?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The beneficial effects of Ocimum gratissimum, have been attributed mainly to its antioxidant and anti-inflammatory properties. This study investigated the protective effects of aqueous extract of Ocimum gratissimum leaves (AEOGL) on acetic acid induced colitis in male rats. Twenty male Wistar rats, 100–180 g were divided into four groups as follows: Group 1 (control) (n = 5) received 2 ml/kg of distilled water for 21 consecutive days. Group 2 (n = 5) received 2 ml of 6% acetic acid solution once intra rectally for induction of colitis. Group 3 and 4 (n = 5 each) were treated as group 2 and thereafter received AEOGL orally at 200 and 400 mg/kg/day respectively for 20 consecutive days. All the animals from each group were sacrificed 24 h after the induction of colitis and administration of AEOGL. The diarrhea score, ulcer score, hematological parameters, nitric oxide (NO), myeloperoxidase (MPO), superoxide dismutase (SOD), reduced glutathione (GSH) markers and histopathological alteration were evaluated. Acetic acid-induced colitis significantly caused alteration in diarrhea score, ulcer score, hematological parameters, MPO and SOD activities, NO and GSH levels (p < 0.05). It induced significant inflammation of the colonic tissue. AEOGL administration significantly decreased diarrhea score and ulcer score to normal (p < 0.05). It prevented alteration effect of acetic acid on hematological parameters and significant decreases the activities of MPO, SOD, NO and increase GSH levels (p < 0.05) in colitis rats. In conclusion, Ocimum gratissimum leaves possesses ameliorative effects against acetic acid-induced colitis as a consequence of its anti-inflammatory and anti-oxidative properties.
Introduction
Ulcerative colitis (UC) is an idiopathic inflammatory bowel disease that affects the colonic mucosa and is clinically characterized by diarrhea, abdominal pain and hematochezia. The prevalence of inflammatory bowel diseases, including ulcerative colitis, is generally higher, with an estimated of 250 cases per 100,000 individuals in western countries but is becoming common in rest of the world due to the adoption of western lifestyle [Citation1,Citation2] . The etiology of inflammatory bowel diseases is still not fully understood but it is widely acknowledged that they result from complex interplay among genetic, environmental, microbial and immune factors Citation[3]. Worsen and inappropriate mucosal immune response mediated by mucosal T cells triggers the release of several pro-inflammatory mediators, including reactive oxygen and nitrogen species, neutrophil infiltration and overproduction of pro- and anti-inflammatory cytokines Citation[3]. All these factors can cause tissue damage and are thought to be critical events in the pathogenesis of ulcerative colitis.
Inflammatory bowel diseases disorders are multifactorial with no therapeutic agents available to control both inflammatory immune response and oxidative stress effectively Citation[1]. However, the available drugs, such as 5-amino salicylic acid (5-ASA) derivatives, monoclonal antibiotics, steroids (glucocorticoid), anti-tumor necrosis factor (TNF)-α (infliximab) and immunosuppressive agents, have exhibited beneficial effects in the treatment of inflammatory bowel disease Citation[4]. Indeed, most of these drugs bring serious side effects with low results and many patients do not respond to these treatment Citation[5]. Based on the dissatisfaction with the current pharmacology treatments, focus has been increased on complementary medicine approaches, including medicinal plant extract or natural active compound extract from the plants Citation[6]. There is a great need to identify effective chemopreventive agents that might improve colitis with fewer or no side effects.
Ocimum gratissimum (OG) is an herbaceous plant which belongs to the Labiatae family. The plant is indigenous to tropical areas especially India and West Africa. In Nigeria, it is found in the Savannah and coastal areas Citation[7]. It is known by various names in different parts of the world. In India it is known by its several vernacular names, the most commonly used ones being Vriddhutulsi (Sanskrit), Ram tulsi (Hindi), Nimmatulasi (Kannada) Citation[8]. In Nigeria, the plant is called “effinrin-nla” by the Yoruba speaking tribe, “Ahuji” by the Igbos, and “Daidoya” by the Hausas Citation[7]. It is commonly used in folk medicine to cure many diseases due to its potent bioactive principles including tannins, saponins, flavonoids, phenols and anthraquinone glycosides Citation[9]. Antioxidant vitamins; alpha-tocopherol and ascorbic acid have been detected in its leaves extracts Citation[9]. The flowers and the leaves of this plant are rich in essential oils so it is used in preparation of teas and infusion Citation[10]. Ocimum gratissimum is used in Nigeria in the management of baby’s cord, to keep the wound surfaces sterile and also used extensively in the treatment of fungal infections, fever, cold and catarrh Citation[11]. Its pharmacological potencies such as anti-inflammatory, antibacterial, antimicrobial, anti-oxidative and antimalarial have also been documented [Citation12Citation[13]–Citation14,Citation10] . Recent evidence has emerged that early initiating time of polyphenol rich extract of OG had pharmacological important against colitis Citation[15]. Despite the vast therapeutic benefit of Ocimum gratissimum in various experimental models, its effect on drugs induced colitis are scarce. This study was therefore designed to examine the beneficial effects of aqueous extract of Ocimum gratissimum leaves on acetic acid-induced colitis in male Wistar rats. Furthermore, we studied macroscopic and histological parameters and analyzed some hematological parameters, oxidative stress markers and inflammatory markers such as NO level and MPO activity.
