Abstract
4-Aminobutyrate aminotransferase (GABA) receptors are the key mediators of quick inhibitory synaptic transmission in the midpoint of the nervous system in mammalian. Dysfunction of GABA receptors contributes the genetic disorders and chronic neurological disorders include childhood absence epilepsy, juvenile myoclonic epilepsy, adolescent myoclonic epilepsy, and seizures. They get affected by mutations of GABA subunit genes from the brain. The neurological diseases of Huntington, Alzheimer, Epilepsy and Parkinsonism are affecting due to the mutation of GABA levels. Thus, this study has chosen 32 molecules from thirty-one medicinal plants, after which we executed significant computational approaches such as homology modeling, molecular docking and absorption, distribution, metabolism, and excretion (ADME). In the computational approaches, the analogue O-[(2E)-3-(4-hydroxyphenyl)-2-propenoyl]pentofuranosyl-(1-3) pentopyranosyl-(1-4)pentose showed the superior docking scores of −9.336 with well binding affinities. Moreover, the natural molecules rosmarinic acid, curcumin and mangiferin were close to that of superior one. Based on this scrutinizes, we concluded that the top ranking molecules can be considered as a suitable drug candidate for GABA causing problems.
Introduction
4-aminobutyrate aminotransferase (GABA) receptors are the key mediators of fast inhibitory synaptic transmission in the central nervous system of mammals [Citation1]. Its dreadful conditions become involved in catalysis by aminobutyrate transaminase (ABAT, GABA transaminase) for the period of the early stage [Citation2]. It is divided into the three kinds of receptors namely GABAA, GABAB, and GABAC [Citation3Citation[4]Citation[5]Citation[6]–Citation7] . These are all mainly affected by the mutations of GABA subunit genes from the nervous system.
Generally, the GABA has the capability to maintain lower blood pressure in rats and human beings as well [Citation8Citation[9]Citation[10]Citation[11]–Citation12] . Moreover, the deficiencies of GABA levels are causing various neuronal problems in central nervous system of mammalians. Especially, the epilepsy is a most commonly causing neurological disease to the people which also originates due to the deficiencies of γ-aminobutyric acid.
Epilepsy is one of the neurological disorders, which mainly affects the region of CNS (central nervous system). Inhibition of GABA-mediated inhibitory neurotransmission or increase in excitation due to glutamatergic or cholinergic transmission has been reported to play a central role in development of epilepsy [Citation13]. Nearly, 50 million people are affected by epilepsy worldwide which affects different age groups and sex [Citation14]. Eighty percent of the burden of epilepsy is in the developing country, where 80–90% of people with epilepsy receive no treatment at all [Citation15]. Currently, lots of drugs have available for seizure and its related problems which includes valproate, lamotrigine and topiramate. These drug molecules are identified to cause several side effects in shorter usage and they contain low bio accessibility [Citation16]. For that reason, this study was seeking alternate drug molecule with good accessibility to the human for epilepsy.
The results of GABA on human health were the challenge of a huge quantity of interest in food mechanized. In the market, lots of GABA containing food items are available, which includes germinated brown rice, chocolate, wine, and other food products [Citation17]. Natural plants and their products have been used against lots of disease and disorders around the world nearly hundreds of years. Recently, many ethnobotanical studies have been reported the potentiality of medicinal plants and their active molecules for diseases includes central nervous system, pathogenic diseases, etc [Citation18]. The medicinal plant-derived secondary metabolites such as alkaloids, flavonoids, tannins, saponins, and other phytochemicals possess potential bio-actions when they are utilized [Citation19].
Worldwide, 33% of the huge human population having developed countries and above 80% of the human population and industrially developing countries has been used the herbal based products for maintaining human health and treat certain climate change, food and water related diseases like cold, fever, heart diseases, diabetes, etc [Citation20Citation[21]–Citation22] .
Nowadays, most of the people are depend and believe the medicinal plants and their products, because those are contain good therapeutic abilities with well bio-availability. Therefore, we carried out the computational studies such as homology modeling, molecular docking, drug designing and ADME analysis, which we carried out for find a suitable drug molecule. Through this analysis, we found certain effective drug analogues from medicinal plants for epilepsy.
