Abstract
A series of benzimidazole derivatives (1–20) was synthesized and evaluated for its in vitro antimicrobial, antitubercular and anticancer activities. Compound 10 was found to be the most active antibacterial agent. The compounds active in in vitro evaluation against M. tuberculosis were further assessed for their in vivo activity in mice and for their capacity to inhibit the vital mycobacterial enzymes viz., isocitrate lyase, pantothenate synthetase and chorismate mutase. The dose of the compounds in antitubercular evaluation that proved fatal and highly toxic to mice was 5.67 mg/kg while lethal dose varied from 1.82 mg/kg to 3.23 mg/kg body weight of the mice. A dose of 1.34 mg/kg was found to be safe for each of the compounds. All compounds inhibited the mycobacterial enzymes but to a lesser extent than streptomycin sulphate used as positive control. Compound 19, exhibiting inhibition of 67.56%, 53.45%, and 47.56% against isocitrate lyase, pantothenate synthetase and chorismate mutase, respectively is the most potent antitubercular compound among the synthesized benzimidazole derivatives. Further, compound 19 also emerged as a potent anticancer agent (IC50 = 0.0013 µM) than 5-flourouracil against breast cancer cell line (MCF 7).
1 Introduction
Chemotherapy has revolutionized the treatment of infectious diseases since the discovery of antibacterial dyes by Ehrlich earlier in the 20th century and paved the way to a great victory for human health and longevity. The emergence of resistance against currently used antimicrobial drugs led to a revitalized interest of the researchers in infectious diseases to develop new chemical entities to combat them [Citation1Citation[2]Citation[3]–Citation4] .
Tuberculosis (TB), caused by Mycobacterium tuberculosis (M. tuberculosis), remains a pivotal cause of high mortality worldwide despite the handiness of highly potent antitubercular drugs due to the development of resistance by the mycobacterium as a result of gene mutation to first-line antitubercular drugs [Citation5]. To combat the mycobacterial resistance, there is a need to identify novel targets unique to M. tuberculosis which are absent in humans whose blockage would either prove lethal to the bacterium or render it extremely susceptible to the host immune response [Citation6]. Chorismate mutase (CM), isocitrate lyase (ICL), and pantothenate synthetase (PS) are few such unique targets for M. tuberculosis [Citation7]. Chorismate is a precursor of important molecules such folic acid, menaquinones, mycobactins and aromatic amino acids. The shikimate pathway utilizes CM as one of the key enzymes for catalyzing the isomerization of chorismate to prephenate for biosynthesis of l-phenylalanine and l-tyrosine in the mycobacteria [Citation8,Citation9] . The glyoxylate metabolism shunt employs ICL as an important enzyme in the main metabolic route for the biosynthesis of cellular material i.e., fatty acids, which might be the major source of carbon for M. tuberculosis during growth on C2 substances [Citation10]. PS catalyzes the condensation of pantothenate from D-pantoate and β-alanine for the biosynthesis of coenzyme A and acyl carrier protein in mycobacterium [Citation11].
Breast cancer is by far the most common cancer of women, comprising 23% of all female cancers, and there were an estimated 1.15 million new cases in 2002. It ranks second overall when both sexes are considered. More than half of all cases occur in industrialized countries — about 361,000 in Europe (27.3% of cancers in women) and 230,000 in North America (31.3%). Incidence rates are high in most of the developed areas of the world (except for Japan, where breast cancer is third after colorectal cancer and stomach cancer), with the highest age-standardized incidence in North America (99.4 per 100,000). The incidence is more modest in eastern Europe, South America, southern Africa, and western Asia, but breast cancer is still the most common cancer of women in these regions [Citation12]. To curb the menace of increasing resistance and unaffordable treatment by breast cancer survivals, there is urgency for new and cost effective chemotherapeutic agents.
Benzimidazole, also known as benzoglyoxaline, is a heterocyclic moiety of choice for the researchers in modern times [Citation13]. The presence of imidazole (a biologically active pharmacophore) makes it a versatile heterocycle with an extensive range of biological activities such as antihistaminic [Citation14], antiulcer, antitubercular [Citation15], antioxidant [Citation16], anti-HIV [Citation17], anti-inflammatory [Citation18], analgesic [Citation19], antimicrobial [Citation20], antiprotozoal, antitrichinellosis [Citation21], antihypertensive [Citation22], anticancer [Citation23] DNA binding [Citation24] and antimicrobial activities [Citation25].
Prompted by the above findings and in continuation of our work on benzimidazole derivatives [Citation26], we herein report the synthesis, antimicrobial, antitubercular and anticancer evaluation of a novel series of benzimidazole derivatives.
2 Experimental
2.1 Materials and method
The chemicals of analytical grade were procured from commercial sources and used as such without further purification. Media for antimicrobial activity was obtained from Hi-media Laboratories. Microbial type cell cultures (MTCC) for antimicrobial activity were purchased from IMTECH, Chandigarh. Infrared (IR) spectra were recorded on Bruker 12060280, Software: OPUS 7.2.139.1294 spectrophotometer using KBr pellet method and expressed in cm−1. The proton nuclear magnetic resonance (1HNMR) and 13CNMR spectra were recorded on Bruker Avance III 600 spectrometer (at 600 and 150 MHz respectively) in deuterated DMSO downfield to tetramethylsilane standard and chemical shifts were recorded as δ (parts per million). Melting points were determined by open glass capillary method and are uncorrected. The progress of reaction was confirmed by TLC performed on silica gel-G plates and the spots were visualized in iodine chamber. The LCMS data were recorded on Waters Q-TOF micromass (ESI-MS), at Panjab University, India. Elemental analysis for synthesized derivatives was performed on CHNN/CHNS/O analyzer (Flash EA1112N series, Thermo finnigan, Italy).
2.2 Synthesis
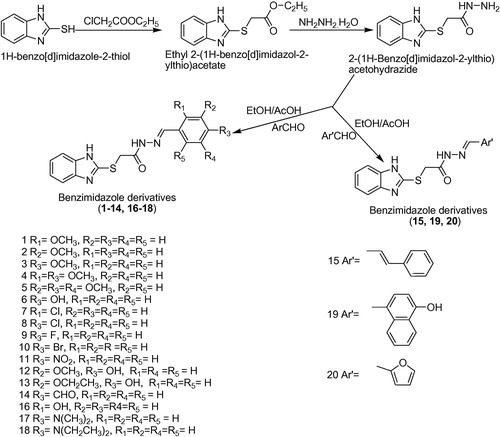
2.2.1 General procedure for synthesis of ethyl-2-(1H-benzo[d]imidazol-2-ylthio)acetate
A mixture containing 2-mercaptobenzimidazole (0.03 mol), potassium hydroxide (0.03 mol) and 60 ml ethanol was stirred and heated at 78–90 °C for 10–15 min. Ethyl chloroacetate (0.03 mol) was then added in one portion that led to arise in temperature of 30–40 °C due to exothermic reaction. After stirring for 24 h at room temperature, reaction mixture was added to ice (100 gm) and stirred further for half an hour, maintaining the temperature at 0–10 °C. The shiny white precipitate so obtained was filtered using suction and rendered free from chloride by repeated washing with water. The dried product was finally recrystallized with ethyl alcohol.
2.2.2 General procedure for synthesis of ethyl-2-(1H-benzo[d]imidazol-2-ylthio)acetohydrazide
Ethyl-2-(1H-benzo[d]imidazol-2-ylthio)acetohydrazide was obtained by gently refluxing a solution of ethyl-2-(1H-benzo[d]imidazol-2-ylthio)acetate (0.01 mol) and hydrazine hydrate (0.06 mol) in rectified spirit on a water bath for 3–4 h. The progress of reaction was confirmed by TLC. The solution was concentrated and kept in refrigerator overnight. The creamish white precipitate formed was filtered, dried and recrystallized from water.
