Abstract
In this study, the pink yeast Rhodotorula sp. strain ATL72 was isolated from salt marches near Mediterranean Sea, Egypt. From phylogenetic analysis, the isolated strain ATL72 was closely related to Rhodotorula bloemfonteinensis EU075187 by similarity of 40%. The biological synthesis of nanosilver (AgNPs) using the marine pink yeast Rhodotorula sp. strain ATL72 was established. The UV–Visible spectral analysis confirmed the synthesis of AgNPs showing a characteristic peak around 400–500 nm. TEM analysis not only confirmed the synthesis of AgNPs but also described the spherical and oval shaped nanoparticle besides size measurements ranged from 8.8 to 21.4 nm. The biosynthesized AgNPs showed a strong antimicrobial activity by causing a complete inhibition of growth for a wide range of Gram positive and Gram negative bacteria as well as fungi with low MIC value. In conclusion, the pink yeast Rhodotorula sp., strain ATL72 isolated from Egypt is a promising new biological source for the synthesis of silver nanoparticles having a potent antimicrobial activity against a wide range of pathogenic bacteria and fungi.
1 Introduction
Rhodotorula sp. is unicellular yeast, characterized by a high growth rate. It has a yellow, orange or red color and known as caretogenic yeast or pink yeast due to its production of carotenoids [Citation1]. It is widely distributed and isolated from several sources such as soil, fresh water, flowers, sea water, and food [Citation2].
A lot of studies have been discussed about the importance of genus Rhodotorula in the field of biotechnology and its vital applications in the future of industry, which negates the thought that genus Rhodotorula is considered as saprophytes [Citation3]. Metal nanoparticles such as silver nanoparticles (AgNPs) possess diverse technological applications due to their particular chemical, optical, electronic and magnetic characteristics that are completely different from the characters of the bulk metals [Citation4]. One of the synthesis techniques of AgNPs is the biological synthesis [Citation5]. It is high yield, low cost, non-toxic and eco-friendly process which can use different biological resources such as plants, bacteria, fungi, yeast, algae and viruses in the synthesis of nanoparticles [Citation6]. Recent studies confirmed the ability of genus Rhodotorula such as Rhodotorula minuta [Citation7] and Rhodotorula glutinis [Citation8] to synthesis metal nanoparticles.
One of the most vital applications of the metal nanoparticles, especially silver nanoparticles, in the field of medicine is using these nanoparticles as antimicrobial agents. The lethal activity of nanoparticles against broad spectrum of Gram-positive bacteria, Gram-negative bacteria and fungi has been approved [Citation9]. This study aimed to add a new biological source for the synthesis of silver nanoparticles besides monitoring the microbial biochemical changes and evaluating the antimicrobial activity of variable silver nanoparticles separated at different centrifugation speeds.
2 Materials and methods
2.1 Isolation and purification of marine pink yeast
Water samples were collected from salt marches near Mediterranean Sea and then diluted via serial dilution method. From the dilutions; 10−5, 10−6 and 10−7, 100 µL were inoculated into 50 ml of liquid enriched medium (yeast extract 5 g/l, glucose 10 g/l and 1 L of sea water) [Citation10]. Antibacterial antibiotics were added by the recommended concentrations immediately before the inoculation process to control the bacterial growth. The flasks were incubated on an incubator shaker at 30 °C and 180 rpm. Each week, samples from each dilution were taken to inoculate a plate of agar enriched medium using the compound streaking method till the appearance of pure pink colonies.
2.2 Molecular identification of the isolated yeast
Total genomic DNA (gDNA) from the strain ATL72was extracted using the Fast DNA® Spin Kit, after that18S rRNA gene was amplified by PCR reaction using the universal forward and reverse primers; 18S rRNA-F:5′-TACCTGGTTGATCCTGCCAG-3′ and 18S rRNA-R: 5′- CCTTCCGCAGGTTCACCTAC-3′ [Citation11]. The sequencing of the PCR product was done using ABI PRISM®BigDyeTM Terminator Cycle Sequencing Kits, followed by basic local alignment search tool (BLAST) and SeqMatch analysis. The quality and quantity of the obtained sequence was examined by Finch TV version 1.4.0. While, assembling the gene contiguous sequences was done by CAP3 software. The 18S rRNA gene sequence of the isolate ATL72 was submitted to the Gene bank under the accession number MG021304 and used for construction of the phylogenetic tree using Sea view software and automated BLAST search for detecting the closest type strain to the isolate ATL72.