Materials and methods
Drugs and chemicals
Acetic acid (Manufactured by Eastman Chemical Company limited) a chemical reagent, often produced by fermentation and subsequent oxidation of ethanol. Ketamine hydrochloride (50 mg/10 ml) injection was purchased from Popular Pharmaceuticals LTD. No. 164, Tongi, Industrial Area, Gazipur-1711, Bangladesh. All other reagents were of analytical grade and purchased from Sigma (St. Luis, USA).
Plant extraction
The Ocimum gratissimum leaves were collected, washed, air dried under shade and grounded into fine powder using a blender. The aqueous extract was prepared by extracting the powdered leaves (520 g) with 5.2 L of distilled water in an electric shaker for 48 h. The extract was filtered through Whatmann No. 1 (Whatmann International Ltd, Maidstone, UK) paper and evaporated under reduced pressure at 40 °C using a rotary evaporator. The resulting concentrate was freeze dried with the aid of a lyophilizer. The residue (52.80 g) was kept in petri dishes with a tight fitting cover until it was needed for the study.
Preliminary phytochemical test
Preliminary phytochemical properties of the aqueous extract of Ocimum gratissimum leaves were tested using the following chemicals and reagents: flavonoids (NaCl and HCl), alkaloids (Mayer and Dragendorff’s reagents), saponins (frothing test), tannins (FeCl3), cardiac glycosides (Salkowski test), terpenoids (Borntrager’s reaction), phenols [FeCl3 and K3Fe(CN6)] Citation[16].
Determination of total phenolics
The total phenol contents in the aqueous extract of Ocimum gratissimum leaves extracts were determined by the method of Singleton and Rossi Citation[17] and as described by Gulcin et al. Citation[18]. Briefly, an aliquot of the extract (1 ml) was mixed with 4 ml of Folin–Ciocalteu’s phenol reagent which already being diluted with distilled water (1:10 v/v). The resulting mixture was voltexed. After 5 min of standing, 3 ml of 7% (w/w) sodium carbonate (Na2CO3) solution was added and thereafter incubated for 90 min at room temperature for color development. Using an ultraviolet (UV)–Vis spectrophotometer (Beckman, DU 7400, USA), the absorbance was read at a wavelength of 765 nm against a negative control containing 1 ml of distilled water. Extracts were evaluated at a final concentration of 1 mg/ml. Total phenolic content was expressed as mg/g gallic acid equivalent using the equation obtained from a calibration curve of gallic acid.
Determination of total flavonoids
Total flavonoids content of the leaves extract was determined using aluminum chloride colorimetric assay method according to Zhilen et al. Citation[19] and as described by Miliauskas et al. Citation[20]. Briefly, to 0.6 ml of distilled water and 0.3 ml of 4% sodium nitrate solution were added to 0.4 ml of the extract samples. After 5 min, 0.5 ml of 10% aluminum chloride and 0.9 ml of sodium hydroxide solutions were added to the resulting mixture. Against blank, the absorbance, at a wavelength of 430 nm, was read using a UV–Vis spectrophotometer (Beckman, DU 7400, USA). The development of yellow color was taking as indication of the presence of flavonoids. Extract samples were evaluated at final concentration of 1.5 mg/ml. Total flavonoid content was calculated as quercetin equivalent (mg/g) using the equation obtained from the calibration curve. Quercetin with varying concentrations 0.1, 0.2, 0.3, 0.4 and 0.5 mg/ml was used as standard in comparison to the extract sample.
Animals
Twenty adult male Wistar rats weighing 100–180 g were used in this study. They were obtained from the Animal House of the College of Health Sciences, Obafemi Awolowo University, Ile-Ife. They were housed in a plastic cages and allowed to acclimatize in the laboratory for two weeks before the commencement of the study. The animals were kept under normal environmental conditions with a 12 h light/dark cycle and had free access to standard rodent diet and water. The animal experimental procedures were conducted in accordance with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978) and approved by Institutional Research Committee.
Experimental design
Twenty male Wistar rats were divided into four groups as follows; Group 1 (control group) consisted of 5 rats, received distilled water for 21 days. Groups 2–4 consisted of 5 rats each. Group 2 received 2 ml of 6% acetic acid solution once intra-rectally for the induction of colitis. Groups 3 and 4 were pre-treated with the acetic acid solution to induced colitis and subsequently treated with 200 and 400 mg/kg/day (p.o) of AEOGL respectively for 20 days. Twenty-four hours after the end of the experiment, the animals were sacrificed under ketamine hydrochloride anesthetic (10 mg/kg/b.w via intramuscular route). The Blood samples from all the rats were drawn via cardiac puncture and collected into EDTA tubes for hematological analysis. The rectum and the descending colon of the rats were removed for antioxidant status, neutrophil infiltration status and histological study using hematoxylin and eosin stain.
