Abstract
The objective of this study was to perform phytochemical screening, to determine the content of phenolic compound, to evaluate antioxidant, anti-inflammatory and antiangiogenic activities of ethanol, water-ethanol and water extracts of Lophira procera. Antioxidant activity was determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) and phosphomolybdenum assay, anti-inflammatory activity by proteins denaturation inhibition and membranes stabilization test and antiangiogenic activity by chicken chorioallantoic membrane (CAM) method. The results showed that this plant is rich in saponins, polyphenols, tannins, total flavonoids, proanthocyanidins and coumarins. Extracts presented a strong antioxidant activity (IC50 values of 5.452 ± 0.119 μg/mL and 6.346 ± 0.544 μg/mL and respective AAI of 9.173 ± 0.203 and 7.919 ± 0.711). Excellent anti-inflammatory activity was also observed (IC50 = 16.952 ± 1.897 and IC50 = 23.172 ± 0.066 μg/mL for inhibition of protein denaturation and membrane stabilization respectively). Finally, extracts manifested a very good anti-angiogenic activity (with inhibitions ranging from 57.142 ± 0.124% to 100%). These biological activities are certainly due to high content of phenolic compound. This is the first study to report the phytochemical screening, the content of phenolic compound, the antioxidant, anti-inflammatory and antiangiogenic activities of extract derived from Lophira procera. The use of this plant in traditional medicine against ulcers, breast cancer, kidney and dental pain is therefore justified and its potential as a candidate for bioactive therapeutic molecule.
1 Introduction
Lophira procera is a plant of family ochnaceaes. its vernacular name (Fang) is Akoga and its trade names are Azobe and Bongossi. It is a giant tree of the damp forest, one of the largest in the African virgin forest, easily recognizable by its slender barrel, fairly light brown and its large oblong, erect leaves and tufts at the ends of the branches at the top. This tree is very widespread in Gabon [Citation1] (). In traditional Gabonese medicine, this plant is used in the treatment of several pathologies. The decoction of barks from this plant is used in lotions against the evil of the kidneys; by an anal route against rheumatism and lumbago, this decoction is also used against chronic gonorrhea, sterility, sexual asthenia, ulcers, rheumatoid arthritis and breast cancers. traditional therapists and phytotherapists define cancer as an accumulation of hard clods in the body and plants that reduce these clods are considered anticancer.
Fig. 1 Lophira procera A. Chev. (Ochnaceae). Synonym Lophira alata Banks ex CF Gaertn. Photo taken in a forest of Douala, a village in the town Mitzic/Gabon (Ngoua-Meye-Misso, 2016).

Cancers are among the leading causes of morbidity and mortality in the world. In 2012, there were approximately 14 million new cases and 8.2 million deaths related to the disease. The number of new cases is expected to increase by about 70% over the next two decades [Citation2]. Cancer, or malignant tumor, is characterized by a rapid proliferation of abnormal cells which, beyond their usual delimitation, can invade adjacent parts of the organism and then swarm into other organs. It was recognized in 1941 that cancers develop from “subliminal neoplastic states” caused by viral or chemical carcinogenic agents that induce somatic changes [Citation3,Citation4] . Today, these states correspond to “initiation”, involve DNA alterations, are irreversible and can persist in a tissue until a second type of stimulation called “promotion” occurs [Citation5]. Several promoters directly or indirectly induce cell proliferation, recruit inflammatory cells, increase the production of reactive oxygen species (ROS) leading to oxidative damage to DNA and reduce DNA repair, resulting in replication of DNA and proliferation of cells that have lost normal growth control [Citation6]. Studies have shown that ROS are activators of pro-angiogenic substances such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMP), the goal of which is to induce angiogenesis [Citation6,Citation7] .
Angiogenesis is a process of formation of new vessels from arterial vascularization created by endothelial cells. It is essential for the continuous growth of the tumor because it supplies the tumor with nutrients and oxygen, and eliminates cellular waste, which can be toxic to cancer cells [Citation8]. Inflammatory cells and soluble factors are present in all tumors. Signs of “burning” inflammation that include tissue remodeling, angiogenesis and other wound healing characteristics are usually used by pathologists as morphological indices of invasive cancer. Recent evidence demonstrates that these stromal processes play a fundamental role in the development and progression of cancer and, at least in some cases, can predict the clinical behavior of cancer better than the characteristics of neoplastic cells themselves [Citation9]. There are biomolecules present in plants that can neutralize ROS [Citation10,Citation11] , prevent inflammation and inhibit tumor angiogenesis [Citation11,Citation12] to finally kill the tumor cells. Also, the plants have been at the origin of many active molecules having shown their effectiveness in the treatment of different cancers, such as breast, ovary and lung treat taxol (paclitaxel) which comes from the bark of Pacific yew (Taxus brevifolia).
