Abstract
Congenital cataract is defined as an opacification of the eye lens appearing at birth or shortly thereafter. Congenital cataract is one of the highest significant causes of blindness or optical impairment in the childhood. Cataract, the cloudiness of the lens is the most frequent reason of blindness worldwide, demonstrating nearly half of all causes of loss of sight worldwide. A significant amount of isolated congenital cataract has monogenic roots. It is genetically heterogeneous, and all the modes of Mendelian inheritance have been described. Numerous genes linked with inherited and paediatric cataracts have been recognised. Inherited congenital cataract has been linked with mutations in particular genes, comprising of crystallins, membrane transport channel proteins, gap junction proteins, the development transcriptional factors and the cytoskeleton. Discovering the mutations and the genes responsible for cataractogenesis are important in achieving a response to the molecular deficiencies and pathophysiologic features of inherited congenital cataracts.
1 Introduction
The human eye is an organ of vision. It plays a major role in life, gives us the sense of sight, and allows to understand about the world around us. Visualization and interpretation of colours, shapes and dimensions of numerous objects are made possible by eye. Inherited eye diseases comprise 1/3 of all reported human genetic disorders [Citation1]. Congenital cataract is the type of cataract, which occurs at the early stages of life [Citation2]. It is described as an opacity of the crystallin lens causing an impaired vision [Citation3], and the abnormality of the lens can impede with the normal development of the eyes [Citation4]. The prevalence varies with socioeconomic rank affecting 1–6 cases out of 10,000 live births in the developed countries and about 5–15 out of 10,000 cases in the developing countries of the world. Autosomal dominant, autosomal recessive and x-linked genetic type of congenital cataracts, which may be sequestered (nonsyndromic) or associated with systemic disorder or syndromes [Citation5]. The phenotypic arrangement is founded over the site & the type of the lens imperviousness that containing posterior polar, anterior polar, lamellar, and nuclear, coralliform, and cerulean, pulverulent, cortical, polymorphic and total cataract [Citation6]. Phenotypically same cataracts might be caused by mutation at different genomic loci and possibly control, unlike inheritance pattern, whereas physically variable cataracts might be seen in a particularly large population [Citation7]. About 50% of cases have a genetic root with other causes counting intrauterine infection, malnourishment and metabolic diseases [Citation3]. Mutation in more than 30 genes is identified to cause non-syndromic forms of innate cataracts [Citation8]. The discovery of the mutations affecting congenital cataract should result in a better way of the procedure concerned in cataractogenesis and give further insights into the normal lens development and structure [Citation9]. Hereditary research has recognised mutations in various genes associated with cataracts like crystalline, which consist of about half of the recognised genetic types of the cataract. Congenital cataracts are either unilateral or bilateral, they are categorised by distinct genetic basis, morphology, the existence of particular metabolic disorders, or linked with other optical abnormalities. Crystallin is concerned about half of the families with recognised mutations [Citation10]. Crystallin compactness and order is critical to the correctness of the lens [Citation2]. Mutations in crystallin that are severe enough to cause accumulation that can lead to congenital cataracts in an exceptionally penetrant Mendelian genetics. In this review, we tried to discuss the description of genetic heterogeneity of congenital cataract disorder and also we shed some light on diagnosis and therapeutics.