Measurement of body weight gain
Weekly body weight of the rats for all groups were measured with the aid of a digital weighing balance (Hanson, China) to assess weekly weight gain or weight loss.
Measurement of colonic ulcer score (colon mucosa damage index (CMDI)
After sacrificing the animals through ketamine hydrochloride anesthetic, the entire rectum and the descending part of the colon were removed and opened longitudinally, and washed with normal saline to remove luminal content, the visible damages were examined with the aid of magnifying lens and score on a 0–5 scale by method of Davies et al. Citation[21]; Zheng et al. Citation[22].
The ulcer score method includes;
Measurement of colonic macroscopic damage
After sacrificing the animals through ketamine hydrochloride anesthetic, the distal colon, (8 cm) was removed and opened longitudinally, and washed to remove luminal contents with normal saline, the weight of the colon was measured with the aid of a sensitive weighing balance and the length of the excised part of the colon was also measured using a meter ruler [Citation22,Citation23] . The Colon Macroscopic Damage was calculated using this formula;
Measurement haematological indices
The haematocrit (HCT), haemoglobin (HB) concentration, red blood cell (RBC), mean Corpuscular volume (MCV), mean corpuscular haemoglobin concentrated (MCHC), white blood cell (WBC), granulocyte (GRAN), monocytes (MON),neutrophil (NEU), lymphocytes (LYMP) and platelet (PL) counts were measured using an auto-analyzer machine (SFRI blood cell Counter, H18 light, France).
Biochemical assays
The colon samples of the rats were homogenized in 50 mM Tris–HCl buffer (pH 7.4) containing 1.15% potassium chloride and the homogenate was centrifuged at 10,000 revolution per minutes for 15 min. at −4 °C. The supernatant was collected for the estimation of superoxide dismutase (SOD) and was assayed by the method described by Misra and Fridovich Citation[24]. Briefly, to 200 μL of the lysate, 2.5 ml of buffer, 30 mM EDTA and 300 μL of 2 mM of pyrogallol was added. An increase in absorbance was recorded at 420 nm for 3 min by spectrophotometer. One unit of enzyme activity is 50% inhibition of the rate of autooxidation of pyrogallol as determined by change in absorbance/min at 420 nm. The activity of SOD was expressed as µg/mg protein.
Reduced glutathione (GSH) was determined using the method described by Beutler et al. Citation[25]. Briefly, 1 ml of the supernatant, obtained above, was added to 0.5 ml of Ellman’s reagent (10 mM). 2 ml of phosphate buffer was, thereafter, added. The yellow colour developed was read at 412 nm against blank containing 3.5 ml of phosphate buffer. A series of standards were also treated similarly and the amount of GSH was expressed in µg/mg protein.
Nitric oxide (NO) was measured by the method of Grisham et al. Citation[26]. 500 μl sample was mixed with 500 μL TCA in 1.5 ml tube to deprotein the sample. 100 μL was of each sample was separated in labeled eppendorf tubes and 0.1 ml of 1% sulphanilamide in 5% phosphoric acid was added and incubated at RT for 10 min, followed by addition of 0.1 ml of 0.1% NED (N-1-napthylethylenediamine dihydrochloride) and incubate for 10 min at 60%. The absorbance of the chromophore formed was measured at 546 nm. The standard curve is prepared using sodium nitrite (100 μm). The amount of NO in the sample is extrapolated from the standard curve.
Myeloperoxidase (MPO) as marker of inflammation and colon injury was measured according to the method of Xia and Zweier Citation[27]. To 2 ml of O-dianisidine mixture (16.7 mg of O-dianisidine, 100 ml of 0.05 M potassium phosphate buffer and 50 μL of diluted H2O2) into the cuvette, 70 μL of PMF was added. The increase in absorbance was monitored every 30 s for 1 min. The absorbance was read at 350 nm. One unit of MPO activity can be defined as the quantity of enzyme able to convert/degrade 1 μmol of H2O2 to water in 1 min at room temperature.
Histological analysis
The colon tissues biopsies of the rats were fixed in 10% formalin, dehydrated in graded alcohol, cleared in xylene and embedded in paraffin wax. The tissues were then cut into 2–3 μm thick sections by a microtome, fixed on the slides and stained with hematoxylin-eosin (H&E). The slides were examined under a light microscope (Olympus CH; Olympus, Tokyo, Japan) and photomicrographs were taken with a Leica DM 750 Camera at ×100 and 400 magnification.
Statistical analysis
The results obtained were expressed as mean ± SEM. Data were analyzed using One-way analysis of variance (ANOVA) followed by post-hoc test using Student-Newman-Keuls and P value less than 0.05 was considered statistically significant. The statistical analysis was performed with the aid of Graph Pad Prism 5.03.
Results
Phytochemicals screening of aqueous extract of Ocimum gratissimum leaves
Quantitative phytochemical analysis of the extract showed the presence of alkaloids, flavonoid, tannins, saponin, cardiac glycoside and terpenoid ().
Table 1 Phytochemical ingredients of aqueous extract of Ocimum gratissimum leaves.