The aim of this study is to evaluate the antioxidant, anti-inflammatory and antiangiogenic activities of extracts of this plant after carrying out a phytochemical screening.
2 Materials and methods
2.1 Plant material
The stem barks of Lophira procera were collected 23 August 2016 in Mitzic (Woleu-Ntem, Northern of Gabon) (). They were identified at National Herbarium of IPHAMETRA, Libreville (Gabon). Voucher specimen has been deposited in the Herbarium of IPHAMETRA and at Laboratory of Biochemistry Research (LAREBIO), Department of Chemistry-Biochemistry, Faculty of Sciences of USTM in Franceville.
2.2 Preparation of plant extract
Barks were dried at ambient temperature of the Laboratory (20–30 °C) and protected from light for several days. After drying, barks were crushed using a grinder (Laboratory Blender, Torrington, CT. USA). This powder was used for extractions by maceration method. Briefly, 200 g of powder was mixed with 2000 mL of solvent (water, water-ethanol (50/50, v/v) and ethanol). After 72 h, the obtained extract was filtered using Whatman N 1 filter paper. Ethanol and water-ethanol extracts were concentrated under reduced pressure at rotavapor (Büchi, Labortechnik, Switzerland) at 40 and 60 °C respectively. Water extract was lyophilized using a lyophilizer (Alpha 1–2 LDplus, Germany). All crude extracts obtained were stored at 4 °C until analysis.
2.3 Phytochemical screening
Each extract was then tested for the presence of flavonoids, coumarins, tannins, total phenolic, saponosids, cardiac glycosides, reducing sugar, sterols and triterpenes, oses and holosides, anthracenics, anthocyans, alkaloids and anthracenosids as described elsewhere [Citation13].
2.4 Total phenolic content
The Folin-Ciocalteu Method [Citation14] with minor modifications was used to determine the total phenolic contents of the different extracts using gallic acid as standard. The absorbance was measured at 735 nm using a Spectrophotometer (Thermo Scientific, Evolution 60S, USA). Results were expressed as gallic acid equivalent per gram of lyophilized sample (average of the triplicate analysis).
2.5 Total flavonoid content
The aluminum chloride (AlCl3) colorimetric assay method [Citation15] was used to determine total flavonoid contents, using quercetin as a standard [Citation16]. The absorbance was measured at 415 nm and total flavonoid contents were expressed as quercetin equivalents in milligrams per gram sample (average of the triplicate analysis).
2.6 Tannins content
Tannins content was determined according the reference method of European community [Citation17]. The absorbance was measured at 525 nm and tannic acid was used as standard.
2.7 Proanthocyanidins (PAs) content
The proanthocyanidins content was determined according the method described by Prigent [Citation18]. The absorbances were read at 550 nm. Apple procyanidins were used as a standard. Results were expressed as apple procyanidins equivalent (APE).
2.8 Antioxidant activity
2.8.1 DPPH test
The method described by Scherer and Godoy [Citation19], based on the DPPH radical test, was used to determine the Antioxidant Activity Index (AAI). Briefly, DPPH solution was prepared by dissolving 10 mg of DPPH powder in 100 mL ethanol. Graded concentrations of extracts ranging from 3.125 to 100 μg/mL obtained by twofold dilutions were prepared and 100 μL of each dilution were mixed with 100 μL of the working solution of DPPH. Absorbencies were measured at 517 nm after 20 min incubation at room temperature in the dark. Ascorbic acid (vitamin C) and Butylated hydroxytoluene (BHT) were used as references. The ability to scavenge DPPH radical (RSA) was calculated by the following equation: %RSA = [(Acontrol–Asample)/Acontrol] × 100.
A = Absorbance at 517 nm. The IC50 (concentration providing 50% inhibition) of extracts and standards was determinate using regression curves in the linear range of concentrations. The AAI was then calculated as follows: AAI = [DPPH]f (μg.mL−1)/IC50 (μg.mL−1), [DPPH]f is the final concentration of DPPH. We considered criteria of Scherer and Godoy (2009) according to which plant extracts show poor antioxidant activity when AAI < 0.5, moderate antioxidant activity when AAI between 0.5 and 1.0, strong antioxidant activity when AAI between 1.0 and 2.0, and very strong when AAI > 2.0.