2.3 Preparation of AgNPs
Into 100 ml of liquid optimized medium (1 g/l yeast extract, 3.7 g/l malt extract, 35 g/l Nacl, 7.7 g/l fructose and 9 g/l urea), pink yeast were inoculated and incubated in shaking incubator for 72 h at 28 °C with 150 rpm. After that, the liquid culture was centrifuged and the pellet washed extensively three times with sterile distilled water to remove any residues from the media. To have cell free water extract, pellet was added to 100 ml of sterile deionized water in a shaker incubator at 27.5 °C at 150 rpm for overnight and then centrifuged to remove the biomass by decantation-centrifugation. AgNO3 (1 mM) was added to the water extract and kept for 24 h in dark conditions [Citation8]. Control flasks contain deionized water and AgNO3 without cell filtrate. After 24 h the samples showed any change in color were subjected to further analysis.
2.4 UV–Visible spectral analysis of AgNPs
The formation of nanoparticles was monitored using both visual observation of the color and UV–visible spectral analysis (Unicam UV–VIS. Spectrometer UV2, U.S.A). It was noticed that the filtrate was colorless before incubation with silver nitrate then the color turned to dark brown after complete reduction of silver ions. Silver nanoparticles were scanned using spectrophotometer at wavelengths from 200 to 800 nm [Citation12]. The filtrate without silver nitrate was used as a blank sample.
2.5 Transmission electron microscopic (TEM) analysis of AgNPs
For determination of size and the shape of AgNPs, Transmission Electron Microscope (JEOL JEM-2100, U.S.A) was used. The process was performed by coating of AgNPs onto carbon coated grid (Type G 200, 3.05 µµdiameter, TAAP, U.S.A) [Citation13].
2.6 Selected area diffraction pattern (SADP) analysis for AgNPs
SADP is a crystallographic technique accomplished using the transmission electron micrographs and requires a very thin sectioned specimen about 100 nm and about 100–400 KeV as an energy electron volt [Citation14].
2.7 Antimicrobial activity and determination of MIC of AgNPs
AgNPs were centrifuged at three different speeds 4000, 8000 and 14000 rpm to study their activity at each speed. The precipitate of nanoparticles was dried, weighted and suspended in 1 ml of deionized water and subjected to sonication for getting homogenized suspension [Citation15].
Streptococcus sp., Bacillus sp., Staph sp., Shigella sp., Escherichia coli, Pseudomonas aeruginosa, Klebsiella sp. and Candida sp. were provided from Genetic Engineering and Biotechnology Unit, Faculty of Science, Mansoura University, Egypt, to study the antimicrobial activity of the biosynthesized AgNPs [Citation16]. These pathogens were activated by culturing 100 µL of each in 10 ml LB medium (10 g/l Pepton, 10 g/l NaCl, 5 g/l Yeast Extract) and incubated at 37 °C for 24 h with shaking at 150 rpm [Citation17]. In 10 ml LB broth, 100 µL of AgNPs from each speed (4000, 8000 and 14,000 rpm) and 100 µL of each pathogen were added. The microbial growth was monitored by measuring the optical density (OD) at 600 nm [Citation18].
For MIC determination [Citation19] of the biosynthesized AgNPs, 100 µL AgNPs from different concentrations (50, 25, 10,5,1,0.5 and 0.25 µg/ml) from each speed (4000, 8000 and 14000 rpm) and 100 µL from each test organism (E. Coli, Candida sp. and Bacillus sp.) were applied to 5 ml LB broth medium and incubated at 37 °C with shaking for 24 h. AgNPs with inoculum in broth media were used as a positive control. The results were monitored by measuring the mean OD at 600 nm [Citation20].