Materials and methods
Software and hardware
The computational analysis was carried in Maestro v10.2 of Schrodinger suite. The docking tools were complexly programmed in single software as Maestro v10.2 which includes prime application for homology modeling, Ligprep for ligand preparation, Sitemap for validate the binding sites, grid generation for fix the target site and Glide Xtra Precision for docking purpose. Centos Linux was used as the operating system for computational examinations.
Target
Human 4-aminobutyrate aminotransferase (GABA) sequence (Accession No. P80404) was retrieved from the established database [Citation23].
Ligand molecules
Totally thirty-two phytoconstituents were selected for docking with a target. The natural molecules were selected with the help of previous literature reports (Table 1) (Supplementary data). Those molecules were obtained from the established chemical database [Citation24].
Homology modeling and protein preparation
Homology modeling was performed for predicting the target by using prime application in Maestro v10.2 [Citation25,Citation26] . During this structure prediction, we face some important steps such as find homolog's, BLAST homology search and show sequence similarity and identity with already known template sequences. Finally, the target model was build which can be further used for docking purposes. The predicted target was then used in protein preparation by wizard tool of Schrodinger suite. During the process, the missing side chains and back bones were added, and update the missing residues also. The wizard tool has contain two important mechanisms namely, preparation and refinement. The X-ray crystallography structure of the target is closely associated with the water molecules. The water molecules occupied target is not eligible to take the further study, hence, it was eliminate. Finally, the optimization and minimization steps were performed on this process [Citation25].
Active site validation and grid generation
The site validation was analyzed on the target inner and surface regions by using sitemap tool [Citation27]. Site map analysis, showed possible active binding sites and one binding site was selected to proceed further. It was chosen based on their site values (site scores and size of site area). Then, the selected site was used for the grid generation. Grid box shape and values were generated in X: 5.3; Y: 3.71; Z:-20.86 coordination. Target site was explained with 10 A°C radius around the ligand binding site [Citation28].
Ligand preparation
The structures of the thirty-two ligand molecules were converted to 3D structure from 2D using ligprep tool [Citation29]. The ligands were drawn geometry optimized via Optimized Potentials for Liquid Simulations 2005 (OPLS2005) force field [Citation30]. All the ligand molecules were generated 3D structures from 1D (Smiles) and 2D (SDF) representation, probing for tautomers and steric isomers and geometry minimization of ligands [Citation31].
Molecular docking
The molecular docking study was used to execute in Glide Xtra precision (XP) docking mode. It was used to predict the binding affinities between the target to ligand, ligand efficiency, and ligand inhibitory constant to the target. The complex of thirty-two ligands is docked with the active site of the target by using Glide Xtra precision (XP) which docks ligands flexibly [Citation32Citation[33]–Citation34] . The energetic small molecules will have accessible poses that avoid these penalties and also get good docking scores. It reveals to have accurate hydrophobic contacts between targets to ligand.
ADMET analysis
This analysis was carried out by Quikprop tool of Masetro v10.2 [Citation35]. All the ligand molecules were analyzed with the parameters of hydrogen bond donor, hydrogen bond acceptor, blood brain barrier and central nervous system followed by Katsila et al., [Citation36].
Results and discussion
Homology modeling
The crystal structure of human 4-aminobutyrate aminotransferase is not available from protein data bank. Therefore, this study predicted the target model with the template of porcine 4-aminobutyrate aminotransferase by homology modeling. During the structure prediction, the analysis represents the sequence similarity and identity between target and template. This structure has been predicted from the sequence of human 4-aminobutyrate aminotransferase. It shows the sequence identity and similarity with the template of pig 4-aminobutyrate aminotransferase. This study target was found together in the sequence of PDB ID: 1OHV ().