2.8.2 Total antioxidant capacity
The assay was based on the reduction of Mo (VI) to Mo (V) and subsequent formation of a green phosphate/Mo (V) complex in acid pH [Citation20]. A total volume of 0.3 mL extract dissolved in methanol was added to 3 mL of reagent solution (0.6 mol/L sulphuric acid, 28 mmol/L sodium phosphate and 4 mmol/L ammonium molybdate). The mixtures were incubated at 70 °C for 90 min then cooled to room temperature. The absorbance was measured at 695 nm. The total antioxidant activity was expressed as the number of equivalence of ascorbic acid, BHT and quercetin.
2.9 In vitro evaluation of anti-inflammatory effect
2.9.1 Test anti-dénaturation of protéins
Protein denaturation methods have been used [Citation21,Citation22] with slight modifications. Briefly, 0.1 mL of albumin from fresh chicken eggs was mixed with 1.9 mL of phosphate buffered saline (PBS, pH 6.4) and 1 mL of varying concentrations of the aqueous extract so that the finals concentrations become 31.25, 62.5, 125, 250 and 500 μg/mL. A similar volume of distilled double water served as a negative control. Then, the mixtures were incubated at 37 °C in an incubator (Ecocell, LSIS-B2V/EC55, Germany) for 20 min and then heated at 70 °C for 5 min. After cooling, the absorbances were measured at 660 nm on the spectrophotometer (Evolution 60S, USA). Diclofenac sodium in the final concentrations of (156.25, 312.5, 625, 1250 and 2500 μg/mL) was used as a reference drug and similarly treated for the determination of absorbance. % Inhibition = [(Abssample–Abscontrol)/Abscontrol] × 100, Abs = absorbance. The concentration of the extract for a 50% inhibition (IC50) was determined by the dose response curve.
2.10 In vivo evaluation of antiangiogenic activity
Antiangiogenic activity was evalued using chick chorioallantoic membrane (CAM) Model according to previously reported method [Citation23,Citation24] with minors modifications. Briefly fertilized chicken eggs were purchased from a local poultry farm, were sterilized with ethanol (70%) and incubated at 37 °C in incubator (Ecocell, LSIS-B2V/EC55, Germany), with 80% relative humidity. On day 2 of post incubation, 3 mL of albumin were withdrawn. A square window of 1 cm2 was opened in the egg shell at the opposite to blunt edge and sealed with an adhesive tape. The eggs were returned for further incubation. At the 8th day, the experimental groups were divided into 5 of each containing 50 numbers of eggs. Group 1, 2, 3 and 4 were treated with water extracts. Sterile discs (diameter: 10 mm) of Whatman N° 1 soaked of 10 μL of the water extract at concentrations ranging from 62.5, 125, 250 to 500 μg/mL was applied to the CAM. In parallel Group 5 treated with phosphate buffered saline (PBS) alone as negative control, a paper disc Whatman N° 1 soaked of 40 μL PBS at pH 7.4 was placed on the CAM of egg. The treated CAM samples were incubated for 48 h. The experiments have been repeated at least four times, and the results were reproducible (see experimental part for details).
After 48 h of incubation at 37 °C and 80% relative humidity, a volume of 10 μL of formaldehyde at 4% was applied to the CAM. 5 min later, the CAM was cut around the disc and all (disc and CAM) was placed in a Petri. Then the photographies were taken with a Nikon digital camera D5100 (made in Thailand) and the images were subsequently analyzed with the software Image J. The numbers of vessel branch points contained in a circular region (equal to the area of each filter disc) were counted manually. The percentage of vascularization (density) is measured relative to a normal control vascularization. The ability to inhibition angiogenesis (AIA) was calculated by the following equation: %AIA = [(Nbvcontrol–Nbvsample)/Nbvcontrol] × 100. Nbv = Number of blood vessel branch points.
2.11 Statistical analysis
The data were expressed as the mean ± standard deviation (SD) of triplicate independent experiments and analyzed using one-way analysis of variance (ANOVA) and Student’s t-test. P < 0.05 was considered to be statistically significant.