2 Embryology
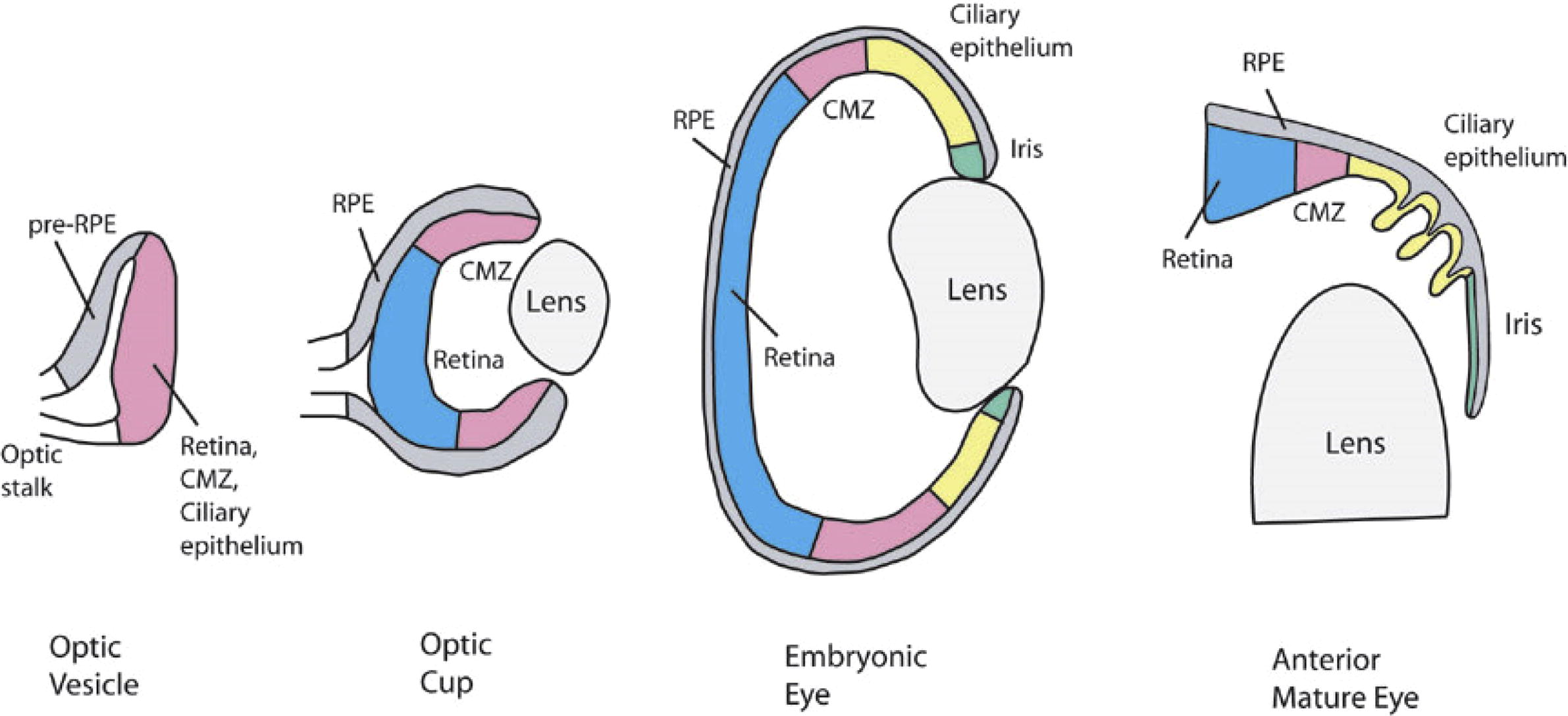
The lens development is happening through the collections of controlled processes. Embryology and morphogenesis of the eye lens in the animal as well as in human beings provided an insight into the sophisticated and innate disorder that may concern in the characteristic eye phenotypes present in congenital cataract. The optical vesicle that causes the lens is covered by ectodermal cells. The lens placode interferes on the optical vesicle initiate from the forebrain, about the 25 days of pregnancy, it is a shortening of the ectoderm, which is the only layer of cuboidal cells that invaginate into the neural ectoderm of the optic vesicle as the lens depths become clear from the ectoderm by the 33rd day. The anterior epithelial cells at that time they move to the equatorial region and produce the lens bow. The cells in the bow area generate secondary lens fibres in the third gestational month, which spread until they cover the primary lens fibres.
Lens sutures are formed in the second month at the anterior extremities [Citation11]. The secondary lens fibres are retained in a determinedly controlled way, such that a distraction grating is, establish with a destructive disorder to decrease the scattering of light. After the birth, the secondary lens fibres are constantly forming and these post-natal fibres make the lens cortex. The mature lens is avascular, aneural and alymphatic. The lens includes a great number of proteins, so-called crystallin. About 90% of the soluble protein in the eye lens is work together by Crystallin, which is responsible for about 38% of the wet mass of the lens. A large quantity of protein in the fibre cells increase larger refractive indices, which give concrete phenotype to the lens, known as transparency. The lens centre is often dehydrated and compressed, for this reason, it inhabits greater protein concentration ().