Structure validation
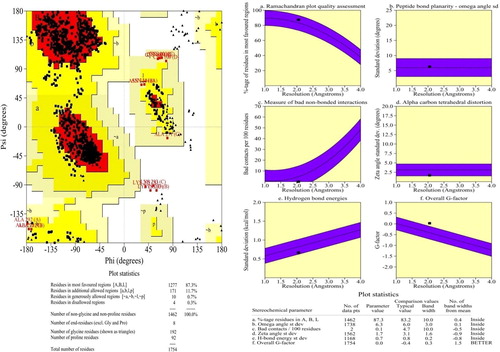
The structural validation was analyzed by Ramachandran plot, which shows the residues occurring regions. It also represents the accuracy and reliability of residues between template and target. In this validation, 87.3% of residues mostly occurred in the favor region, 11.7% of residues in additional region, 0.7% of residues in generously allowed region and the remaining 0.3% of the residues were present in disallowed regions (a). Whereas, the main chain parameters in terms of conformation of the predicted model were checked for deviations from the template using Procheck tool. Previously, Iftikhar et al., [Citation37] predicted the structure of human 4-aminobutyrate transaminase with the template of 4-aminobutyrate-aminotransferase of pig. Through, the homology modelling, they have analyzed the sequence similarity and identity in same target and template. Also, they have reported that the Ramachandran plot for structure validation.
Fig. 2 Structural validation of modelled GABA of Homo sapiens. (a) Ram Phage shows the accuracy and reliability of sequence with the template of porcine 4-aminobutyrate-aminotransferase. The plot shows the 87.3% of residues mostly occurs in favoured region; (b) Main chain parameters validate by using Procheck.
Drugable site analysis
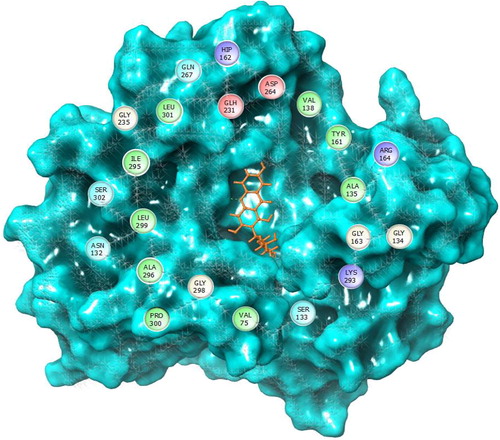
In this study, the site analysis has produced five active drugable binding sites from the target. The site map shown their binding cavity residues were Gln267, Hip162, Glh231, Asp264, Val138, Tyr161, Arg164, Gly163, Gly134, Lys293, Ser133, Val75, Gly298, Pro300, Ala296, Asn132, Leu299, Ser302, Ile295, Gly235, Leu301 and Gln267 (). Similarly, Iftikhar et al., [Citation37] have analyzed the ligand binding sites in 4-aminobutyrate aminotransferase for docking with known analogues. Recently, many computational studies have been carried out this binding site analysis method in many kinds of target molecules [Citation38,Citation39] . Moreover, Vijayakumar et al., [Citation40] has described that the potentiality of the binding site analysis in Maestro v 10.2, Schrodinger suite. As a result of this analysis, the qualified binding site was taken for grid generation, which is help to fix the possible binding site in target [Citation41].