2.2.3 General procedure for synthesis of benzimidazole derivatives (1–20)
A solution of ethyl-2-(1H-benzo[d]imidazol-2-ylthio)acetohydrazide (0.01 mol) was poured into a solution of appropriate aromatic aldehyde (0.01 mol) in boiling ethanol and refluxed for an appropriate time using 1–2 drops of glacial acetic acid as catalyst. The progress of reaction was monitored by thin layer chromatography. Excess solvent was removed by distillation after completion of reaction and the concentrate was kept aside for precipitation. The obtained product was filtered followed by washing with dilute ethanol and recrystallization with ethanol.
2.2.4 Spectral data of benzimidazole derivatives (1–20)
2.2.4.1 2-(1H-Benzo[d]imidazol-2-ylthio)-N′-(2-methoxybenzylidene) acetohydrazide (1)
Peach coloured crystalline powder; mp 145–147 °C; yield 94.08%; Rf 0.54 (Benzene: Chloroform 6:4); IR (cm−1): 3425 N–H str. for 2° amide, 2838 N–H str. for imidazole, 1669 C=O str for 2° amide, 745 C–S str of thiol, 656 OCN deformation of amides; 1HNMR (DMSO, δ): 3.85 (s, 3H of methoxy), 4.98 (s, NH of benzimidazole), 7.12–7.77 (m, 8H, aromatic), 8.13 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.90 CH2 aliphatic, 55.54 CH3 aliphatic, (112.59, 119.90, 121.37, 130.18, 132.24, 158.71) C of benzene, (120.54, 123.01, 139.29, 149.83) C of benzimidazole, 142.52 CH aliphatic, 168.97 C of amide; EIMS m/z 341 [M + 1]+; Anal. Calcd. for C17H16N4O2S: C, 59.98; H, 4.74; N, 16.46; S, 9.42. Found: C, 59.97; H, 4.72; N, 16.47; S, 9.40.
2.2.4.2 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(3-methoxybenzylidene) acetohydrazide (2)
Yellow crystalline powder; mp 158–160 °C; yield 94.08%; Rf 0.41(Benzene: Chloroform 6:4); IR (cm−1): 3431 N–H str. for 2° amide, 3047 N–H str for imidazole, 1668 C=O str for 2° amide, 1052 C–O–C str of arylalkyl ether, 750 C–S str of thiol; 1HNMR (DMSO, δ): 3.79 (s, 3H of methoxy), 4.59 (s, NH of benzimidazole), 7.12–8.00 (m, 8H of benzimidazole), 8.18 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.03 CH2 aliphatic, 55.18 CH3 aliphatic, (116.26, 119.97, 121.30, 129.97, 135.15, 159.48) C of benzene, (115.73, 122.25, 140.74, 149.81) C of benzimidazole, 143.38 CH aliphatic, 169.84 C of amide; EIMS m/z 341 [M + 1]+; Anal. Calcd. for C17H16N4O2S: C, 59.98; H, 4.74; N, 16.46; S, 9.42. Found: C, 59.96; H, 4.73; N, 16.45; S, 9.41.
2.2.4.3 2-(1H-Benzo[d]imidazol-2-ylthio)-N′-(4-methoxybenzylidene) acetohydrazide (3)
Brown powder; mp 208–212 °C; yield 94.08%; Rf 0.36 (Benzene: Chloroform 6:4); IR (cm−1): 3395 N–H str. for 2° amide, 3065 N–H str. for imidazole, 1651 C=O str for 2° amide, 1104 C–O–C str of arylalkyl ether, 728 C–S str of thiol; 1HNMR (DMSO, δ): 3.81 (s, 3H of methoxy), 6.92–7.75 (m, 8H aromatic), 7.98 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.90 CH2 aliphatic, 55.18 CH3 aliphatic, (114.05, 127.76, 127.87, 153.58, 159.90) C aromatic, 140.59 CH aliphatic, 170.21 C of amide; EIMS m/z 341 [M + 1]+; Anal. Calcd. for C17H16N4O2S: C, 59.98; H, 4.74; N, 16.46; S, 9.42. Found: C, 59.97; H, 4.71; N, 16.45; S, 9.39.
2.2.4.4 2-(1H-Benzo[d]imidazol-2-ylthio)-N′-(2,4-dimethoxybenzylidene) acetohydrazide (4)
Peach coloured powder; mp 206–208 °C; yield 65.76%; Rf 0.51 (Benzene); IR (cm−1): 3439 N–H str. for 2° amide, 3019 N–H str. for imidazole, 1653 C=O str for 2° amide, 1273 C–O–C str of aralkyl ether, 888 C–H out of plane bending, 743 C–S str of thiol; 1HNMR (DMSO, δ): 6.64–8.03 (m, 7H aromatic), 8.28 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.90 CH2 aliphatic, (55.34, 55.64) CH3 aliphatic, (98.02, 106.20, 126.48, 158.18, 161.49) C of benzene, (115.87, 126.48, 136.24) C of benzimidazole, 153.60 CH aliphatic, 170.15 C of amide; EIMS m/z 371 [M + 1]+; Anal. Calcd. for C18H18N4O3S: C, 58.36; H, 4.90; N, 15.12; S, 8.66. Found: C, 58.34; H, 4.92; N, 15.09; S, 8.62.
2.2.4.5 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(3,4,5-trimethoxybenzylidene) acetohydrazide (5)
Peach coloured crystals; mp 152–155 °C; yield 43.27%; Rf 0.24 (Benzene); IR (cm−1): 3460 N–H str for 2° amide 3057 N–H str for imidazole, 1577 C=O str for 2° amide, 1125 C–O–C str for asymm. ether, 761 C–S str of thiol; 1HNMR (DMSO, δ): 3.82 (s, 2H of methylene), 4.61 (s, NH of benzimidazole), 6.96–7.96 (m, 6H aromatic), 8.13 (s, NH of 2° amide); 13C NMR (DMSO, δ): 34.22 CH2 aliphatic, (39.79, 39.92, 40.04) CH3 aliphatic, (108.42, 128.16, 139.61, 139.61, 149.63) C of benzene, (112.35, 122.24, 136.57, 149.45) C of benzimidazole, 142.89 CH aliphatic, 169.23 C of amide; EIMS m/z 401 [M + 1]+; Anal. Calcd. for C19H20N4O4S: C, 56.99; H, 5.03; N, 13.99; S, 8.01. Found: C, 56.98; H, 5.01; N, 13.96; S, 8.00.
2.2.4.6 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-hydroxybenzylidene) acetohydrazide (6)
Mustard yellow powder; mp 104–106 °C; yield 92.28%; Rf 0.19 (Benzene: Chloroform 6:4); IR (cm−1): 3621 O–H str for phenol, 3368 N–H str for 2° amide, 2884 N–H str. for imidazole, 1593 C=O str for 2° amide, 832 C–H out of plane bending, 735 C–S str of thiol; 1HNMR (DMSO, δ): 3.97 (s, 2H of methylene), 4.56 (s, NH of benzimidazole), 6.95–7.94 (m, 8H aromatic), 8.10 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.91 CH2 aliphatic, (115.59, 128.39, 131.77, 161.54) C of benzene, (115.44, 128.34, 131.79, 153.46) C of benzimidazole, 139.55 CH aliphatic, 163.17 C of amide; EIMS m/z 342 [M + 1]+; Anal. Calcd. for C17H17N4O2S: C, 58.88; H, 4.32; N, 17.17; S, 9.82. Found: C, 58.86; H, 4.29; N, 17.16; S, 9.83.