Fig. 1 Typical eye development (http://faculty.washington.edu/tomreh/eyedev.html).
3 Genetic factors of congenital cataract
The genesis of congenital cataract is still not discovered well and very little is identified because of lack of modern techniques, long-term accurate data required and the lack of intense investigative techniques. Various set of symptoms and infections prior birth suggestion to the malformations in the eye and helps in congenital cataracts development. Although various causes have been bringing into being, the particular aetiology is often difficult to determine, mostly in the patient’s sufferings from unilateral hereditary cataracts, clinically and genetically heterogeneous situation [Citation12]. In addition, different mutations in the identical gene are able to cause similar cataract forms, in spite of the fact that the similar mutation in a distinct gene might clue to dissimilar phenotypes are indicated by variable morphologies of cataracts in few families. Isolated congenital cataracts are likely to be expressively autosomal dominant with penetrant Mendelian charismas are more common than autosomal recessive cataract. Many of these are related with additional abnormalities, mainly as a portion of developing syndromes. The key cytoplasmic proteins of human lens are encoded by mutations in different genes and are linked with cataracts of different morphologies, containing genes encoding crystallin (CRYA, CRYB, and CRYG), lens specific connexin (Cx43, Cx46, and Cx50), major intrinsic protein (MIP) or Aquaporin, paired-like homeodomain transcription factor 3 (PITX3), avian musculoaponeurotic fibrosarcoma (MAF), cytoskeletal structural proteins and heat shock transcription factor 4 (HSF4) [Citation13]. An important concern is an αB-crystallin gene, CRYAB, which is extensively appeared in different tissues mainly in the muscle. Mutations in CRYAB can cause a range of defects ranging from isolated cataracts to minor cataracts linked with myopathy. Another example is the ferritin gene that gives rise to the hyperferritinemia-cataract syndrome [Citation13].
4 Congenital cataracts with inherited ailment set of symptoms
The genes up to date recognized to cause autosomal recessive congenital cataract is crystallin, the heat shock transcription factor, the lens intrinsic membrane protein, the connexin and the cytoskeletal protein.
4.1 Crystallin
The crystallin lens of the eye has been a model system for the intricacies of tissue induction and morphogenesis as well as cell specification and differentiation. Crystallin is main essential constituents of the vertebrate eye lens and exhibit more than 90% of the water-soluble total lens proteins; they play important roles in maintaining the clearness and diversion function of the lens [Citation14]. Crystallin has been categorised into α, β, γ crystallin [Citation15]. They are soluble protein and own an important role in developing the transparency of the lens.
4.2 Alpha (α)-crystallin
α-Crystallin (αA-crystallin and αB-crystallin) belong to the chaperone-like small heat shock protein family (sHSPs). They block the formation of stress protein aggregates to avoid toxic effects [Citation16]. Alpha-crystallin produces 40% of the human lens crystallin [Citation15]. The alpha-crystallin in the lens is enormous, complex and comprises of two subunits: the αA polypeptide and αB polypeptide. These subunits possess mechanical roles within the lens but also the member of minor heat shock protein family representing a crucial molecular chaperone action [Citation17].
4.3 CRYAA and CRYAB: genes for α-crystallins
There are two α-crystallin genes, CRYAA and CRYAB, coding α- and α-Crystallin in humans (and superficially most terrestrial vertebrates) [Citation18]. They are situated on the chromosomes (21 and 11), but they are closely combined in a sequence, gene structure and are clearly the consequence of initial gene replication. Both of the genes are 3 to 4 Kb long with three exons. They possess open reading frames (ORFs) of 173 (α) and 175 codons (α). Genetically the gene for αA-crystallin controlled three exons, and this is replicated in the great number of species from fish to birds. However, during the evolution of the mammalian lineage, there was a novelty with the assemblage of an alternatively spliced exon, giving rise to a greater protein product (αAins) [Citation19]. The ‘insert exon’ is involved in many species, mostly in the rodents. In primates, this alternative splice form was recovered, but the remaining of the insert exon continue in human CRYAA as a pseudoexon. Whereas the sequence is no longer spliced into developed mRNA, it is largely sustaining; raising the curious possibility that mutation could re-establish splicing and possibly lead to a novel cataractogenic deficiency.