Total phenolic and flavonoid contents
The results of the total phenol and flavonoid contents of the aqueous extract of Ocimum gratissimum leaves are presented in . The total phenol content reported as gallic acid equivalent was 90.03 mg GAE/100 g, while the total flavonoid content reported as quercetin equivalent was 50.04 mg QUE/100 g.
Body weight change
The body weight change is show in the .
Fig. 1 Effects of AEOGL on percentage weight gain of rats induced colitis by acetic acid. Values are given as Mean ± SEM (n = 5) * = significantly different from control group (p < 0.05); α = significantly different from acetic acid group (AA) (p < 0.05).
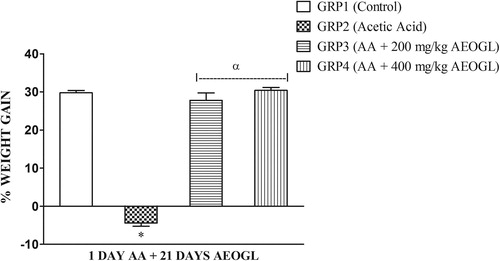
The group 2 (AA) had a significantly lower (p < 0.05) body weight change when compared the Group 1 (control). There was a significantly lower (p < 0.05) body weight change in Group 2 when compared with Groups 3 and 4 respectively. However, Groups 3 and 4 showed no significant different in body weight change when compared with the control.
Effects on diarrhea score and ulcer parameters
The diarrhea score, colon mucosa damage index and colon macroscopic damage of the rats treated with AA (Group 2) was significantly higher (p < 0.05) when compared with AEOGL treated groups (groups 3 and 4). There was a significantly lower of colon mucosa damage index in Group 4 when compared with Group 3. Thus, AEOGL prevent or reduce the increased in diarrhea score, colon mucosa damage index and colon macroscopic damage induced by acetic acid treatment ().
Hematological parameters
The mean values of hematological parameters in rats treated with AEOGL are presented in . Acetic acid induced colitis significantly increased white blood cell (WBC) count, neutrophil count (NEU) and granulocyte count. However, administration of AEOGL significantly lower (p < 0.05) white blood cell (WBC) count, neutrophil count (NEU), lymphocyte count (LYM), and granulocyte counts when compared with colitis control group. Acetic acid treatment significantly lower (p < 0.05) lymphocyte count (LYM), red blood cell (RBC) count and hemoglobin (Hb) concentration when compared with AEOGL treated groups. .
Table 2 Effects of AEOGL on diarrhea score and ulcer parameters of rats induced colitis by acetic acid.
Table 3 Effects of AEOGL on hematological parameters of rats induced colitis by acetic acid.
Effects on nitric oxide (NO) and MPO
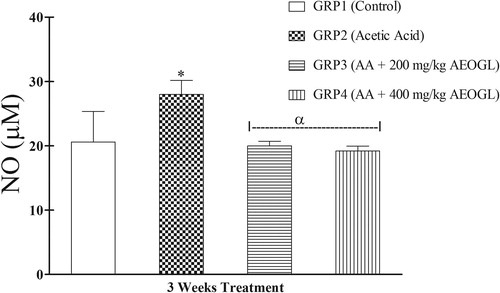
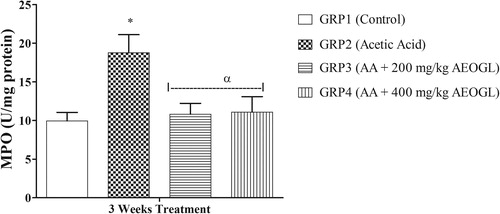
The mean values of nitric oxide level and MPO activity in rats treated with AEOGL are presented in and respectively. The induction of colitis with AA was accompanied with significant increase in the plasma nitric oxide system in rats. The NO level in the plasma increased significantly in the rats treated with acetic acid (colitis control group) compared with normal control animals. AEOGL treatment prevented the increased in the NO level and maintained its normalcy in acetic acid-exposed rats. Ulcerative colitis group (AA) had a significantly higher MPO in the colonic tissue compared with the control. However, AEOGL had no significant different in MPO when compared with the control. AEOGL groups had a lower MPO activity when compared with the colitis group (AA). Thus, AEOGL prevented increased in MPO in the colon mucosa.
Colitis-induced colon oxidative stress
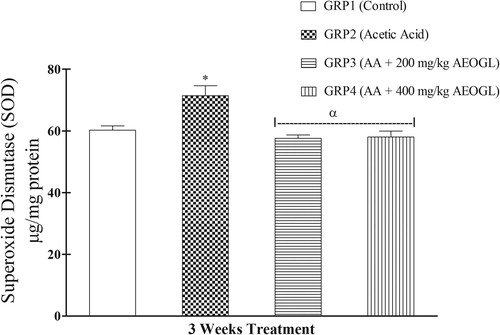
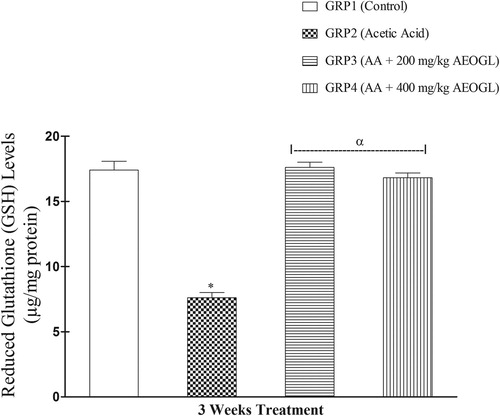
The induction of colitis was accompanied with significant depletion of colon mucosa antioxidant system in rats. The SOD activity in the colon tissue increased significantly while the GSH level decreased significantly in the rats treated with acetic acid (colitis control group) compared with normal control animals ( & ). AEOGL treatment prevented the increased in the SOD activity and decreased GSH level and maintained their normalcy in acetic acid-exposed rats ( & ).