Molecular docking
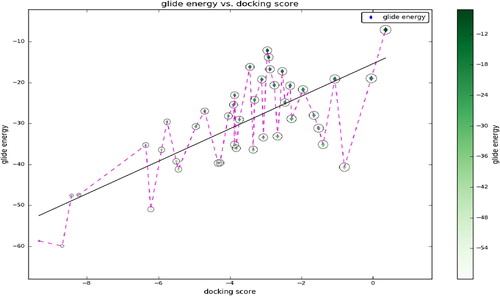
The molecular docking was performed in human 4-aminobutyrate aminotransferase molecule with experimentally reported and known bioactive molecules. Among them, 5-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl] pentofuranosyl-(1-3) pentopyranosyl-(1-4) pentose is placed superior docking scores of −9.336, when compared to other ligand molecules (). It also shows better binding affinities with a protein molecule. Followed by rosmarinic acid, curcumin and mangiferin shows the nearest docking scores when compared to the superior docking score placed bioactive molecule. Through molecular docking, we found that of the ligands four bioactive molecules have received the favorable docking score with the target of human 4-aminobutyrate aminotransferase. These ligands demonstrate favorable docking scores with target receptor, which are originated with the docking score of −8.224. Through the scatter plot, entire ligand molecules docking scores with their glide were represented in . In recent scenario, lots of computational researches have been done for finding out the effective drug molecules against certain globally challenged micro-organisms and its diseases as well. The computational analysis provides us some important facts such as docking score, binding energy, binding affinities and also indicated the ligand potentiality. In general, binding affinities were shown particularly the ligand contribution and that flexibility with the protein active site. Taken together, we found such kinds of docking parameters and binding affinities contact with ligands to target, which are mainly involved in the hydrogen bond side chain, back chain and Pi-Pi stacking contacts.
Table 2 Docking scores and glide energy values of ligand molecules received with the human 4-aminobutyrate-aminotransferase.
5-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl] pentofuranosyl-(1-3) pentopyranosyl-(1-4)pentose
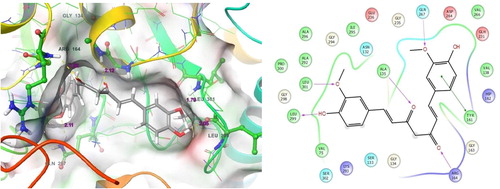
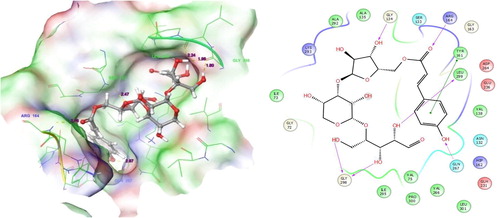
This ligand molecule had the potent docking scores of −9.336. shows the ligand docking score and their energy values. It has shown good countable binding affinities with the residues of human 4-aminobutyrate aminotransferase. At this point, so much of binding contacts were involved in the target residues. Some of the interactions depend on both specific interactions with the ligand binding site and non-specific forces to the outside of the target binding cavity. Different binding affinities contacts were involved between ligand to target. Precisely, hydrogen bond side chain, back chain and Pi-Pi stacking contacts were involved between both molecules. The complex of ligand and protein was investigated for analyzing their contacts formation. An examination of the docked complex represents six bond formations between target to ligand. There, some active residues contacts with the ligand atoms were Gly134, Arg164, Tyr161, Leu299, Gln267 and Gly298 (a). Among the six residues, four residues contacts belong to hydrogen bond back chain such as Gly134 (OH), Arg164 (O), Leu299 (OH) and Gly298 (OH) (b). This molecule interacts by forming a π-π stacking contacts. Gln267 (OH) by forming a side chain contacts with the functional group of ligand. Here, the dotted blue straight line is indicated hydrogen bond side chain. The solid blue straight line is indicated hydrogen bond back chain. And, the dumbbell shaped green line is indicated the Pi-Pi stacking contact. Likewise, our previous computational study reported that the analogue has shown better docking scores with good binding contacts in Acetylcholinesterase (AChE) enzymes. AChE, which is an organic substance and it, is contributing in the transfer of neuronal signals in the brain. AChE is a recognized enzyme that acting an essential function in the central nervous system (CNS). Already, we reported that molecule 5-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl] pentofuranosyl-(1-3) pentopyranosyl-(1-4) pentose has showed good docking scores with well contacts to AChE enzyme. Hence, we suggested that the molecule could be containing good neuronal disorder inhibiting abilities [Citation25].
Fig. 5 Molecular interactions of Target with 5-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl] pentofuranosyl-(1-3) pentopyranosyl-(1-4)pentose: Residues and hydrogen bond contacts (yellow dotted line) with their distance values (pink values), and the 2D template representing the types of contacts formed between the ligand and target.