2.8 Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE)
SDS-PAGE was used to investigate the effect of AgNPs collected at different centrifugation speeds (4000 and 14,000 rpm) on selected pathogens E. coli, Candida sp. and Bacillus sp.. Total proteins from pellets of the treated microorganisms were extracted in 100 mM phosphate buffer at pH 7. For loading protein over the acrylamide gel, 10 µg protein concentrations from each sample were boiled in 2X sample buffer (2.5 ml Tris-HCl pH 6.8, 10 ml Distilled water, 4 ml 10%SDS, 2 ml Glycerol and 1 ml β-mercaptoethanol) for 2 min. Acrylamide gel was prepared in two layers; 4%stacking gel above 12% separating gel [Citation21].The electrophoresis was applied for 2 h at 100 V, then the gel was stained in Commassie Brilliant Blue R250 overnight, followed by de-staining on shaker for few hours. Using Gel Analyzer3 Program, the gel was analyzed.
3 Results
3.1 Isolation and purification of marine pink yeast
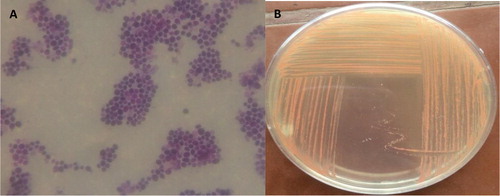
Under light microscope, the stained cells of the isolated yeast strain were circular in shape and the colonies were smooth, soft and orange in color ().
3.2 Molecular identification
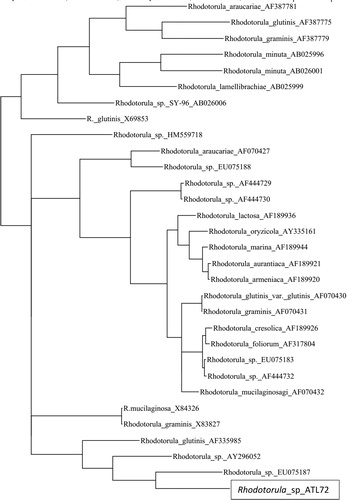
By analysis of the phylogenetic tree, the phylogenetic position of the isolate ATL72 was compared to the closely related species of the genus Rhodotorula. Strain ATL72. It was found that, the closely related species is Rhodotorula bloemfonteinensis EU075187 with percentage similarity of 40% as shown in .
3.3 Biosynthesis of AgNPs
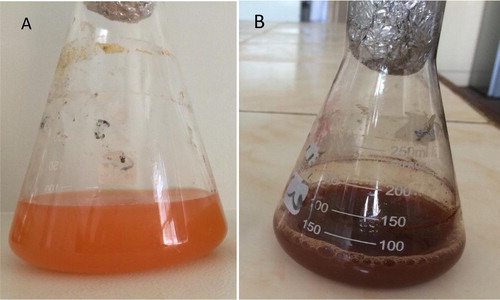
After incubation of cell free water extract with 1 mM silver nitrate, the color changed from orange into dark brown color which indicates for the formation of nanosilver as shown in .
3.4 UV–Visible spectral analysis of AgNPs
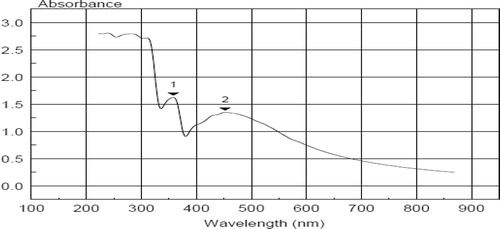
The absorption spectrum of the dark brown AgNPs suspension represented in showed a surface Plasmon absorption band with a peak of at450nm, which indicates the formation of nano-sized particles and another beak ∼350.
3.5 TEM analysis of AgNPs
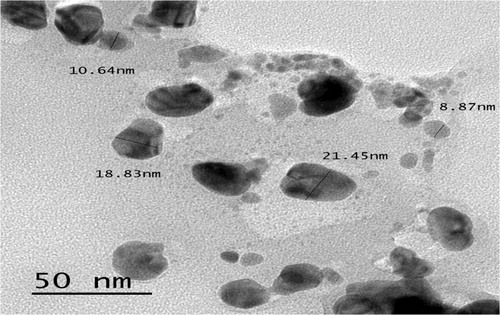
The TEM analysis of AgNPs showed that, the nanoparticles have variable shapes as spherical or oval shaped and the size is ranged between 8.8 and 21.4 nm as shown in .