Histological observations
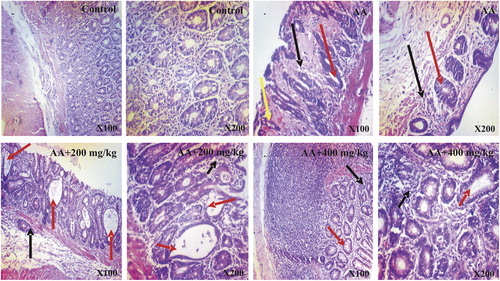
The representative photomicrographs of colon sections of control and treated rats are shown in . The histology of the colon sections of the control rats was structurally normal having the normal epithelial architecture and laminal propria, submucosa and muscularis propria. In contrast, treatment with acetic acid alone resulted in severe epithelial erosion, necrotic and distorted cryptic glands accompanied by marked cellular infiltration by mononuclear cells, degenerative changes in the regions of the colon. The morphological characteristics of the colon of rats treated with AEOGL showed significant amelioration of acetic acid-induced colitis and were comparable to those in control group.
Fig. 6 Photomicrographs of colon tissues showing the effects of AEOGL administration following acetic acid-induced colitis. Photomicrograph of the colon of Control (CN), Acetic Acid (AA), AA + 200 mg/kg and AA + 400 mg/kg. CN shows presence of intact T-lymphocytes and intraepithelial lymphocytes. Abundant or proportionate goblet cells and absorptive cells. In contrast, AA shows severe mucosal atrophy, hyperchromatic nuclei (black arrow), loss of goblet cells, crypts distortions and abscesses (red arrow), hyperemia and tissue hemorrhage (yellow arrow). 200 and 400 mg/kg (Groups 3 and 4) AEOGL treated group showed greater signs of improvement, revealing higher/greater numbers of crypts/glands. They showed high glandular/mucosal height (black arrow), prominent intraepithelial lymphocytes, signs of crypt distortions and abscesses (red arrow). (H & E Stain; ×100 & 400).
Discussion
Induction of colitis in rats using acetic acid is a classical and well-established method to produce an experimental model of colitis similar to human colitis [Citation28,Citation29] . The biochemical changes are mainly excessive production of reactive oxygen and nitrogen metabolites, neutrophil infiltration, increasing local oxidative stress that aggravate tissue injury in inflammatory diseases, weight loss and diarrhea [Citation28,Citation29] .
Weight loss and excessive bleeding of the colonic mucosal wall are one of the major symptoms of colitis. In the present study, animals that were induced with acetic acid (AA) showed a decreased in body weight gain when compared with the control group. In addition, the diarrhea scoring of rats treated with AA was significantly higher when compared with the control group. Compared with the AA group, rats treated with AEOGL at 200 and 400 mg/kg showed an accelerated weight gain toward control group. In this study, AEOGL increase body weight by preventing the fistulae in the gastrointestinal tract e.g. diarrhea. It reduced the inflammation of the large intestine and excessive nutrient losses through diarrhea. Study has shown that administration of AEOGL in rats caused significant histological changes in the intestines, revealing the presence of increased villi and larger goblets cells Citation[30]. The increase in the numbers of villi in the intestine facilitates increase nutrient absorption due to increase in the surface area of the intestine Citation[31]. Furthermore, AEOGL enhances anti-diarrhea effect by inhibiting intestinal motility, partly via muscarinic receptor inhibition Citation[32], thereby facilitating nutrient absorption in the intestine. The significant increase in body weight and decrease in diarrhea scores that were observed in AEOGL groups, may be as a result of AEOGL inhibited the intestinal motility, by increasing number of villi and larger goblets cells of the intestine, which favor rapid absorption of nutrient from the gastro-intestinal tract and subsequently prevented diarrhea.
Alteration in hematological parameters due to tissue damage is an important clinical manifestation of inflammatory bowel diseases. Thus, an assessment of hematological parameters can be used to determine the degree of disease state of colitis Citation[33].