Rosmarinic acid
Among the 32 ligands, rosmarinic acid had the second valuable docking scores (-8.687). Analysis of the docked complex demonstrates that the residue contacts were Gly298, Leu299, Gln267, Gln231, Asp264 and Tyr16. a shows the residue contacts and their distance values in pink color. Among the seven residue contacts, most of the residues were Gln267 (OH), Glh231 (OH), Asp264 (OH) and Tyr261 (O) formed in hydrogen bond side chain contacts with ligand. Gly298 (OH) and Leu299 (OH) were involved in the hydrogen back chain contacts with rosmarinic acid. Surprisingly, the residue Tyr261 is involved in both side chain as well as the π-π stacking contacts with the ligand (b). Rosmarinic acid is a specific bioactive molecule in the plant species of Salvia officinalis which also contains other medicinal plant species like Prunella vulgaris and Melissa officinalis. It reduces the number of harmful actions in induced by Aβ, which include reactive oxygen species configuration, lipid peroxidation, DNA destruction, caspase-3 establishment and protein hyperphosphorylation [Citation42]. Medicinal plants that contain this molecule have been exploited as an effective neurological activity. Salvia officinalis is treated eleven mild to moderate symptoms having Alzheimer disease patients, there it has significantly improved the cognitive functions [Citation43]. This shows the favorable docking score with the human 4-aminobutyrate aminotransferase target ().
Fig. 6 Molecular interactions of target with (R)-(+)-rosmarinic acid: Residues and hydrogen bond contacts (yellow dotted line) with their distance values (pink values), and the 2D template representing the types of contacts formed between the ligand and target.
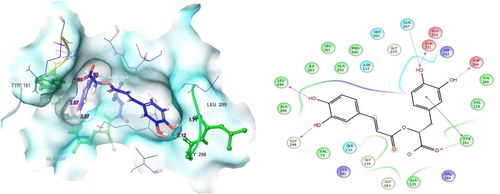
Table 3 Human 4-aminobutyrate-aminotransferase (GABA) residues interactions with tested molecules.
Curcumin
Curcumin had the third valuable docking score (-8.427). Investigation of the docked complex demonstrates that the contacts were Leu299, Leu301, Ala135, Gln267, Arg164 and Tyr161. In which, the residues Leu299 (OH), Leu301 (O), Ala135 (O) and Arg164 (O) were involved in hydrogen bond back chain contacts with the ligand. Gln267 by forming a hydrogen bond side chain contacts and the residue TYR161 is involved in π-π stacking contacts with Curcumin. a is shown the residue contacts and their distance values are in pink color. b shows their binding contact types. Curcumin is mainly present in the medicinal species of Curcuma longa. The pharmacological action of turmeric has been recognized principally. The curcuminoids consists of curcumin and two associated molecules demethoxy curcumin (DMC) and bisdemethoxycurcumin [Citation44,Citation45] . It has effective antimicrobial properties against certain harmful pathogens. Curcumin has been consumed as many as human health complaints of both diseases and disorders. Curcumin, which suppress the type II diabetes and its associated symptoms, rheumatoid arthritis, multiple sclerosis (MS) and Alzheimer’s disease. It is inhibits human immunodeficiency, virus (HIV) replication, enhances wound healing properties, protects from liver injury, increases bile secretion, which also protects from cataract formation, protects from pulmonary toxicity and fibrosis. Besides, it has restrained anti-leishmaniasis and anti-atherosclerotic activities. Additionally, the wide range of literature suggests that curcumin has contains effectual biological activities for the prevention and treatment of numerous health complaints [Citation42]. Previously, Erfani et al., [Citation46] stated that the effect of curcumin on epilepsy. They are pointed out the effectiveness of curcumin at different concentration levels on various clinical experimentation namely Albino rat, Swiss mice and Zebra fish. Firstly, we found that the biological potential of curcumin for epilepsy, which has identified by molecular docking approach. In this study, the molecule curcumin is placed better docking scores with good binding affinities than mangiferin and other molecules.