3 Results
3.1 Phytochemical screening
Phytochemical screening of the extracts was first performed to detect the major chemical groups occurring in the extracts. In view of the results in , it appears that extracts contain abundant saponosids, polyphenols, sterols, triterpenes, oses, holosides, tannins, alkaloids, total flavonoids, proanthocyanidins, coumarins, reducing sugar and gitoxins.
Table 1 Results of the preliminary phytochemical screening.
3.2 Total phenolic contents
The contents of total phenolic, total flavonoids, total tannins and total proanthocyanidins of extracts from Lophira procera are presented in . The contents of total phenolic in terms of gallic acid equivalent (standard curve equation: Y = 0.0012X–0.0004, R2 = 0.998), ranged from 3446.444 ± 8.388 to 4205.333 ± 6.666 mg GAE/g of extract and were abundant in all extracts.
Table 2 Results of phenolic compounds dosage.
3.3 Total flavonoid contents
Total flavonoids (standard curve equation: Y = 0.0032X + 0.0077, R2 = 1) ranged from 730.093 ± 1.362 to 644.364 ± 1.262 mg QE/g of extract. There were abundant in water extracts than water-ethanol and ethanol extracts.
3.4 Tannins contents
Levels of tannins were expressed in terms of tannic acid equivalent (TAE). The equation of the right-hand side of the proportioning of the total tannins by the reference method of European Community (1994) gave Y = 0.0009X + 0.2088 with R2 = 1. Total tannins are ranged from 3025.777 ± 2.222 to 3298.370 ± 1.697 mg TAE/g of extract and were abundant in water extracts.
3.5 Proanthocyanidins contents
Levels of proanthocyanidins were expressed in terms of apple proanthocyanidins equivalent (APE). The equation of the right-hand side of the proportioning of the proanthocyanidins by the HCl-Butanol method gave Y = 0.0006 X + 0.0024 with R2 = 0.999. Among extracts, proanthocyanidin contents had ranged between 4589.888 ± 7.515 and 5178.777 ± 2.545 mg APE/g of extract. The proanthocyanidins are very abundant in water-ethanol extract.
3.6 Antioxidant activity
3.6.1 DPPH test
The method described by Scherer and Godoy [Citation19], based on the DPPH radical test, was used to determine the Antioxidant Activity Index (AAI). The results showed that the extracts had an antioxidant capacity similar to standard products (). The IC50 value was defined as the concentration of the sample that inhibited 50% of the DPPH. The antioxidant activities of the ethanol and water-ethanol extract were superior to those of the water extract with IC50 values of 5452 ± 0.119 μg/mL and 6346 ± 0.544 μg/mL and respective AAI of 9173 ± 0.203 and 7919 ± 0.711. This antioxidant capacity was close to the ability to inhibit BHT positive controls (IC50 = 6.59 ± 0.301 μg/mL, AAI = 7.58 ± 0.567) and vitamin C (IC50 = 7.12 ± 0.605 μg/mL, AAI = 7.02 ± 0.678).
Table 3 Results of antioxidant activity of extracts from Lophira procera.
3.6.2 Total antioxidant capacity
Antioxidant capacity of the Lophira procera extracts was confirmed with the phosphomolybdenum test which was a quantitative assay [Citation25]. because the antioxidant activity was expressed as vitamin C (VtCE), quercetin (QE) and BHT (BHTE) equivalent, the method was based on the reduction of Mo (VI) to Mo (V) by the extracts resulting in the formation of a Mo (V) complex of green phosphate at acid pH. according to the results, the water-ethanol and ethanol extracts had a capacity greater than that of the water extract. Water-ethanol extract had the highest values with (117.686 ± 0.202) mg/g VtCE, (77.231 ± 0.156) mg/g QE, (504.758 ± 0.974) mg/g BHTE (mg) dry extract followed by ethanol extract with (85.673 ± 0.727) mg/g VtCE, (52.462 ± 0.562) mg/g QE and (350.515 ± 3.502) mg/g BHTE (mg)/g dry extract ().
3.7 Anti-inflammatory effect
3.7.1 Test anti-denaturation of proteins
The Anti-inflammatory activity is summarized in . These results showed inhibition of protein denaturation (albumin) dependent on the concentration of aqueous extract. The aqueous extract was used with concentrations ranging from 31.25 to 500 μg/mL, the percentage inhibition increased with the concentration ranging from 230 ± 0.531% to 4360 ± 1.078%. Diclofenac sodium, used at concentrations ranging from 156.25 to 2500 μg/mL, was used as a reference drug which also showed inhibition of concentration-dependent protein denaturation. The effect of diclofenac sodium was found to be rather low compared to that of the aqueous extract tested.