2.2.4.7 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(2-chlorobenzylidene) acetohydrazide (7)
Yellow crystalline powder; mp 113–116 °C; yield 58.89%; Rf 0.26 (Benzene); IR (cm−1): 3426 N–H str for 2° amide, 3025 N–H str. for imidazole, 1609 C=O str for 2° amide, 742 C–Cl str of monochlorinated aromatic, 634 C–S str of thiol; 1HNMR (DMSO, δ): 3.73 (s, 2H of methylene), 6.98–7.97 (m, 8H aromatic), 8.16 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.04 CH2 aliphatic, (104.26, 105.63, 129.22, 153.14, 153.16) C aromatic, 140.25 CH aliphatic, 161.11 C of amide; EIMS m/z 346 [M + 1]+; Anal. Calcd. for C16H13ClN4OS: C, 55.73; H, 3.80; N, 16.25; S, 9.30. Found: C, 55.70; H, 10.27; N, 16.24; S, 9.28.
2.2.4.8 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-chlorobenzylidene) acetohydrazide (8)
Dull yellow powder; mp 210–213 °C; yield 71.63%; Rf 0.26 (Benzene: Chloroform 6:4); IR (cm−1): 3466 N–H str. for 2° amide, 3074 N–H str. for imidazole, 1622 C=O str for 2° amide, 822 C–H out of plane bending, 733 C–Cl str of monochlorinated aromatic, 669 C–S str of thiol; 1HNMR (DMSO, δ): 6.95–7.85 (m, 8H aromatic), 8.01 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.05 CH2 aliphatic, (109.42, 129.01, 129.96, 132.23) C of benzene, (119.95, 122.24, 134.18, 153.45) C of benzimidazole, 139.40 CH aliphatic, 168.14 C of amide; EIMS m/z 346 [M + 1]+; Anal. Calcd. for C16H13ClN4OS: C, 55.73; H, 3.80; N, 16.25; S, 9.30. Found: C, 55.72; H, 3.77; N, 16.23; S, 9.29.
2.2.4.9 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-fluorobenzylidene) acetohydrazide (9)
Light brown crystals; mp 174–176 °C; yield 72.39%; Rf 0.23 (Benzene: Chloroform 6:4); IR (cm−1): 3542 N–H str. for 2° amide, 3258 N–H str. for imidazole, 3056 C–H aromatic str, 1638 C=O str for 2° amide, 1010 C–F str of monochlorinated compound, 799 C–S str of thiol; 1HNMR (DMSO, δ): 4.14 (s, 2H of methylene), 6.91–7.78 (m, 8H aromatic), 7.96 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.06 CH2 aliphatic, (109.41, 110.59, 131.55, 153.24) C of benzene, (111.97, 122.25, 132.21, 150.22) C of benzimidazole, 143.73 CH aliphatic, 168.13 C of amide; EIMS m/z 329 [M + 1]+; Anal. Calcd. for C16H13FN4OS: C, 58.52; H, 3.99; N, 17.06; S, 9.77. Found: C, 58.50; H, 3.97; N, 17.03; S, 9.76.
2.2.4.10 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-bromobenzylidene) acetohydrazide (10)
Lemon yellow powder; mp 182–185 °C; yield 70.54%; Rf 0.43 (Benzene: Chloroform 6:4); IR (cm−1): 3452 N–H str. for 2° amide, 3157 N–H str. for imidazole, 1650 C=O str for 2° amide, 817 C–H out of plane bending, 738 C–S str of thiol, 604 C–Br str aromatic; 1HNMR (DMSO, δ): 4.17 (s, 2H of methylene), 6.99–7.99 (m, 8H aromatic), 8.19 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.04 CH2 aliphatic, (122.25, 131.73, 131.97, 133.26) C of benzene, (120.04, 123.08, 139.61, 149.51) C of benzimidazole, 142.37 CH aliphatic, 169.13 C of amide; EIMS m/z 390 [M + 1]+; EIMS m/z 390 [M + 1]+; Anal. Calcd. for C16H13BrN4OS: C, 49.37; H, 3.37; N, 14.39; S, 8.24. Found: C, 49.36; H, 3.35; N, 14.38; S, 8.23.
2.2.4.11 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-nitrobenzylidene) acetohydrazide (11)
Brick red crystalline powder; mp 208–211 °C; yield 83.57%; Rf 0.39 (Benzene: Chloroform 6:4); IR (cm−1): 3338 N–H str. for 2° amide, 2946 N–H str. for imidazole, 1641 C=O str for 2° amide, 1564 NO2 str (asym) of aromatic nitro group, 1452 C–C str of phenyl nucleus, 1330 NO2 str (sym) of aromatic nitro group, 737 C–S str of thiol, 577 CNO bending of nitro compound; 1HNMR (DMSO, δ): 4.18 (s, 2H of methylene), 7.01–8.05 (m, 8H aromatic), 8.16 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.03 CH2 aliphatic, (123.96, 132.20, 140.25, 149.58) C of benzene, (122.25, 123.83, 138.67, 147.67) C of benzimidazole, 142.05 CH aliphatic, 169.48 C of amide; EIMS m/z 356 [M + 1]+; Anal. Calcd. for C16H13N5O3S: C, 54.08; H, 3.69; N, 19.71; S, 9.02. Found: C, 54.06; H, 3.67; N, 19.70; S, 9.01.
2.2.4.12 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-hydroxy-3-methoxybenzylidene) acetohydrazide (12)
Light orange crystalline powder; mp 102–104 °C; yield 55.36%; Rf 0.40 (Benzene: Chloroform 6:4); IR (cm−1): 3663 O–H str of phenol, 3374 N–H str. for 2° amide, 2880 N–H str. for imidazole, 1652 C=O str for 2° amide, 868 C–H plane bending of 1,3,5- trisubstituted benzene ring, 612 C–S str of thiol; 1HNMR (DMSO, δ): 3.80 (s, 2H of methylene), 6.95–7.93 (m, 7H aromatic), 8.09 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.96 CH2 aliphatic, 56.04 C of OCH3 aliphatic, (115.38, 119.86, 122.27, 126.52, 148.13, 149.98) C of benzene, (115.55, 123.48, 141.60, 147.60) C of benzimidazole, 144.01 CH aliphatic, 170.42 C of amide; EIMS m/z 357 [M + 1]+; Anal. Calcd. for C17H16N4O3S: C, 57.29; H, 4.52; N, 15.72; S, 9.00. Found: C, 57.27; H, 4.51; N, 15.71; S, 9.01.
2.2.4.13 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(3-ethoxy-4-hydroxybenzylidene)aceto hydrazide (13)
Dark brown crystals; mp 176–178 °C; yield 54.08%; Rf 0.43 (Benzene); IR (cm−1): 3306 N–H str. for 2° amide, 2942 N–H str. for imidazole, 2830 C–H str aralkyl ether, 1669 C=O str for 2° amide, 1277 C–O–C str aralkyl asymm, 864 C–H bending of 1,3,5- trisubstituted benzene ring, 747 C–S str of thiol; 1HNMR (DMSO, δ): 3.98 (s, 2H of methylene), 4.56 (s, NH of benzimidazole), 7.07–7.93 (m, 7H of benzimidazole), 8.08 (s, NH of 2° amide); 13C NMR (DMSO, δ): 14.74 C of OCH2CH3, 39.88 CH2 aliphatic, 63.80 C of OCH2CH3 aliphatic, (111.41, 115.55, 123.25, 125.48, 150.05) C aromatic, 147.06 CH aliphatic, 160.55 C of amide; EIMS m/z 371 [M + 1]+; Anal. Calcd. for C18H18N4O3S: C, 58.36; H, 4.90; N, 15.12; S, 8.66. Found: C, 58.35; H, 4.91; N, 15.10; S, 8.64.