Mangiferin
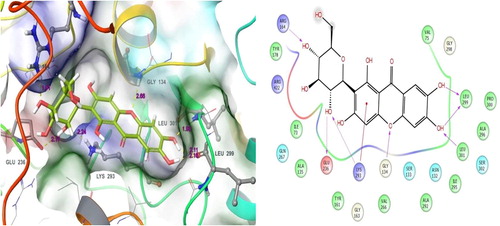
Mangiferin had the fourth valuable docking score (−8.224). It was also shown good binding affinities with target residues. This docked complex showed that the residue contacts between target as well as the Mangiferin. This examination shows that there are six binding affinities between ligand to target. There, the residues Arg164, Glu236, Lys293, Gly134, Leu299 and Leu301 were made different contacts formation with the small molecule of mangiferin. Particularly, the residues Arg164 (OH), Glu236 (OH) and Lys293 (OH) were involved in hydrogen bond side chain contacts. Whereas, following residues Gly134 (O), Leu299 (OH) and Leu301 (OH) were involved in hydrogen bond back chain contacts. But, the residue Leu299 was involved ionic binding with the ligand hydroxyl group. a. represents the residues contacts and their residue distance values. b displayed the residue contacts in ligand atoms and their contacts types. In this study, all the tested compounds contacts were shown in . Mangiferin is mainly found in Mangifera indica. It has an effective pharmacological properties, which also contain lots of health benefits includes immunoregulation, cardio protective, memory enhancement, antiviral, laxative and the list goes on. Important and potentiality of the Mangifera indica and their molecule mangiferin have been clearly reported in a previous finding [Citation47].
ADMET-analysis
In the beginning stage of drug discovery physico-chemical indicators were used in order to find the vital properties affecting to the biological functions (ADME) . There are some important physico-chemical properties were measured such as permeability, solubility, lipophilicity, integrity and stability. But the concept of ADME has been expanded by the toxicity. Right from the beginning of drug discovery in silico method has been used to give an accurate prediction of pharmacokinetics properties for instant ADMET.
Table 4 Physico-chemical properties and biological function values of tested molecules analyzed by QuikProp.
Conclusion
γ-aminobutyric acid is an important neurotransmitter in the region of central nervous system of mammalian. Due to the deficiency of GABA levels the people are affected by certain neurological disorder and diseases especially epilepsy. But, these GABA enzymes are available more in germinated brown rice, chocolate, wine, etc. Based on the availability of GABA in medicinal plant parts the phytoconstituents were tested with GABA by using computational approaches. As a result of this computational finding some molecules such as 5-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl]pentofuranosyl-(1-3)pentopyranosyl-(1-4)pentose, (R)-(+)-rosmarinic acid, curcumin and mangiferin shows favorable docking scores and displayed good contacts to the target. In order to the computational outcome, we have revealed certain active novel drug analogues for the neurological disorder of epilepsy. Especially, 5-O-[(2E)-3-(4-Hydroxyphenyl)-2-propenoyl] pentofuranosyl-(1-3)pentopyranosyl-(1-4)pentose has displayed effective computational results than other superior three analogues. Based on the finding, we conclude that the above molecules could be suitable drug candidates for epilepsy. And also this study will be very helpful for new herbal drug discovery to the pharmaceutical sector.
Supplementary Table S1
Download MS Word (76 KB)Acknowledgement
The authors are grateful to the DST-SERB (SB/YS/LS-109/2014) for providing financial assistance in this project. We specially express our thanks to the management of A.V.V.M. Sri Pushpam College (Autonomous), Poondi, for providing them necessary facilities and support to carry out this work.