Table 4 Effect of Lophira procera A. Chev. and diclofenac sodium against protein denaturation.
3.8 Antiangiogenic activity
The antiangiogenic potential of the extracts was assessed in vivo with the chicken chorioallantoic membrane (CAM) the eighth embryonic day. The fertilized eggs were treated with aqueous extracts (62.5–500 μg/mL). The degree of vessel training on CAM was scored 48 h later. The density of vessels represents the percentage of vascularization of the analysis zone. It is inversely proportional to the degree of inhibition. In the presence of phosphate buffer saline (PBS) taken as control, the target zone has a percentage of vascularization of 100%, corresponding to a normal vascularization or control with a number of vessels equal to 7 (b). Analysis of the images revealed two zones on the CAM: a vascularized zone (absence of extract) and an avascularized zone (a). The inhibition of the formation of blood vessels is increased with the concentration of the extract. In the presence of 62.5 μg/mL, 125 μg/mL, 250 μg/mL and 500 μg/mL extract, the inhibition percentage was 57.142 ± 0.124%, 71.428 ± 0.106%, 85.714 ± 0.029% and 100% (). The proportion of eggs exhibiting antiangiogenic effects is summarized in .
Fig. 2 Inhibition of blood-vessels formation on the CAM by the aqueous extract of Lophira procera. The CAM of an 8 days old chick embryo was separately exposed to PBS (control). Extracts were introduced on top of the CAMs. After 48 h of incubation, the CAM tissue directly beneath each filter disc was resected, and digital images of the CAM sections were captured. (a) shows two zones: a vascularized zone around the disc and another avascularized zone, where the disc imbibed with extract has been deposited; (b) control (PBS); (c) water extracted at 62.5 μg/mL; (d) water extracted at 125 μg/mL; (e) water extracted at 250 μg/mL; and (f) water extracted at 500 μg/mL.
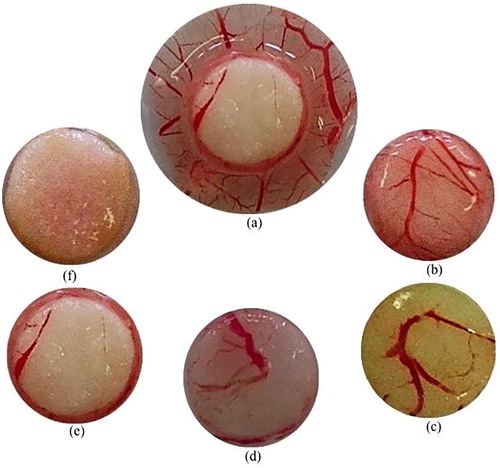
Table 5 Antiangiogenic effect of water extract from Lophira procera.
4 Discussion
It is clearly established that free radicals or ROS are involved in the inflammatory and angiogenic process and that inflammation and angiogenesis are among the main phenomena responsible for growth and tumor invasion [Citation5]. The present study consisted of phytochemical screening, antioxidant, anti-inflammatory and antiangiogenic activities of extracts of Lophira procera, a Gabonese medicinal plant used in traditional medicine to treat ulcers, kidney, dental pain, and breast cancer.
All extracts of this plant showed strong antioxidant activities marked by the inhibition of the DPPH radical and confirmed with reduction of Mo (VI) to Mo (V) by the extracts resulting in the formation of a Mo (V) complex of green phosphate. Ethanol and water-ethanol extract had the highest activity substantially equal to that of vitamin C and BHT. Extracts of this plant can be precursor of potential antioxidant drugs protecting the organism of the free radicals which are at the origin of serious pathologies like the cancers.
In addition to the antioxidant effect, by inhibition of protein denaturation (albumin), water extract shows a very strong anti-inflammatory activity compared to that of the reference molecule, diclofenac sodium. This was confirmed by comparing IC50 values. It is a well known fact that denaturation of tissue proteins leads to inflammatory and arthritic diseases [Citation26]. The production of autoantigens in inflammatory diseases may be due to the denaturation of proteins in vivo. Non-steroidal anti-inflammatory drugs (NSAIDs) may not only inhibit the synthesis of pro-inflammatory prostaglandins but also inhibit protein denaturation [Citation27]. They prevent the denaturation of thermally treated albumin at physiological pH (pH 6.2–6.5) [Citation22]. Therefore, according to the results of this study, the water extract of Lophira procera is able to control the production of autoantigen by inhibiting the denaturation of proteins and can prevent the release of lytic enzymes and active mediators of inflammation by the stabilization of lysosomal membranes. Its use in traditional medicine against renal and dental pain can therefore be justified.