2.2.4.14 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-formylbenzylidene) acetohydrazide (14)
Turmeric yellow crystalline powder; mp > 300 °C; yield 69.64%; Rf 0.37 (Benzene); IR (cm−1): 3271 N–H str. for 2° amide, 2948 N–H str. for imidazole, 1619 C=O str for 2° amide, 1345 C–CHO skeletal aldehydes group, 936 C–H in plane bending of aldehyde group, 748 C–S str of thiol; 1HNMR (DMSO, δ): 3.99 (s, 2H of methylene), 6.98–7.85 (m, 8H aromatic), 8.05 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.91 CH2 aliphatic, (109.42, 121.37, 122.26, 126.58, 126.68 127.08) C aromatic; EIMS m/z 339 [M + 1]+; Anal. Calcd. for C17H14N4O2S: C, 60.34; H, 4.17; N, 16.56; S, 9.48. Found: C, 60.35; H, 4.15; N, 16.55; S, 9.47.
2.2.4.15 2-(1H-Benzo[d]imidazol-2-ylthio)-N′-((E)-3-phenylallylidene) acetohydrazide (15)
Lemon yellow colour; mp 202–204 °C; yield 48.91%; Rf 0.47 (Benzene: Chloroform 6:4); IR (cm−1): 3415 N–H str. for 2° amide, 3032 N–H str. for imidazole, 1652 C=C str vibration of R1CH=CHR2 (cis), 736 C–S str of thiol; 1HNMR (DMSO, δ): 6.95–7.89 (m, 9H aromatic), 7.91 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.91 CH2 aliphatic, (125.51, 135.32, 136.29) CH aliphatic, (126.53, 128.20, 128.81, 128.84, 143.39) C aromatic 153.09 C of amide; EIMS m/z 337 [M + 1]+; Anal. Calcd. for C18H16N4OS: C, 64.26; H, 4.79; N, 16.65; S, 9.53. Found: C, 64.23; H, 4.76; N, 16.64; S, 9.51.
2.2.4.16 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(2-hydroxybenzylidene) acetohydrazide (16)
Light yellow powder; mp 178–180 °C; yield 87.04%; Rf 0.35 (Benzene: Chloroform 6:4); IR (cm−1): 3698 O–H str of phenol, 3373 N–H str. for 2° amide, 2861 N–H str. for imidazole, 1669 C=O str for 2° amide, 749 C–H plane bending of disubstituted benzene ring, 666 C–S str of thiol; 1HNMR (DMSO, δ): 4.18 (s, 2H of methylene), 6.98–7.77 (m, 8H aromatic), 8.32 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.87 CH2 aliphatic, (116.12, 118.54, 121.36, 130.88, 132.23, 162.84) C of benzene, (115.94, 126.46, 141.15, 147.36) C of benzimidazole, 141.34 CH aliphatic, 168.71 C of amide; EIMS m/z 327 [M + 1]+; Anal. Calcd. for C16H14N4O2S: C, 58.88; H, 4.32; N, 17.17; S, 9.82. Found: C, 58.85; H, 4.30; N, 17.15; S, 9.81.
2.2.4.17 2-(1H-Benzo[d]imidazol-2-ylthio)-N′-(4-(dimethylamino)benzylidene)acetohydrazide (17)
Bright yellow powder; mp 175–178 °C; yield 47.16%; Rf 0.48 (Benzene); IR (cm−1): 3515 N–H str. for 2° amide, 3089 N–H str. for imidazole, 1601 C=O str for 2° amide, 1359 C–N str of aryl tertiary amine, 766 C–S str of thiol; 1HNMR (DMSO, δ): 4.11 (s, 2H of methylene), 7.11–7.89 (m, 8H aromatic), 8.05 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.04 CH2 aliphatic, (39.70, 39.71) aliphatic CH3 at N, (111.65, 119.90, 128.12, 128.42, 151.93) C of benzene, (111.72, 121.52, 129.44, 147.80) C of benzimidazole, 144.39 CH aliphatic, 168.42 C of amide; EIMS m/z 354 [M + 1]+; Anal. Calcd. for C18H19N5OS: C, 61.17; H, 5.42; N, 19.81; S, 9.07. Found: C, 61.17; H, 5.41; N, 19.79; S, 9.05.
2.2.4.18 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(4-(diethylamino)benzylidene) acetohydrazide (18)
Peach coloured powder; mp 220–222 °C; yield 54.46%; Rf 0.41 (Benzene: Chloroform 6:4); IR (cm−1): 3308 N–H str. for 2° amide, 3142 N–H str. for imidazole, 1647 C=O str for 2° amide, 1345 C–N str of aryl tertiary amine, 729 C–S str of thiol; 1HNMR (DMSO, δ): 3.51 (s, 2H of methylene), 6.94–7.88 (m, 8H aromatic), 8.02 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.85 CH2 aliphatic, 56.04 C of CH2CH3, 18.50 C of CH2CH3, (115.75, 124.98, 130.05, 153.43) C of benzene, (115.48, 124.96, 141.42, 147.46) C of benzimidazole, 144.01 CH aliphatic, 172.09 C of amide; EIMS m/z 382 [M + 1]+; Anal. Calcd. for C20H23N5OS: C, 62.97; H, 6.08; N, 18.36; S, 8.41. Found: C, 62.95; H, 6.06; N, 18.34; S, 8.39.
2.2.4.19 2-(1H-Benzo[d]imidazol-2-ylthio)-N′-((4-hydroxynaphthalen-1-yl)methylene) acetohydrazide (19)
Mustard yellow crystalline powder; mp 188–190 °C; yield 78.07%; Rf 0.35 (Benzene); IR (cm−1): 3675 O–H aromatic, 3381 N–H str. for 2° amide, 3196 N–H str. for imidazole, 1668 C=O str for 2° amide, 841 naphthacene, 704 C–S str of thiol; 1HNMR (DMSO, δ): 4.25 (s, 2H of methylene), 6.98–7.93 (m, 10H aromatic), 8.03 (s, NH of 2° amide); 13C NMR (DMSO, δ): 40.05 CH2 aliphatic, (109.83, 121.05, 121.59, 127.33, 127.74, 127.94, 128.12, 128.69, 132.78, 163.61) C of naphthalene, (118.15, 123.51, 132.78, 149.39) C of benzimidazole, 143.02 CH aliphatic, 168.24 C of amide; EIMS m/z 377 [M + 1]+; Anal. Calcd. for C20H16N4O2S: C, 63.81; H, 4.28; N, 14.88; S, 8.52. Found: C, 63.80; H, 4.26; N, 14.86; S, 8.50.