References
- P.L.McGeerT.KawamataD.G.WalkerH.I.AkiyamaE.G.Tooyama McGeerMicroglia in degenerative neurological diseaseGlia719938492
- L.K.Medina-KauweA.J.TobinL.De MeirleirJ.JaekenC.JakobsW.L.Nyhanet al.4-Aminobutyrate aminotransferase (4-aminobutyrate aminotransferase-transaminase) deficiencyJ Inherit Metab Dis221999414427
- B.Sabeega BegumS.SubashiniP.HemalathaP.ArchanaN.BharathiP.Nagarajanet al.In silico screening of phytochemical compounds targeting childhood absence epilepsy (CAE)Int J Pharm Pharm Sci62014430433
- L.M.GunneJ.E.HaggstromB.SjoquistAssociation with persistent neuroleptic-induced dyskinesia of regional changes in brain 4-aminobutyrate aminotransferase synthesisNature3091984347359
- J.ButterworthC.M.YatesJ.SimpsonPhosphate-activated glutaminase in relation to Huntington’s disease and agonal stateJ Neurochem411983440447
- S.YoshikaY.AsoY.TakedaStatical evaluation of accelerated stability data obtained at a single temperature. Effect of experimental errors in evaluation of stability dataChem Pharma Bullet38199017571759
- R.A.E.BakayA.HarrisNeurotransmitter receptor and biochemical changes in monkey cortical epileptic fociBrain Res2061981 387-04
- N.NishinoH.FujiwaraS.A.Noguchi-KunoC.Tanaka4-Aminobutyrate aminotransferase A receptor but not musculleric receptor density was decreased in the brain of patients with Parkinson's diseaseJap J Pharmacol481988331339
- K.A.C.ElliottF.HobbigerGamma aminobutyric acid: circulatory and respiratory effects in different species; re-investigation of the anti-strychnine action in miceJ Physiol119597084
- Y.AokiN.Saint-GermainM.GydaE.Magner-FinkY.E.LeeC.Credidioet al.Sox10 regulates the development of neural crest-derived melanocytes in XenopusDev Biol25920033339
- K.InoueT.ShiraiH.OchiaiM.KasaoK.HayakawaM.Kimuraet al.Blood-pressure-lowering effect of a novel fermented milk containing gamma-aminobutyric acid (4-aminobutyrate aminotransferase) in mild hypertensivesEur J Clin Nutr572003490495
- A.NoguchiS.MatsumuraM.DezawaM.TadaT.YanazawaA.Itoet al.Isolation and characterization of patient-derived, toxic, high mass amyloid beta-protein (Abeta) assembly from Alzheimer disease brainsJ Biol Chem28420093289532905
- O.C.SneadBasic mechanisms of generalized absence seizuresAnn Nuerol371995146157
- http://www.who.int/mediacentre/news/releases/2007/pr04/en/.
- E.PeruccaThe new generation of antiepileptic drugs: advantages and disadvantagesBrit J Clin Pharmacol421996531543
- D.S.HillB.J.WlodarczykA.M.PalaciosR.H.FinnellTeratogenic effects of antiepileptic drugsExpert Rev Neurother102010943959
- TakashiAkihiroSatoshiKoikeRyojiTaniTakehiroTominagaShinWatanabeYokoIijimaKohAokiDaisukeShibataHiroshiAshiharaChiakiMatsukuraBiochemical mechanism on 4-aminobutyrate aminotransferase accumulation during fruit development in tomatoPlant Cell Physiol499200813781389
- G.Phani KumarF.KhanumNeuroprotective potential of phytochemicalsPharmacogn Rev620128190
- A.G.AtanasovB.WaltenbergerE.M.P.Wenziget al.Discovery and resupply of pharmacologically active plant-derived natural products: a reviewBiotechnol Adv332015 1582-14
- A.M.AbdouS.HigashiguchiK.HorieM.KimH.HattaH.Yokogoshiet al.Relaxation and immunity enhancement effects of gamma-aminobutyric acid (4-aminobutyrate aminotransferase) administration in humansBiofact262006201208
- V.ChintamunneeM.F.MahomoodallyHerbal medicine commonly used against infectious diseases in the tropical island of MauritiusJ Herb Med1432012113125
- J.TahraouiZ.El-HilalyH.IsrailiZ.LyoussiEthnopharmacological survey of plants used in the traditional treatment of hypertension and diabetes in south-eastern Morocco (Errachidia province)J Ethnopharmacol1102007105117
- www.uniprot.org.
- www.chemspider.com.