Since the idea of tumor angiogenesis was suggested by Folkman [Citation8], the blocking of tumor-induced angiogenesis was considered an attractive anticancer strategy because anti-angiogenic agents can be used independently of cancer types. The aqueous extract also showed strong antiangiogenic activity by the inhibition of blood vessel formations on chick embryo chorioallantoic membrane (CAM). Inhibition was dose dependent. In the range of doses tested, no dead embryos were recorded, indicating that the antiangiogenic effect observed was not due to the toxicity of the plant. This extract shows a stronger anti-angiogenic activity than the aqueous extracts of Oncoba welwitschii, Tetrorchidium oppositifolium, which showed a percentage inhibition of 83.334% at 500 μg/mL [Citation14]. The CAM is an extraembryonic membrane whose main function is to ensure the exchange of gases and nutrients. Because of its large vascularity and ease of use, CAM is a popular research tool widely used to study glioma angiogenesis, growth, invasiveness and screening of anti-tumor drugs [Citation28]. Therefore, according to the results, the aqueous extract of Lophira procera may have good inhibitory activity on tumor growth by blocking angiogenesis. Thus the use of Lophira procera in traditional medicine against breast cancer can be justified.
According to the phytochemical screening, Lophira procera is rich in phenolic compounds including total polyphenols, total flavonoids, tannins and proantocianidins. It has been reported that there is a correlation between total polyphenol content and antioxidant activity [Citation29]. The present invention relates to a method for the preparation of antiangiogenesis agents [Citation30,Citation31] .
5 Conclusion
In conclusion, this is the first study carried out on Lophira procera. It has been shown that water, water-ethanol and ethanol extracts have a strong antioxidant activity marked by the inhibition of DPPH radical and by reduction of Mo (VI) to Mo (V), a strong anti-inflammatory activity manifest by the inhibition of protein denaturation and a strong antiangiogenic activity marked by the inhibition of vascularization in CAM. The study also identified the different chemical groups present in this plant. These biological activities are due to the high content of phenolic compounds in this plant. The use of this plant in traditional medicine as anti-ulcers, anti-breast cancer, anti-pain is therefore justified. The isolation of the phenolic compounds responsible for these activities will be the subject of next study.
Acknowledgements
The authors are very much thankful to Shell-Gabon for the financial support of materials in Laboratory of Research in Biochemistry of University of Sciences and Technology of Masuku, Franceville-Gabon (Grant No. SG/CIS/SDM/SA/ sa n° 77). We are very much grateful to local informants and Mr. MEYE MISSO Paul-Edouard who shared their knowledge on the use of medicinal plants with us.
References
- Walker R, Sillans S., Plantes utiles du Gabon. Ed Lechevalier. Sepia, 1961, p. 614.
- WHO. Cancer Media Center. Fact sheet N° 297, 2017.
- P.RousJ.KiddConditional neoplasms and subthreshold neoplastic states: a study of the tar tumors of rabbitsJ Exp Med731941365389
- I.C.MackenzieP.RousThe experimental disclosure of latent neoplastic changes in tarred skinJ Exp Med731941391415
- L.M.CoussensZena-Werb. Inflammation and cancerNature4202002860867
- Y.W.KimT.V.ByzovaOxidative stress in angiogenesis and vascular diseaseBlood1232014625631
- Bhatia M, Karlenius TC, Di Trapani G, Tonissen KF. The interaction between redox and hypoxic signalling pathways in the dynamic oxygen environment of cancer cells. In: tonissen KF, editor. Carcinogenesis. Rijeka, Croatia: InTech; 2013. p. 125–152.