2.2.4.20 2-(1H-Benzo[d]imidazol-2-ylthio)-N'-(furan-2-ylmethylene) acetohydrazide (20)
Brown coloured crystals; mp 158–160 °C; yield 66.98%; Rf 0.49 (Benzene: Chloroform 6:4); IR (cm−1): 3350 N–H str. for 2° amide, 3103 C–H str for furan, 2973 N–H str. for imidazole, 1595 C=O str for 2° amide, 755 C–S str of thiol; 1HNMR (DMSO, δ): 6.96–7.83 (m, 7H aromatic), 7.89 (s, NH of 2° amide); 13C NMR (DMSO, δ): 39.93 CH2 aliphatic, (109.42, 110.53, 127.97, 153.62) C of furan, (115.05, 122.24, 132.23, 148.02) C of benzimidazole, 141.78 CH aliphatic, 172.01 C of amide. EIMS m/z 301 [M + 1]+; Anal. Calcd. for C14H12N4O2S: C, 55.99; H, 4.03; N, 18.65; S, 10.68. Found: C, 55.97; H, 4.04; N, 18.64; S, 10.65.
2.3 In vitro antimicrobial evaluation
2.3.1 Determination of MIC
The in vitro antimicrobial potential of the synthesized benzimidazole derivatives was assessed by tube dilution method against Escherichia coli MTCC 1652 (Gram-negative bacterium); Bacillus subtilis MTCC 2063, Staphylococcus aureus MTCC 2901 (Gram-positive bacteria); Candida albicans MTCC 227 and Aspergillus niger MTCC 8189 (fungal strains) [Citation27] using Cefadroxil and fluconazole as standard antibacterial and antifungal drugs respectively. The stock solution of 100 µg/ml concentration for each test and standard drugs was prepared in dimethyl sulfoxide. These were then serially diluted in double strength nutrient broth I.P. for bacteria and Sabouraud dextrose broth I.P. for fungi [Citation28]. The bacterial cultures were incubated for a period of 24 h at 37 ± 2 °C. The incubation time for C. albicans was 48 h at 37 ± 2 °C and for A. niger was 7 d at 25 ± 2 °C. The results of antimicrobial activity were determined in terms of minimum inhibitory concentration (MIC).
2.3.2 Determination of MBC/MFC
The minimum bactericidal concentration (MBC) and fungicidal concentration (MFC) of the synthesized benzimidazole derivatives was determined by subculturing 100 µL of culture from each tube that showed no growth in MIC determination onto sterilized petri-plates containing fresh agar medium. The petri-plates were incubated and analyzed for microbial growth visually [Citation29].
2.4 In vitro antitubercular activity evaluation
The antimycobacterial activities of the compounds were performed in three level safety laboratories at National Centre of Fungal Taxonomy (NCFT), New Delhi in association with HIHT University, Jolly Grant, Dehradun (U.K). Middle brook 7H10 agar (Becton Dickinson Company (DifcoTM), 7 Loveton Circle, Sparks, Maryland, USA; Lot No. 8175150) supplemented with oleic acid-albumin- catalase (OADC) (Becton Dickinson Company Lot 8136781) was used for reviving and culturing the mycobacteria for sensitivity testing. Drugs viz. Streptomycin (S) (500 mg) was obtained as gift samples from Shalina Laboratories Pvt. Ltd., Navi Mumbai, Maharashtra. Alamar blue dye (Accumed International, Westlake Ohio), microtiter plates (Falcon, 3072: Becton Dickinson, Lincoln Park NJ), sterilized glass wares, UV-cabinets with reverse pressure gas system. The preserved strains of M. tuberculosis viz., mycobacterium sensitive to streptomycin (S), isoniazid (H), rifampin (R) and pyrazinamide (PZA)- H37Rv (NCFT/TB/537) was used in order to assess the antimycobacterial activity of the compounds.
2.4.1 Preparation of the drugs/compounds dilutions
Each of the synthesized compound was dissolved in DMSO to obtain a concentration of 50 µg/ml and diluted further to concentrations of 25 µg/ml and 12.5 µg/ml. Similarly, stock solution of 50 µg/ml concentration was prepared for standard antitubercular drug, streptomycin and diluted to 25 µg/ml in order to check the antitubercular activity.
2.4.2 Preparation of growth media
It was prepared by adding dehydrated medium (19 g) to purified water (900 ml) containing glycerol (15 ml). The mixture was stirred well to dissolve and autoclaved at 121 °C for 10 min. Oleic acid-albumin catalase (100 ml) was aseptically added to the medium after cooling to 45 °C. No adjustment for pH was made.
2.4.3 Preparation of inoculum for drug sensitivity testing
Preserved strains of M. tuberculosis viz. mycobacterium sensitive to S, H, R and PZA-H37Rv (NCFT/TB/537) were revived on Middle brook 7H10 agar, prior to antituberculosis susceptibility testing. Cells were scraped from freshly growing colonies (three weeks old) on Middle brook 7H10 plates and introduced into saline (10 ml). Bacterial suspensions of 0.5 McFarland standard turbidity equivalent to 108 CFU were prepared by dilution with saline. The mixture was vortexed for 30 s in a glass bottle containing glass beads and the particles were allowed to settle [Citation30].
2.4.4 Random screening of the isolated compounds for antitubercular activity (Alamar-blue assay)
The antimycobacterial activity of compounds was assessed against mycobacterium sensitive to S, H, R and PZA-H37Rv (NCFT/TB/537) using the microplate alamar blue assay (MABA) [Citation31]. This methodology is nontoxic, uses a thermally-stable reagent and is suitable for random screening of the antimycobacterial activity. Briefly, 200 μL of sterile deionized water was added to all outer-perimeter wells of sterile 96 well plates to minimize evaporation of the medium in the test wells during incubation. The 96 well plates received 100 μL of the Middlebrook 7H9 broth (having loopful inoculum of bacteria-108 CFU) and different dilutions of the respective compounds were made directly on the plate. The maximum concentration of the compounds tested was 50 µg/ml. Plates were covered and sealed with parafilm and incubated at 37 °C for five days. After this time, 25 μL of a freshly prepared 1:1 mixture of Alamar blue reagent and 10% tween 80 was added to the plate and incubated for 24 h. A blue colour in the well was interpreted as no bacterial growth (antimycobacterial activity) and a pink colour was scored as growth.
2.4.5 Bioassay protocol for susceptibility tests of the compounds by well diffusion method
The well diffusion method was used to determine susceptibility [Citation30,Citation32] . The agar well diffusion method was modified [Citation33]. Middle brook 7H10 agar medium was used for bacterial cultures. The culture medium was inoculated with loopful bacteria separately suspended in Middle brook 7H10 broth. Wells of 8 mm diameter were punched into the agar and filled each well separately with 50 µg/ml of test compounds and 25 µg/ml of standard drug. The petri dishes were then left in the hood overnight to allow diffusion of the drug and then sealed with a carbon dioxide-permeable tape. These were then incubated at 37 °C in a carbon dioxide incubator for four weeks. The wells were flooded with alamar-blue dye in highly sterilized chamber and de-stained further to observe the zones of inhibition. The sensitivity of the strains to the compounds was determined by measuring the diameter of zones of inhibition surrounding the well using millimetre scale.
2.4.6 Determination of minimum inhibitory concentration (MIC) by alamar blue assay
The compounds were serially diluted to determine the minimum inhibitory concentration of the drug in Middle brook 7H9 medium using micro titre plate method [Citation30,Citation34,Citation35] . The compounds which were found to be satisfactory by the above two methods at a maximum concentration of 50 µg/ml were diluted further to concentrations viz. 25, 12.5, 6.25, 3.125 and 1.56 µg/ml respectively. Similarly, streptomycin was further diluted to 25 µg/ml in order to check the antitubercular activity. The MIC of the potent compounds was performed in microtiter plates by alamar blue assay. Plates were covered and sealed with parafilm and incubated at 37 °C for five days. After this time, 25 μL of a freshly prepared 1:1 mixture of alamar blue reagent and 10% tween 80 was added to the plate and incubated for 24 h. A blue colour in the well was interpreted as no bacterial growth (antimycobacterial activity), and a pink colour was determined as growth. MIC is defined as the lowest drug concentration which prevented a colour change from blue to pink.