- Prime 2011 version 2.1 Schrödinger, LLC, NewYork.
- V.K.VyasR.D.UkawalaM.GhateC.ChinthaHomology modeling a fast tool for drug discovery: current perspectivesInd J Pharm Sci742012117
- Site Map version 2.5 Schrodinger, LLC, New York, 2016.
- K.GinalskiV.NickV.GrishinA.GodzikL.RychlewskiPractical lessons from protein structure predictionNucleic Acid Res33200518741891
- J.C.RobertsonN.C.HurleyM.TortoriciG.CiossaniM.T.BorrelloN.T.Velloreet al.Expanding the druggable space of the LSD1/CoREST epigenetic target: new potential binding regions for drug-like molecules, peptides, protein partners, and chromatinJ Compu Biol92013e1003158
- LigPrep, 2011. Version 2.3 Schrödinger, LLC, NewYork.
- S.K.TripathiR.MuttineniS.K.SinghExtra precision docking, free energy calculation and molecular dynamics simulation studies of CDK2 inhibitorsJ Theor Biol334201387100
- K.K.KakaralaJ.KaiserD.VinodStructure and putative signaling mechanism of Protease activated receptor 2 (PAR2) – a promising target for breast cancerJ Mol Graph Model542014179199
- Glide, 2011.version5.7, Schrödinger, LLC, NewYork.
- K.M.ElokelyR.J.DoerksenDocking challenge: protein sampling and molecular docking performanceJ Chem Inf Model53201319341945
- QikProp, version 4.3, Schrödinger, LLC, New York, NY, 2014.
- T.KatsilaG.A.SpyrouliasG.P.PatrinosG.T.MatsoukasComputational approaches in target identification and drug discoveryComp Struct Biotechnol142016177184
- T.IftikharS.BatoolA.DeepB.NarasimhanP.C.SharmaM.Malhotraet al.In silico analysis of the inhibitory activities of 4-aminobutyrate aminotransferase derivatives on 4-aminobutyrate transaminaseArab J Chem10201712671275
- S.SubhaniJ.ArchanaK.JamilHomology modelling and molecular docking of MDR1with chemotherapeutic agents in non-small cell lung cancerBiomed Pharmaco71201545
- S.SubhaniK.JamilMolecular docking of chemotherapeutic agents to CYP3A4 in non-small cell lung cancerBiomed Pharmaco7320156574
- S.VijayakumarP.ManogarS.PrabhuPotential therapeutic targets and the role of technology in developing novel cannabinoid drugs from cyanobacteriaBiomed Pharmaco832016362371
- A.O.M.PatschullB.GooptuP.AshfordT.DaviterI.NobeliIn silico assessment of potential druggable pockets on the surface of α1-antitrypsin conformersPLoS One72012e36612
- T.LiuF.XuX.DuD.LaiT.LiuY.Zhaoet al.Establishment and characterization of multi-drug resistant, prostate carcinoma-initiating stem-like cells from human prostate cancer cell lines 22RV1Mol Cell Biochem3402010265273
- S.AkhondzadehM.NoroozianM.MohammadiS.OhadiniaA.H.JamshidiM.Khaniet al.Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer's disease: a double blind, randomised, placebo controlled trialJ Neurol Neurosurg Psychia742003863866
- B.B.AggarwalA.KumarA.C.BhartiAnticancer potential of curcumin: preclinical and clinical studiesAntican Res232003363398
- M.ParamasivamR.PoiH.BanerjeeA.BandyopadhyayHigh-performance thin layer chromatographic method for quantitative determination of curcuminoids in Curcuma longa L germplasmFood Chem1132009640644
- M.ErfaniF.ArshrafzadehJ.AkhondianH.RahimiM.B.ToosiH.L.Kalatet al.The effect of curcumin on epilepsy: an experimental reviewRev Clinical Med42017131135
- G.M.Masud ParvezCurrent advances in pharmacological activity and toxic effects of various capsicum speciesJ Pharmaco Phytochem3201719001912
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.ejbas.2018.05.008.