Decrease in red blood cell count (RBC) could reveal an imbalance between its production and loss Citation[34]. The results obtained in this study revealed that AA induced colitis caused significant reduction in red blood cell counts (RBC) with subsequent decline in hemoglobin (HB) concentration. The observed decrease in the number of RBCs, accompanied by a decreased HB, seems to confirm that colitis probably caused excessive blood loss as a result of serious gastrointestinal tract bleeding, hemolysis of red blood cell and poor absorption of iron in the intestine. The results of this study showed that, the aqueous extract of Ocimum gratissimum leaves administered at the dosages used and for the duration of the experiment appeared to improve the red blood cells and HB counts in rats toward control. However, there was a significant decrease in RBC count and HB concentrations in rats treated with AEOGL when compared with the control. The reduction in the RBCs and HB count in the experimental groups, as compared with the control is an indication that AEOGL failed to completely inhibit the effect of acetic acid on colonic blood loss.
In this study, the rats induced with colitis showed a significant increase in white blood cell counts (WBC), neutrophil (NEU), monocyte (MON) and granulocyte (GRAN) counts when compared with the control group, but the lymphocyte counts significantly decrease when compared with the control group. The observed decrease in lymphocyte counts may be as a result of the immunosuppressive effects of colitis on the bone marrow through the inhibition of T cell activity. The administration of 200 and 400 mg/kg doses of the AEOGL significantly normalized the white blood cells, lymphocyte, monocyte and granulocyte counts. It is possible that the extract contained agents that stimulated the bone marrow to produce leucocyte and release them into the blood. Leucocyte are activated when the body is invaded by bacteria and they provide the first line of defense against invading microorganisms Citation[35]. The granules of the granulocytes, particularly neutrophil contain many enzymes, which makes it a powerful and effective defensive agent and hence, deficiency of neutrophils in the body leads to myriad defects, including conditions such as colitis Citation[35]. However, administration of AEOGL significantly ameliorated the hematological parameters of the colitis rats toward control.
In this study, acute exposure to acetic acid significantly increased the colonic tissue nitric oxide (NO) concentration in the rats when compared with the control. Under normal physiological conditions, NO produces an anti-inflammatory effect. However, it is considered as a proinflammatory mediator that induces inflammation due to overproduction by inducible nitric oxide synthase (iNOS) in abnormal situations Citation[36]. The elevated concentration of NO in AA-exposed rats observed in this study could lead to NO reaction with superoxide anion (O2−) to form potent oxidant peroxynitrite (ONOO–) and impair the colonic mucosa. The present study showed that administration of AEOGL significantly inhibited NO production which prevented peroxynitrite formation from inflammatory cells and countered inflammation. The apparent suppression in AA-mediated increase in NO concentration by AEOGL suggests its ability to prevent neutrophil infiltration and inflammation in the colon [Citation37,Citation38] as it was also seen in the photomicrograph of groups treated with AEOGL.
Myeloperoxidase (MPO) is found primarily in the azurophilic granules of neutrophils Citation[39]. The assay of this enzyme has been widely used as an index of severity of digestive inflammation Citation[39]. Accumulation of granulocytes in the colon could contribute to granulocyte-mediated mucosal tissue damage, breakdown of the mucosal barrier and subsequent inflammatory response Citation[40]. Relative to the normal control group, there was marked elevation in the activities of MPO in the acetic acid treated group. The observed increase in the colonic MPO activity indicates colonic inflammation damage as a result of neutrophil infiltration into the colons of AA-treated rats. However, the results of the rats post-treated with AEOGL showed marked reduction in the activities of MPO and histopathological changes induced by acetic acid only showed less colonic mucosal injury with less infiltration of leucocytes and cellular changes in AEOGL treated rats.
Superoxide dismutase (SOD) is an enzyme existing in mitochondria as well as cytosol which is responsible for the maintenance of redox balance in the tissue [Citation41,Citation42] . The increased level of reactive oxygen species caused deregulation of cellular redox balance Citation[42]. Superoxide dismutase (SOD) reduces superoxide to water (O2– to H2O) thus playing a pivotal role in scavenging of oxidative free radicals Citation[43]. In this study, SOD was found to be higher in the acetic acid treated group when compared with the control group. The increased in SOD in AA-induced colitis reflects uncontrolled oxidative stress. Aqueous extract of Ocimum gratissimum leaves treatment brought back SOD activities to normal, thus restoring oxidative balance in the colonic mucosa.
Reduced glutathione (GSH) is an important intracellular antioxidant agent in mammalian gut and involved in the repair mechanism of mucosal damage by free radicals. It plays an important role in protecting the intestinal cells and act as a defense mechanism against inflammation Citation[44]. During inflammation, GSH level decreases resulting in severe colon mucosal injury. In this study, acetic acid treatment resulted to reduction of colonic tissue GSH level. However, treatment with AEOGL significantly normalized the colonic tissue GSH level. The improvement of GSH showed that AEOGL might have depleted ROS level, relieved the inhibition of the antioxidant enzymes and/or might have triggered their synthesis which in turn attenuated the oxidative damage in AA-treated rats.