2.5 In vivo antitubercular activity evaluation
The LD50 (lethal dose) and ED50 (effective dose) doses were determined for the active compounds in mice models infected with Mycobacterium H37Rv via ethical permission no., NCFT/EC/16/2313 assigned to Collaborative Research Group (CRG), NCFT, New Delhi, India.
2.6 Enzyme assays for antitubercular activity
The compounds found potent in in vivo evaluation were assayed for inhibition of mycobacterial enzymes viz., isocitrate lyase, pantothenate synthetase and chorismate mutase.
2.6.1 Mycobacterial isocitrate lyase assay
Isocitrate lyase activity was assayed according to the protocol reported by Dixon and Kornberg (glyoxylate phenyl hydrazone formation) [Citation36] at 10 µM concentration of the compounds. Isoniazid was employed as a negative control (inhibition of 0%) and streptomycin sulphate (25 µg) served as a positive control [Citation37].
2.6.2 Mycobacterial pantothenate synthetase assay
About 60 µL of the PS reagent, including NADH, pantoic acid, β-alanine, ATP, phosphoenolpyruvate, MgCl2, myokinase, pyruvate kinase, and lactate dehydrogenase in buffer, was added to each well of a 96-well plate. The compounds were then added to plates in 1 µL volumes. 39 µL PS diluted in buffer was added to initiate the reaction. The final concentrations in the reaction contained 0.4 mM NADH, 5 mM pantoic acid, 10 mM MgCl2, 5 mM β-alanine, 10 mM ATP, 1 mM potassium phosphoenolpyruvate, and 18 units/ml each of chicken muscle myokinase, rabbit muscle pyruvate kinase, and rabbit muscle lactate dehydrogenase diluted in 100 mM HEPES buffer (pH 7.8), 1% DMSO, and 5 µg/ml PS in a final volume of 100 µL. The test plate was immediately transferred to a microplate reader and absorbance was measured at 340 nm after every 12 s for 120 s. Each plate had 16 control wells in the two outside columns, of which 12 contained the complete reaction mixture with a DMSO carrier control (full reaction) and four without PS. The following formula was used to calculate percent inhibition % inhibition = 100 × (1 − compound rate − background rate)/(full reaction rate − background rate) [Citation38,Citation39] .
2.6.3 Mycobacterial chorismate mutase (MtCM) assay
Reaction volumes of 0.4 ml of chorismate (typically 1 mM) in 50 mM Tris HCl (pH 7.5), 0.5 mM EDTA, 0.1 mg/ml bovine serum albumin, and 10 mM β-mercaptoethanol were incubated at 37 °C for 5 min. The reaction was started with the addition of 10 µL 5 pM of MtCM (i.e., 185 ng of CM equivalent to 12.5 nM final concentration of the dimer based on the molecular mass of 36,948 Da). The reaction was allowed to proceed at 37 °C and was terminated after 1–5 min with 0.4 ml 1 M HCl. After a further incubation for 10 min at 37 °C, 0.8 ml 2.5 M NaOH was added to convert prephenate formed in the enzymatic reaction to phenyl pyruvate. The absorbance of phenylpyruvate chromophore was taken at 320 nm. A blank with no enzyme for every reaction was also set to account for the nonenzymatic conversion of chorismate to prephenate and enzyme was added after the addition of NaOH. The absorbance at 320 nm for the blank varied from 0.1 to 0.3, depending upon the concentration of chorismate and the duration of the reaction [Citation40].
2.7 In vitro anticancer screening
The in vitro cytotoxicity screening of the synthesized benzimidazole derivatives was assessed on MCF7 (human breast adenocarcinoma cancer) cell line using Sulforhodamine-B (SRB) assay with minor modifications [Citation41]. The results of anticancer activity were expressed as IC50 (concentration of compound required to inhibit cell viability by 50%) and compared with the standard anticancer drugs, 5-fluorouracil and carboplatin.
The cells were allowed to attach for a period of 24 h to the wells of the 96-multititre plates before treatment with the test compounds. Solution of the test and standard compounds were prepared in DMSO and made to appropriate volume with media. Monolayer cells were then incubated at 37 °C for 72 h with different concentrations (0.01, 0.1, 1, 10, 100 µg/ml) of the test compounds in an atmosphere of 5% carbon dioxide. After fixing with trichloroacetic acid for 30 min followed by washing with water, the cells were stained with 0.4% w/v solution of pink coloured aminoxanthene dye, Sulforhodamine-B, in acetic acid for 15 min. The cultures were washed with 1% acetic acid to get rid of excess stain and attached stain was recovered with Tris base solution. The colour intensity was measured using spectrophotometer. The asay was done in atleast triplicates.
3 Results and discussion
3.1 Chemistry
The benzimidazole derivatives (1–20) were synthesized according to and characterized by physicochemical and spectral means. The spectral data of the synthesized compounds is found in agreement with the assigned molecular structures. The formation of ester from 2-mercaptobenzimidazole was confirmed by the absence of S–H stretching at 2600–2550 cm−1. The appearance of C=O stretch in the range of 1680–1630 cm−1 and N–H stretch 3100–3070 cm−1 indicated the formation of secondary amide (1–20) formed by the reaction of ester and hydrazine hydrate. The absence N–H stretching of free primary amine at 3500 cm−1 in the target compounds confirmed their formation. The presence of heterocyclic furan moiety in compound 20 is demonstrated by the presence of CH stretch at 3103 cm−1 which is higher for furan than most aromatics. The multiplet corresponding to 6.9–7.9 δ ppm confirmed the presence of protons of benzimidazole and aryl nucleus. The compounds 1, 2 and 3 showed singlet at δ 3.78 ppm corresponding to a proton of the OCH3. Further confirmation was made on the basis of mass analysis and 13CNMR data. The elemental (CHN) analysis results are within acceptable limits (± 0.4%). Few of the benzimidazole derivatives 3, 6, 8, 11, 15, 16, and 20 have been reported earlier [Citation42Citation[43]–Citation44] but their antitubercular/anticancer activities are not explored.
3.2 In vitro antimicrobial activity
The results of in vitro antimicrobial activity of the synthesized compounds are presented in . Most of the synthesized derivatives were found to be highly efficient as antimicrobial agents in comparison to the standard drug cefadroxil and fluconazole as depicted by their low MIC values compared to standard drugs. Amongst the synthesized benzimidazole derivatives, compound 10 was found to be the potent antibacterial agent against S. aureus (MIC = 0.032 µM).
Table 1 MIC of synthesized benzimidazole derivatives.
In case of B. subtilis, lowest MIC values of 0.021 and 0.031 µM were observed for compounds 20 and 5, respectively. Compound 10 (MIC = 0.0321 µM) showed highest inhibitory action against E. coli (a Gram negative bacterium). Compounds 5, 10 and 18 exhibited most effective antifungal activity against C. albicans, having MIC value of 0.016 µM against each compound while compound 17 (respectively) possessed maximum activity against A. niger with MIC of 0.018 µM. Compound 10 emerged as the best antibacterial agent against tested Gram positive and Gram negative bacteria. All the synthesized compounds showed high antifungal activity than the standard drug fluconazole.
The results of minimum bactericidal concentration/minimum fungicidal concentration () conveyed that the synthesized benzimidazole derivatives were neither bactericidal nor fungicidal except compound 3 which was fungicidal against both fungi (As a general rule, a compound is said to be bactericidal/fungicidal if its MBC/MFC is less than three times of its MIC) [Citation45].
