracheal instillation of single-walled carbon nanotubes induced early lung fibrosis and subchronic tissue damage in miceArch Toxicol859201111211131
- B.G.KimP.H.LeeS.H.LeeY.E.KimM.Y.ShinY.Kanget al.Long-term effects of diesel exhaust particles on airway inflammation and remodeling in a mouse modelAllergy Asthma Immunol Res832016246256
- L.DoughtyJ.A.CarcilloS.KaplanJ.JanoskyThe compensatory anti-inflammatory cytokine interleukin 10 response in pediatric sepsis-induced multiple organ failureChest1136199816251631