- J.FolkmanTumor angiogenesis: Therapeutic implicationsN Engl J Med285197111821186
- G.FinchN.BertosF.PepinS.SadekovaM.SouleimanovaH.Zhaoet al.Stromal gene expression predicts clinical outcome in breast cancerNat Med142008518527
- C.Sima-ObiangJ.P.OndoG.R.Ndong-AtomeL.C.Obame-EngongaJ.F.Djoba-SiawayaE.Nsi-EmvoPhytochemical screening, antioxidant and antimicrobial potential of stem barks of Coula edulis Baill. Pseudospondias longifolia Engl. and Carapa klaineana Pierre. from GabonAsian Pac J Trop Dis62016557563
- R.L.Ngoua-Meye-MissoJ.P.OndoE.J.N.AssamB.J.O.OrangoC.Sima-ObiangJ.D.L.C.Ndonget al.Phytochemical screening, antioxidant and antiangiogenic properties of Oncoba welwitschii (Oliv.) Gilgn. and Tetrorchidium oppositifolium (Pax. and Khoffm.), medicinal plants from GabonInt J Innov Res Sci Eng Technol62017110
- O.SwastikaW.NastitiR.BambangAnti-angiogenic effect of Artocarpus heterophyllus seed methanolic extract in ex ovo chicken chorioallantoic membraneAsian Pac J Trop Biomed72017240244
- I.CuleiMethodology for the analysis of vegetable drugs: practical manual on the industrial utilization of medicinal and aromatic plants1982Center BuildingRomania6781
- L.S.VernonR.OrthoferL.R.M.RaventosAnalysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagentMethods Enzymol2991999152178
- C.Quettier-DeleuB.GressierJ.VasseurT.DineC.BrunetPhenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flourJ Ethnopharmacol7220003542
- C.Sima-ObiangE.L.C.ObameJ.P.OndoC.ZongoE.E.NsiS.A.TraoréEthnotherapy study, phytochemical screening and antioxidant activity of Antrocaryon klaineanum Pierre and Anthocleista nobilis G. Don. medicinal plants from GabonInt J Adv Res32015812819
- Methods of reference for the determination of tannins J. official of the European Communities. 1984; 197: p. 18–20.
- Prigent S. Interactions of phenolics compounds with globular proteins and their effects on food related functional properties. PhD Thesis, Wageningen University, Wageningen, The Netherlands 2005; p. 131–133.
- R.SchererH.T.GodoyAntioxidant activity index (AAI) by 2,2-diphenyl- 1-picrylhydrazyl methodFood Chem1122009654658
- J.KubolaS.SiriamornpunPhenolic content and antioxidant activities of bitter gourd (Momordica charantia L.) leaf stem and fruit fraction extracts in vitroFood Chem1102008881890
- C.PriyankaC.SangitaD.ProtapadityaB.SanjibEvaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro studyJ Adv Pharm Technol Res32012136138
- L.A.D.WilliamsA.O'ConnarL.LatoreO.DennisS.RingerJ.A.Whittakeret al.The in vitro Anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (Immunogenic) Bovine Serum Albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery processWest Indian Med J572008327331
- D.RibattiB.NicoA.VaccaM.PrestaThe gelatin sponge-chorioallantoic membrane assayNat Protoc120068591
- D.RibattiChick embryo chorioallantoic membrane as a useful tool to study angiogenesisInt Rev Cell Mol Biol2702008181224
- P.PrietoM.PinedaM.AguilarSpectrophotometric quantitation of antioxidant capacity through the formation of a formation of phosphomolybdenum complex: specifics application to the determination of vitamin EAnal Biochem2691999337341
- E.L.OpieOn the relation of necrosis and inflammation to denaturation of proteinsJ Exp Med1151962597608
- J.SadiqueAL-Rqobahs WABughait MF, El Gindi AR, Fitoterapia601989525532
- Y.J.YuanK.XuW.WuQ.LuoJ.L.YuApplication of the chick embryo chorioallantoic membrane in neurosurgery diseaseInt J Med Sci11201412751281
- A.BenabdallahC.RahmouneM.BoumendjelO.AissiC.MessaoudTotal phenolic content and antioxidant activity of six wild Mentha species (Lamiaceae) from northeast of AlgeriaAsian Pac J Trop Biomed62016760766
- S.MahmoudiM.KhaliA.BenkhaledK.BenamiroucheI.BaitiPhenolic and flavonoid contents, antioxidant and antimicrobial activities of leaf extracts from ten Algerian Ficus carica L. varietiesAsian Pac J Trop Biomed62016239245
- J.S.LeeS.ShuklaJ.A.KimM.KimAnti-angiogenic effect of Nelumbo nucifera leaf extracts in human umbilical vein endothelial cells with antioxidant potentialPLoS One102015e0118552