Table 2 MBC/MFC (µg/ml) of synthesized benzimidazole derivatives.
3.3 In vitro antitubercular activity
All the synthesized compounds were evaluated for their in vitro antitubercular activity against M. tuberculosis H37Rv (NCFT/TB/537). The zone of inhibition as well as MIC values of the test compounds was determined. The MIC and MLC (minimum lethal concentration) were determined for compounds showing zone of inhibition of >20 mm. The results of in vitro antitubercular activity compared with streptomycin are presented in .
Table 3 Antimycobacterial activity, MIC and MLC of synthesized compounds against M. tuberculosis H37Rv.
3.4 In vivo antitubercular activity
The LD50 and ED50 for the active compounds were determined in mice models infected with Mycobacterium tuberculosis H37Rv (). It was found that the toxic dose of the compounds which proved fatal and highly toxic to mice was 5.67 mg/kg while LD50 varied from 1.82 mg/kg to 3.23 mg/kg body weight of the mice. LD50 is the dose that killed 50% of the mice population within the group. Thus, ED50 of 1.34 mg/kg was considered safe for each of the compounds. It was observed that this dose was effective and safe for mice in different groups before infecting the mice models with specific TB bacteria as no mortality of any single animal was recorded.
Table 4 LD50 (mg/kg) of potent compounds.
3.5 Mycobacterial enzyme assays
The results of mycobacterial enzyme assays were expressed in terms of percent inhibition of mycobacterial enzymes i.e., isocitrate lyase, chorismate mutase and pantothenate synthetase, by the mycobacterium. The inhibition of the enzyme activity by the tested compounds was less than that of streptomycin sulphate used as positive control (). Compound 19 emerged as the best compound that inhibited the mycobacterial isocitrate lyase, pantothenate synthetase and chorismate mutase to 67.56%, 53.45% and 47.56% respectively which was comparable to inhibition of 75.12%, 77.06%, and 79.56%, respectively by streptomycin sulphate.
Table 5 In vitro percent inhibition of enzymes in Mycobacterium tuberculosis H37Rv by potent compounds.
3.6 In vitro anticancer activity
Most of the synthesized compounds possessed more cytotoxicity as compared to 5-fluorouracil (). Compound 19 (IC50 = 0.0013 µM) showed extremely potent cytotoxicity against MCF7 cell line as compared to 5-fluorouracil (IC50 = 0.0461 µM). Majority of the compounds were more active than standard drug carboplatin while compounds 4, 5, and 18 were as active as carboplatin (IC50 = 0.2694 µM).
Table 6 IC50 (µM) values of synthesized benzimidazole derivatives.
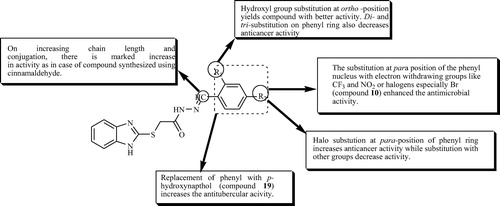
3.7 Structure activity relationship (SAR)
From the comparison of antimicrobial, antitubercular and anticancer activities of synthesized benzimidazole derivatives, the following SAR may be deduced.
The good antimicrobial activity (minimum MIC values) of the synthesized benzimidazole derivatives compared to the standard drugs cefadroxil and fluconazole may draw an attention that the synthesized benzimidazole derivatives have a very good interaction with target sites and there is a need for further in vivo studies to confirm the antimicrobial activity by taking the most active benzimidazole derivative (compound 10) as a lead compound to develop novel antimicrobial agent.
The appreciable antitubercular activity of the synthesized benzimidazole derivatives compared to the standard drug streptomycin revealed a fact that there is a need for minor structural modifications of benzimidazole derivatives to improve the binding of molecule to tubercular target.
The excellent anticancer activity of the synthesized benzimidazole derivatives compared to the standard drug 5-flourouracil and carboplatin indicated a fact that there is a need for further in vivo studies to confirm the anticancer activity and for developing novel anticancer agent based on synthesized benzimidazole derivatives.
The above results also indicated a fact that different structural requirements are necessary for a compound to show different activities.
The other SAR findings are summarized in .
4 Conclusion
A series of benzimidazole derivatives was synthesized and assessed for its in vitro antimicrobial and anticancer activities. The compounds were also assessed for their in vitro and in vivo antitubercular activity against M. tuberculosis H37Rv. The compounds found to be active in in vivo evaluation in mice models infected with M. tuberculosis were further assessed for their capacity to inhibit the vital mycobacterial enzymes viz., isocitrate lyase, pantothenate synthetase and chorismate mutase. All compounds inhibited these enzymes but to a lesser extent than streptomycin sulphate taken as positive control. Compound 19, the most potent one among the synthesized benzimidazole derivatives exhibited inhibition of 67.56%, 53.45%, and 47.56% against isocitrate lyase, pantothenate synthetase and chorismate mutase, respectively which is comparable to the inhibition of these enzymes by streptomycin sulphate. Most of the synthesized derivatives emerged out as excellent antimicrobial agents as compared to standard antibacterial (cefadroxil) and antifungal (fluconazole) drugs. Compound 10 was found to be the most active antibacterial agent against Gram positive as well as Gram negative bacteria. The results of anticancer activity displayed that majority of the derivatives inhibited the viability of MCF7 cell line, especially; compound 19 was highly potent one among the series (IC50 = 0.0013 µM).
Conflict of interest
There is no conflict of interest among the authors.
Acknowledgement
This research work was supported by the Indian Council for Medical Research, New Delhi, India (Grant No. 45/14/2011/PHA/BMS).
References
- S.S.MorseFactors in the emergence of infectious diseasesEmerg Infect Dis11995715
- Kent MM, Yin S. Controlling infectious diseases. Popul Bull 2006; 61: 1–20.