3.6 SADP analysis of AgNPs
The diffraction pattern of the nanosilver particles () appeared as lighted spots on the dark field also reflect the crystalline structure and the diffraction rings of the nanoparticles of silver.
3.7 Antimicrobial activity of AgNPs and determination of its MIC
At OD600, the growth of all pathogens including Gram positive bacteria (Streptococcus sp., Bacillus sp., Staphylococcus sp.) and Gram negative bacteria (Shigella sp., Escherichia coli, Pseudomonas areuginosa and Klebsiella sp.) as well as one fungus Candida sp. were completely inhibited after treatments by all AgNPs collected at the three different centrifugation speeds 4000, 8000 and 14,000 rpm ().
Table 1 Effect of AgNPs synthesized by pink yeast on microbial growth.
The concentration 1 µg/ml of AgNPs precipitated by any speed is the MIC concentration that completely inhibits the growth of the three selected pathogens Bacillus sp., E. coli and Candida sp. ().
Table 2 MIC of AgNPs synthesized by pink yeast on growth (OD600) of E. coli, Bacillus sp. And Candida sp.
3.8 Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE)
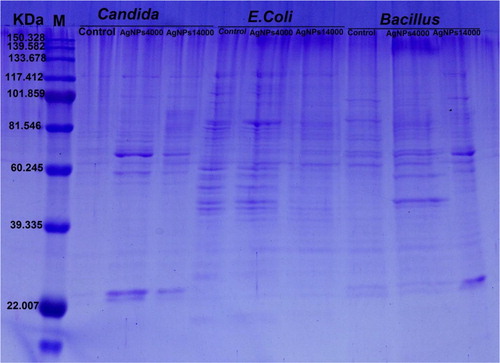
SDS-PAGE analysis of proteins extracted from Candida sp., E. coli and Bacillus sp. control and treated with AgNPs at two separation speeds showed variable changes in the protein pattern (). These changes were highly pronounced with treatments of AgNPs at the high speed separation whereas the change is low at the low speed separation. Generally, the presence of new protein bands and increasing in band intensity of samples treated with AgNPs were more than in untreated samples. In E. coli, there was no change in the protein profile or the intensity of the bands in treated and untreated samples of that separated at 4000 rpm speed.
4 Discussion
Nanobiotechnology is a branch of the science which manipulates the properties and the behavior of materials at nanoscale for numerous applications for serving the mankind [Citation5]. In the present study, a Rhodotorula sp., strain ATL72, a carotenoid producing yeast, was isolated from different localities of salt marches near Mediterranean sea, Egypt. Morphological and molecular tools were used for identification of the strain. The dendogram analysis of its 18S rRNA gene showed the similarity between it and Rhodotorula bloemfonteinensis EU075187 with percentage of 40%. This result indicated the possibility that the isolated Rhodotorula Strain ATL72 might be a new strain.
Biosynthesis of silver nanoparticles from the isolated strain Rhodotorula sp. ATL72 was performed using the cell free water extract approach. The dark brown color of the supernatant raised due to the bio-reduction of AgNO3 by the reducing proteins in the water extract of the yeast [Citation22] and other microorganisms [Citation23]. A novel technique depending on differential freezing and thawing of samples was applied for separating metallic nanoparticles from a culture of yeast MKY3 [Citation24].Yeast strains possess more benefits over bacteria in the mass production of NPs due to the easiness of controlling yeasts in laboratory circumstances besides the synthesis of numerous enzymes and rapid growth with the use of simple nutrients [Citation25].
Silver nanoparticles were characterized by UV–vis spectroscopy, giving a strong broad surface Plasmon peak ∼450 which has been documented for various metal nanoparticles [Citation26]while the other peak ∼ 350 may attributed to the presence of contaminating proteins in the sample. TEM analysis of silver nanoparticles showed irregular oval or rounded shapes exhibiting sizes ranging from 8.8 to 21.4 nm as pronounced in several publications [Citation7,Citation8,Citation24] .
The results of antimicrobial activity of AgNPs on different Gram positive, Gram negative bacteria and yeast indicated the complete inhibition of growth of these organisms. These results were confirmed by previous extensive studies on Candida albicans, Salmonella enterica, E. coli, and Vibrio parahaemolyticus [Citation27]. Also, AgNPs can be definitely used as effective antibiotics against E. coli, Salmonella typhimurium, Staphylococcus epidermidis and Staphylococcus aureus [Citation28]. The effect of AgNPs as antimicrobial agent depends on the shape of nanoparticles as encountered from the work monitoring the effect of different shapes of AgNPs (spherical, rod-like and triangular) on E. coli and found that triangular-shaped nanoparticles are the most effective shape [Citation29].