The aqueous extract of Ocimum gratissimum leaves are rich in flavonoids and phenolic compounds (). Previous studies have reported the protective action of flavonoids and phenols against oxidative stress induced cellular damage [Citation45,Citation46] . Flavonoids and phenols can exert their anti-inflammatory and anti-oxidative activities by various mechanisms, e.g. by scavenging or quenching free radicals, by chelating metal ions, or by inhibiting enzymatic systems responsible for free radical generation Citation[47].The anti-inflammatory and anti-oxidative properties of AEOGL can also be due to the presence of saponin, terpenoids, glycoside and alkaloids present in this plant Citation[48]. In this study, AEOGL intervention shows restoration of oxidant and/or antioxidant balance and this may be suggests that the phytochemical components of the extract have favorable effects on the antioxidant defense systems and hematological parameters. These resultant mechanisms explained the involvement of the extract in colonic tissue integrity preservation observed in this study. Thus, AEOGL treatment effectively ameliorated the induction of oxidative stress, inflammatory cells infiltration, hematological alteration and histological damage in the AA-treated rats. Overall, the findings of this study show the efficacy of AEOGL to prevent AA-induced colonic mucosa injury. The anti-colitis effect of AEOGL is related to its anti-inflammatory and anti-oxidative properties. However, it can be suggested that the therapeutic effect of AEOGL can also be associated with improved blood supply to the mucosa and the whole intestinal wall under the influence of the extract and other effects. Hence more studies has to been done to ascertain the mechanism behind anti-colitis effect of Ocimum gratissimum leaves.
Conflict of interest
The authors of this manuscript declare no conflict of interest.
References
- E.V.LoftusJr.Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influencesGastroenterology126200415041517
- D.C.BaumgartW.J.SandbornInflammatory bowel disease: clinical aspects and established and evolving therapiesLancet369200716411657
- A.KaserS.ZeissigR.S.BlumbergInflammatory bowel diseaseAnn Rev Immunol282010573621
- H.YukitakeH.KimuraH.SuzukiY.TajimaY.SatoT.Imaedaet al.BTZO-15, an ARE-Activator Ameliorates DSS- and TNBS-Induced colitis in ratsPLoS One682011e23256
- D.K.PodolskyInflammatory bowel diseaseN Engl J Med3472002417429
- S.C.KongD.P.HurlstoneC.Y.PocockL.A.WalkingtonN.R.FarquharsonM.G.Brambleet al.The incidence of self-prescribed oral complementary and alternative medicine use by patients with gastrointestinal diseasesJ Clin Gastroenterol392005138141
- K.D.EffraimT.W.JacksO.A.SodipoHistopathological studies on the toxicity of Ocimum gratissimum leave extract on some organs of rabbitAfr J Biomed Res620032125
- K.M.NadkarniIndian materia medicathird ed.1999Popular Prakashan Pvt LtdIndia
- A.C.AkinmoladunE.O.IbukunA.EmmanuelE.M.ObuotorE.O.FarombiPhytochemical constituent and antioxidant activity of extract from the leaves of Ocimum gratissimumSci Res Essays2007163166
- M.RabeloE.P.SouzaP.M.G.SoaresA.V.MirandaF.J.A.MatosD.N.CriddleAntinociceptive properties of the essential oil of Ocimum gratissimum L. (Labiatae) in miceBraz J Med Biol Res362003521524
- I.I.IjehO.D.OmodamiroI.J.NwannaAntimicrobial effects of aqueous and ethanolic fractions of two spices, Ocimum gratissimum and Xylopia aethiopicaAfr J Biotechnol42005953956
- G.B.SahouoZ.F.TonziboB.BotiC.ChopardJ.P.MahyY.T.N’guessanAnti-inflammatory and analgesic activities: Chemical constituents of essential oils of Ocimum gratissimum, Eucalyptus citriodora and Cymbopogon giganteus inhibited lipoxygenase L-1 and cyclooxygenase of PGHSBull Chem Soc Ethiop1722003191197
- T.T.AdeboluA.O.SalauAntimicrobial activity of extracts of Ocimum gratissium on selected diarrhea causing bacteria in southwestern NigeriaAfr J Biotechnol472005682684
- L.O.OrafidiyaS.K.AdesinaO.IgbeneghuE.O.AkinkunmiG.E.AdetogunA.O.SalauThe effect of honey and surfactant type on the antibacterial properties of the leaf essential oil of Ocimum gratissmum Linn. Against common wound-infecting organismsInt J Aromather1620065762
- Q.K.AlabiR.O.AkomolafeJ.G.OmoleM.A.AdefisayoO.L.OgundipeA.Aturamuet al.Polyphenol rich extract of Ocimum gratissimum leaves ameliorates colitis via attenuating colonic mucosa injury and regulating pro-inflammatory cytokines production and oxidative stress in ratsBiomed Pharmacother1032018812822
- G.E.TreaseW.C.EvansPharmacognosy1983Ballière Tindall PressLondon