- N.GautamA.GargA.K.BishnoiS.AgarwalD.C.GautamAntioxidant and antimicrobial assessment of synthesized and spectrally characterized new nitrophenothiazines and their sulfone analoguesPhosphorus Sulfur Silicon Relat Elem1902015528536
- H.W.HethcoteThe mathematics of infectious diseasesSIAM Rev422000599653
- J.CamachoA.BarazarteN.GamboaJ.RodriguesR.RojasA.Vaisberget al.Synthesis and biological evaluation of benzimidazole-5-carbohydrazide derivatives as antimalarial cytotoxic and antitubercular agentsBioorg Med Chem19201120232029
- P.PrakashB.ArunaA.A.SardesaiS.E.HasnainPurified recombinant hypothetical protein coded by open reading frame Rv1885c of Mycobacterium tuberculosis exhibits a monofunctional aroq class of periplasmic chorismate mutase activityJ Biol Chem28020051964119648
- H.AgarwalA.KumarN.C.BalM.I.SiddiqiA.AroraLigand based virtual screening and biological evaluation of inhibitors of chorismate mutase (Rv1885c) from Mycobacterium tuberculosis H37RvBioorg Med Chem Lett17200730533058
- A.AroraN.R.ChandraA.DasB.GopalS.C.MandeB.Prakashet al.Structural biology of Mycobacterium tuberculosis proteins: the Indian effortsTuberculosis912011456468
- H.GuoN.RaoChorismate-mutase catalysed Claisen rearrangementU.NubbemeyerM.HiersemannThe Claisen Rearrangement- Methods and applications2007Wiley-VCH Verlag GmbH & Co. KGaAWeinheim123
- J.M.LiN.LiD.Y.ZhuL.G.WanY.L.HeC.YangIsocitrate lyase from Mycobacterium Tuberculosis promotes survival of Mycobacterium Smegmatis within macrophage by suppressing cell apoptosisChin Med J121200811141119
- V.C.C.UyJ.B.BillonesTowards antituberculosis drugs: virtual screening for potential inhibitors of pantothenate synthetase of Mycobacterium tuberculosisPhilipp Sci Lett52012122130
- American Cancer SocietyBreast Cancer Facts & Figures 2015–20162015American Cancer Society, Inc.Atlanta138
- J.B.WrightThe chemistry of the benzimidazolesChem Rev481951397541
- C.H.SrideviK.BalajiA.NaiduR.SudhakaranSynthesis of some phenylpyrazolo benzimidazolo quinoxaline derivatives as potent antihistaminic agentsE- J Chem (online)72010234238
- P.K.RanjithP.RajeeshK.R.HaridasN.K.SusantaT.N.RowR.Rishikesanet al.Design and synthesis of positional isomers of 5 and 6-bromo-1-[(phenyl)sulfonyl]-2-[(4-nitrophenoxy)methyl]-1H-benzimidazoles as possible antimicrobial and antitubercular agentsBioorg Med Chem Lett23201352285234
- R.K.AroraN.KaurY.BansalG.BansalNovel coumarin-benzimidazole derivatives as antioxidants and safer anti-inflammatory agentsActa Pharm Sin B42014368375
- T.PanX.HeB.ChenH.ChenG.GengH.Luoet al.Development of benzimidazole derivatives to inhibit HIV-1 replication through protecting APOBEC3G proteinEur J Med Chem952015500513
- K.VasanthaG.BasavarajaswamyM.V.RaiP.BojaV.R.PaiN.Shruthiet al.Rapid ‘one-pot’ synthesis of a novel benzimidazole-5-carboxylate and its hydrazone derivatives as potential anti-inflammatory and antimicrobial agentsBioorg Med Chem Lett25201514201426
- M.GabaP.GabaD.UppalN.DhingraM.S.BahiaO.Silakariet al.Benzimidazole derivatives: search for GI-friendly anti-inflammatory analgesic agentsActa Pharm Sin B52015337342
- J.WenY.LuoH.ZhangH.ZhaoC.ZhouG.CaiA green and convenient approach toward benzimidazole derivatives and their antimicrobial activityChin Chem Lett272016391394
- R.S.KeriA.HiremathadS.BudagumpiB.M.NagarajaComprehensive review in current developments of benzimidazole-based medicinal chemistryChem Biol Drug Des862015196510.1111/cbdd.12462
- Y.BansalO.SilakariThe therapeutic journey of benzimidazoles: a reviewBioorg Med Chem20201262086236
- S.YadavB.NarasimhanH.KaurPerspectives of benzimidazole derivatives as anticancer agents in the new eraAnticancer Agents Med Chem16201614031425
- R.A.HaqueS.BudagumpiS.Y.ChooM.K.ChoongB.E.LokeshK.SudeshNitrile-functionalized Hg(II)- and Ag(I)-N-heterocyclic carbene complexes: synthesis, crystal structures, nuclease and DNA binding activitiesAppl Organometal Chem262012689700
- K.F.AnsariC.LalSynthesis physicochemical properties and antimicrobial activity of some new benzimidazole derivativesEur J Med Chem44200940284033
- S.YadavP.KumarE.D.ClercqJ.BalzariniC.PannecouqueS.K.Dewanet al.4-[1-(Substituted aryl/alkyl carbonyl)-benzoimidazol-2-yl]-benzenesulfonic acids: synthesis, antimicrobial activity, QSAR studies, and antiviral evaluationEur J Med Chem45201059855997
- J.G.CappucinoN.ShermanMicrobiology: a laboratory mannual1999Addison Wesley Longman IncCalifornia263
- Pharmacopoeia of India, vol. II. Ministry of Health Department New Delhi: Govt. of India; 1996; p. A-88.
- M.C.Rodriguez-ArguellesE.C.Lopez-SilvaJ.SanmartinP.PelagattiF.ZaniCopper complexes of imidazole-2-pyrrole-2- and indol-3-carbaldehyde thiosemicarbazones: inhibitory activity against fungi and bacteriaJ Inorg Biochem99200522312239
- Parish T, Stroker NG. Mycobacteria Protocols: Methods in molecular Biology. (Vol. 101) Totowa NJ: Humana Press Totowa; 1998, p. 395–422.
- L.A.CollinsS.G.FranzblauMicroplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium aviumAntimicrob Agents Chemother41199710041009
- C.KirimuhuzyaP.WaakoM.JolobaO.OdyekThe antimycobacterial activity of Lantana camara a plant traditionally used to treat symptoms of tuberculosis in South-western UgandaAfr Health Sci920094045
- C.PerezC.AnesiniIn vitro antimicrobial activity of Argentine folk medicinal plants against Salmonella typhiiJ Ethnopharmacol4419944146
- M.J.SanjayNatural products: an important source for antitubercular drugsCRISP520041
- L.TonaK.KambuN.NgimbiK.CimangaA.J.VlietinckAntiamoebic and phytochemical screening of some Congolese medicinal plantsJ Ethnopharmacol6119985765
- G.H.DixonH.L.KornbergAssay methods for key enzymes of the glyoxylate cycleBiochem J7219593P
- M.KratkyJ.VinaovaN.G.RodriguezJ.StolarikovaAntimycobacterial activity of salicylanilide benzenesulfonatesMolecules172013492503
- S.SamalaP.B.DeviR.NallangiP.YogeeswariD.SriramDevelopment of 3-phenyl-4,5,6,7-tetrahydro-1??-pyrazolo[4,3-c]pyridine derivatives as novel Mycobacterium tuberculosis pantothenate synthetase inhibitorsEur J Med Chem692013356364
- S.WangD.EisenbergCrystal structures of a pantothenate synthetase from M. tuberculosis and its complexes with substrates and a reaction intermediateProtein Sci12200310971108
- R.AdepuK.Shiva KumarS.SandraD.RambabuG.Rama KrishnaC.Malla Reddyet al.C-N bond formation under Cu-catalysis: synthesis and in vitro evaluation of N-aryl substituted thieno[2,3-d]pyrimidin-4-(3H)-ones against chorismate mutaseBioorg Med Chem20201251275138
- P.SkehanR.StorengD.ScudieroA.MonksJ.McMahonD.Visticaet al.New colorimetric cytotoxicity assay for anticancer-drug screeningJ Natl Cancer Inst82199011071112
- K.M.HosamaniR.V.ShingalapurSynthesis of 2-mercaptobenzimidazole derivatives as potential anti-microbial and cytotoxic agentsArch Pharm (Weinheim)3442011311319
- A.K.ZainabSynthesis of some new 1, 2, 4-triazoles derived from 2-mercaptobenzimidazoleUm-Salama Sci J62009200208
- Budeanu CH, Rusu G, Cojocaru Z, Nistor, C. Investigations on a series of five-membered nitrogen heterocycles. Synthesis of some new benzimidazolyl-2-mercaptoacetohydrazidehydrazones and testing of their cytostatic action on experimental tumors. Revista Medico-Chirurgicala 1976; 80: 605–9.
- S.EmamiM.FalhatiA.BanifafemiA.ShafieeStereoselective synthesis and antifungal activity of (Z)-trans-3-azolyl-2-methylchromanone oxime ethersBioorg Med Chem12200458815889