The obtained MIC results (1µg/ml) of AgNPs are the same at any separating speed. This result is more or less similar to what reported for Candida albicans treated with a MIC value of 2 µg/ml AgNPs [Citation30] and with that for both E. coli and Bacillus sp. treated with MIC value of 1 µg/ml AgNPs[Citation16]. On the other hand, high MIC value of 10 µg/ml was reported for E. coli [Citation31,Citation20] .
The SDS-PAGE analysis showed qualitative and quantitative changes in protein profile after treatment of Candida sp. and Bacillus sp. by AgNPs. The appearance of new bands and the increasing in band intensity might be attributed to the ability of AgNPs to induce new proteins as a stress response [Citation32]. While in case of E. coli treated with AgNPs the protein profile remain unchanged, although the band intensities were comparatively less than those of the control. This implies that the affinity of AgNPs to the thiol groups of the proteins causing unfolding of the protein chain might cause degradation of the proteins. Also, previous studies reported that AgNPs may implicate in preventing the synthesis of the proteins by inhibiting many translation factors [Citation33,Citation34] .
In conclusion, the pink yeast Rhodotorula sp., strain ATL72 isolated from Egyptis a promising new biological source for the synthesis of silver nanoparticles having a potent antimicrobial activity against a wide range of pathogenic bacteria and fungi.
Acknowledgments
Many thanks to Prof. Samia Ali Haroun, the former head of Botany Department, Faculty of Science, Mansoura University, for continuous support and valuable discussion.
Competing interests
The authors declare that they have no competing interests.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Marova I, Certik M, Breierova E. Production of enriched biomass by carotenogenic yeasts-application of whole-cell yeast biomass to production of pigments and other lipid compounds. In: Dr. Darko Matovic, editor. Biomass-detection, production and usage. InTech; 2011. ISBN: 978-953-307-492-4.
- A.A.E.El-bannaA.M.El-razekA.R.El-mahdyIsolation, identification and screening of carotenoid-producing strains of Rhodotorula glutinisFood Nutr Sci32012627633
- A.M.KotS.BłażejakA.KurczI.GientkaM.KieliszekRhodotorula glutinis—potential source of lipids, carotenoids, and enzymes for use in industriesAppl Microbiol Biotechnol10014201661036117
- R.DasS.GangandS.NathPreparation and anti-bacterial activity of silver nanoparticlesJ Biomater Nanobiotechnol242011472
- V.SarsarK.K.SelwalM.K.SelwalNanosilver: potent antimicrobial agent and its biosynthesisAfr J Biotechnol1342014546554
- K.N.ThakkarS.S.MhatreR.Y.ParikhBiological synthesis of metallic nanoparticlesNanomed Nanotechnol Biol Med622010257262
- M.SubramanianN.M.AlikunhiK.KandasamyIn vitro synthesis of silver nanoparticles by marine yeasts from coastal mangrove sedimentAdv Sci Lett342010428433
- M.ZahranA.MohamedF.MohamedM.El-RafieOptimization of biological synthesis of silver nanoparticles by some yeast fungiEgypt J Chem561201391110
- V.DhandL.SoumyaS.BharadwajS.ChakraD.BhattB.SreedharGreen synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activityMater Sci Eng, C5820163643
- N.Gunde-CimermanP.ZalarS.de HoogA.PlemenitašHypersaline waters in salterns–natural ecological niches for halophilic black yeastsFEMS Microbiol Ecol3232000235240
- A.GuptaJ.VongsvivutC.J.BarrowM.PuriMolecular identification of marine yeast and its spectroscopic analysis establishes unsaturated fatty acid accumulationJ Biosci Bioeng11442012411417
- M.A.MahmoudS.A.Al-SohaibaniM.R.Al-OthmanA.Abd El-AzizS.A.EifanSynthesis of extracellular silver nanoparticles using Fusarium semitectum (KSU-4) isolated from Saudi ArabiaDigest J Nanomat Biostruct822013589596