- V.L.SingletonJ.A.RossiColorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagentsAm J Enol Viticult161965144158
- I.GulcinM.OktayE.KirecciO.I.KufreviogluScreening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extractsFood Chem832003371382
- J.ZhilenT.MengehengW.JianmingThe determination of flavonoids contents in mulberry and their scavenging effects on superoxide radicalsFood Chem641999555559
- G.MiliauskasP.R.VenskutonisT.A.van BeekScreening of radical scavenging activity of some medicinal and aromatic plant extractsFood Chem852004231237
- R.P.DaviesW.E.M.MorrowD.C.MillerJ.DeenEpidemiologic study of decubital ulcer in sowJ Am Vet Med Assoc2087199610581062
- L.ZhengZ.GaoS.WangChronic ulcerative colitis model in ratsWorld J Gastroenterol612000150152
- W.G.DongS.P.LiuB.P.YuD.F.WuH.S.LuoJ.P.YuAmeliorative effects of sodium ferulate on experimental colitis and their mechanisms in ratsWorld J Gastroenterol911200325332538
- H.P.MisraI.FridovichThe role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutaseJ Biol Chem247197231703175
- E.BeutlerO.DurgunB.M.KellyImproved method for the determination of blood glutathioneJ Lab Clin Med511963882888
- M.B.GrishamG.G.JohnsonJ.R.LancasterJrQuantitation of nitrite and nitrite in extracellular fluidsMethods Enzymol2681996237246
- Y.XiaJ.L.ZweierMeasurement of myeloperoxidase in leukocyte-containing tissuesAnal Biochem24519979396
- R.FabiaR.WillenA.A’rRajabR.AnderssonB.AhrenS.BengmarkAcetic acid-induced colitis in the rat: a reproducible experimental model for acute ulcerative colitisEur Surg Res2441992211225
- R.KojimaS.HamamotoM.MoriwakiK.IwadateT.OhwakiThe new experimental ulcerative colits model in rats induced by subserosal injection of acetic acidNihon Yakurigaku Zasshi11822001123130
- E.E.J.IwealaO.ObidoaStudies on some biochemical and histological changes associated with long term consumption of leaves of Ocimum gratissimum L. in male ratsAm J Food Technol52010376384
- J.P.PappenheimerIntestinal absorption of hexoses and amino acids: from apical cytosol to villus capillariesJ Membr Biol1842001233
- V.N.OffiahU.A.ChikwenduAntidiarrhoeal effects of Ocimum gratissimum leaf extract in experimental animalsJ Ethnopharmacol681999327330
- A.G.JagtapP.V.NiphadkarA.S.PhadkeProtective effect of aqueous extract of Bombax malabaricum DC on experimental models of inflammatory bowel disease in rats and miceIndian J Exp Biol492011343351
- Q.K.AlabiR.O.AkomolafeO.S.OlukiranA.O.NafiuJ.G.OmoleA.M.Adefisayoet al.Assessment of haematological and biochemical effects of Kolaviron in Male Wistar RatsBr J Pharm Res1632017114
- W.F.GanongReview of medical physiology22nd ed.2005McGraw HillSingapore515517
- R.K.CrossK.T.WilsonNitric oxide in inflammatory bowel diseaseInflamm Bowel Dis92003179189
- L.GillbergM.VarsanyiM.SjostromM.LordalJ.LindholmP.M.HellstromNitric oxide pathway-related gene alterations in inflammatory bowel diseaseScand J Gastroenterol201247201212831297
- W.WadieH.Abdel-AzizH.F.ZakiO.KelberD.WeiserM.T.KhayyalSTW 5 is effective in dextran sulfate sodium-induced colitis in ratsInt J Colorectal Dis272012144153
- S.J.KlebanoffMyeloperoxidase: friend and foeJ Leukoc Biol7752005598625
- A.MantovaniM.MuzioC.GarlandaS.SozzaniP.AllavenaMacrophage control of inflammation: negative pathways of regulation of inflammatory cytokinesNovartis Found Symp2342010120131
- M.T.AbreuThe pathogenesis of inflammatory bowel disease: translational implications for cliniciansCurr Gastroenterol Rep42002481489
- A.D.KandhareK.S.RaygudeP.GhoshA.E.GhuleT.P.GosaviS.L.Badoleet al.Effect of hydroalcoholic extract of Hibiscus rosa sinensis Linn. leaves in experimental colitis in ratsAsian Pac J Trop Biomed52012337344
- L.KruidenierI.KuiperW.Van DuijnM.A.Mieremet-OomsR.A.van HogezandC.B.Lamerset al.Imbalanced secondary mucosal antioxidant response in inflammatory bowel diseaseJ Pathol20120031727
- S.ChavanL.SavaV.SaxenaS.PillaiA.SontakkeD.IngoleReduced glutathione: importance of specimen collectionIndian J Clin Biochem202005150152
- V.SharmaG.P.RajaniEvaluation of Caesalpinia pulcherrima Linn. for anti-inflammatory and antiulcer activitiesIndian J Pharmacol4322011168171
- Q.K.AlabiO.S.OlukiranM.A.AdefisayoB.A.FadeyiEffects of treatment with Nauclea latifolia root decoction on sexual behavior and reproductive functions in male rabbitsJ Dietary Suppl201710.1080/19390211.2017.1380105
- A.LukacinovaJ.MojzisR.BenackaJ.KellerT.MaguthP.KurilaPreventive effects of flavonoids on alloxan-induced diabetes mellitus in ratsActa Vet772008175182
- Mishra J, Srivastava RK, Shukla SV, Raghav CS. Antioxidants in aromatic & medicinal plants. Science Tech entrepreneur; 2007.