- Z.WangTransmission electron microscopy of shape-controlled nanocrystals and their assembliesJ Phys Chem B1046200011531175
- A.AhmadP.MukherjeeS.SenapatiD.MandalM.IslamKhanR.Kumaret al.Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporumColloids Surf, B282003313318
- J.R.MoronesJ.L.ElechiguerraA.CamachoK.HoltJ.B.KouriJ.T.Ramírezet al.The bactericidal effect of silver nanoparticlesNanotechnology1610200523462353
- S.ShahrokhG.EmtiaziToxicity and unusual biological behavior of nanosilver on gram positive and negative bacteria assayed by microtiter-plateEur J Biol Sci1320092831
- M.ZareiA.JamnejadE.KhajehaliAntibacterial effect of silver nanoparticles against four foodborne pathogensJundishapur J Microbiol71201420084161
- W.R.LiX.B.XieQ.S.ShiH.Y.ZengO.Y.You-ShengY.B.ChenAntibacterial activity and mechanism of silver nanoparticles on Escherichia coliAppli Microbiol Biotechnol854201011151122
- R.KrishnanV.ArumugamS.K.VasaviahThe MIC and MBC of silver nanoparticles against enterococcus faecalis-a facultative anaerobeJ Nanomed Nanotechnol63201521577439
- W.R.LiX.B.XieQ.S.ShiH.Y.ZengO.Y.You-ShengY.B.ChenAntibacterial activity and mechanism of silver nanoparticles on Escherichia coliAppl Microbiol Biotechnol854201011151122
- U.K.LaemmliCleavage of structural proteins during the assembly of the head of bacteriophage T4Nature2271970680685
- N.AsmathunishaK.KathiresanA review on biosynthesis of nanoparticles by marine organismsColloids Surf, B1032013283287
- Rai M, Duran N. Biogenic nanoparticles: an introduction to what they are, how they are synthesized and their applications. In: Rai M, Duran N, editors. Metal nanoparticles in microbiology. Springer Science & Business Media; 2011. ISBN: 978-3-642-18311-9.
- M.KowshikS.AshtaputreS.KharraziW.VogelJ.UrbanS.K.Kulkarniet al.Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3Nanotechnology141200295100
- D.KumarL.KarthikG.KumarK.B.RoaBiosynthesis of silver anoparticles from marine yeast and their antimicrobial activity against multidrug resistant pathogensPharmacologyonline3201111001111
- A.HengleinPhysicochemical properties of small metal particles in solution: microelectrode reactions, chemisorption, composite metal particles, and the atom-to-metal transitionJ Phys Chem9721199354575471
- Wang C., Kim Y.J., Singh P., Mathiyalagan R., Jin, Yang DC. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol 2016;44(4):1127–113.
- J.JainS.AroraJ.M.RajwadeP.OmrayS.KhandelwalK.M.PaknikarSilver nanoparticles in therapeutics: development of an antimicrobial gel formulation for topical useMol Pharm65200913881401
- S.PalY.K.TakJ.M.SongDoes the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the gram-negative bacterium Escherichia coliAppl Environ Microbiol736200717121720
- J.KimS.KukE.YuK.NamKimJ.HoParkS.Jin Leeet al.Antimicrobial effects of silver nanoparticlesNanomed Nanotechnol Biol Med3200795101
- M.SadeghiS.K.JamaliA.AmininiaS.GhafariSynthesis and characterization of silver nanoparticles for antibacterial activityInt J Nano Dimension122010119124
- K.MarkowskaA.M.GrudniakK.KrawczykI.WrobelandK.I.WolskaModulation of antibiotic resistance and induction of a stress response in Pseudomonas aeruginosa by silver nanoparticlesJ Med Microbiol6362014849854
- P.VelusamyG.V.KumarV.JeyanthiJ.DasR.PachaiappanBio-inspired green nanoparticles: synthesis, mechanism, and antibacterial applicationToxicological Res322201695102
- S.K.GogoiP.GopinathA.PaulA.RameshS.S.GhoshA.ChattopadhyayGreen fluorescent protein-expressing escherichia c oli as a model system for investigating the antimicrobial activities of silver nanoparticlesLangmuir2222200693229328