5 Beta (β)-crystallin
β-Crystallin is the most prominent protein of the lens, where their firmness and association into innovative track developments are intense for the lens clarity and refraction. The dimerization is an initial step in the determination of β-crystallin complexes. β-Crystallin connection into dimmers is significantly preferred, but rapidly revocable under physical circumstances. β-Crystallin dimmers can interchange monomers, maybe through a brief and extremely not preferred monomer intermediate circumstance.
5.1 Mapping of crystallin, BETA-B3; CRYBB3 ON CHR22q11.23
BB3 is the 11th most important soluble proteins in the young human lens. The BB3 is present on mouse chromosome number 5 [Citation20]. Mutation in the CRYBB3 genes identified in two Pakistani families with autosomal recessive congenital nuclear cataract and G-C exchange at nucleotide 493 in exon 6 of the CRYBB3 gene [Citation21].
5.2 Lens intrinsic membrane protein 2 (LIM2)
Mammalian lens integrity cell membrane surroundings five main proteins [Citation22]. One of the most essential membrane proteins of a lens integrity cell is the MP19. It works in certain ways as a combining component possibly gets connected with the lens cell messages and has been demonstrating to be linked with cataractogenesis.
5.3 Mapping of LIM 2 ON CHR 19q l3.33
The gene for the MP19 was linked to human chromosome number 19. Mapping the L1M2 gene to mouse chromosome number 7 in an area of reserved synteny with human chromosome number 19 [Citation22]. Another research identified an innate Iraqi Jewish family of autosomal recessive manner with a late pulverulent cortical cataract at 19q. Sequencing of LIM2 gene discovered a homozygous T-to-G change, and this declared to be the first report of cataract development in humans associated with a mutation in LIM2.
5.4 Glucosaminyl (N-acetyl) transferase 2 gene (GCNT2)
In the human erythrocytes in the embryonic growth, the foetal (i) antigen is changed by the adult (I) antigen as the significance of the expression of the (beta-1), 6-N-acetylglucosaminyltransferase, I-branching (GCNT2) enzyme, which translates the linear Poly-N-acetyllactosaminoglycans into branched Poly-N-acetyllactosaminoglycans. Commonly the fully-grown erythrocytes entirely express the (I) antigen, however, in a small number of peoples, the erythrocytes are ironic in the I antigen except I, and this is known as the mature I-phenotype and it is suggested to be influenced by the absence of I-branching transferase activities.
5.5 Mapping of GCNT2 on Chr 6p24.2
Genetic study of the mature I phenotype in Taiwanese peoples who also suffered from congenital cataracts [Citation23]. Mutation detection has indicated an association among molecular discrepancies in the gene (IGnT) of the mature I-phenotype. Conserved the locus for the autosomal recessive congenital cataract in the 4 Arabic families to the region of 6p24 containing I blood type locus [Citation24]. A novel nonsense mutation, G-A exchange was identified in the GCNT2 gene.
5.6 Heat shock transcription factor HSF4
HSF4 is a mammalian factor that changes the makeup of DNA-binding homotrimer and regulates the minor and the major heat shock proteins. Heat-shock transcription factors (HSFs) activate heat-shock response genes in the phases of temperature or other pressures [Citation25].
5.7 Mapping of HSF4 ON CHR 16q22.1
By using fluorescence, in situ hybridization processing map the HSF4 gene to chromosome I6q2I. Mapping of the locus causing autosomal recessive congenital total cataract while in the large number peoples in the Tunisian family at chromosome number1 [Citation26]. Sequencing discovered a homozygous A-G mutation in the 5′ splice site [Citation27]. Mutation analysis of HSF4 display homozygous mutations in the two consanguineous Pakistani families [Citation28].
6 Connexin
A peculiar behaviour of the human lens is the distinguished organization of the low-resistance gap junctions between lens fibre cells. Connexin are gap junction protein that is necessary for a cell to cell transmission in a number of tissues. Three different connexins (CX) genes (CX43, the CX46, and the CX50) are discovered in the lens [Citation29].
7 Mapping of CX50 (GJA8) CHR lq21.1
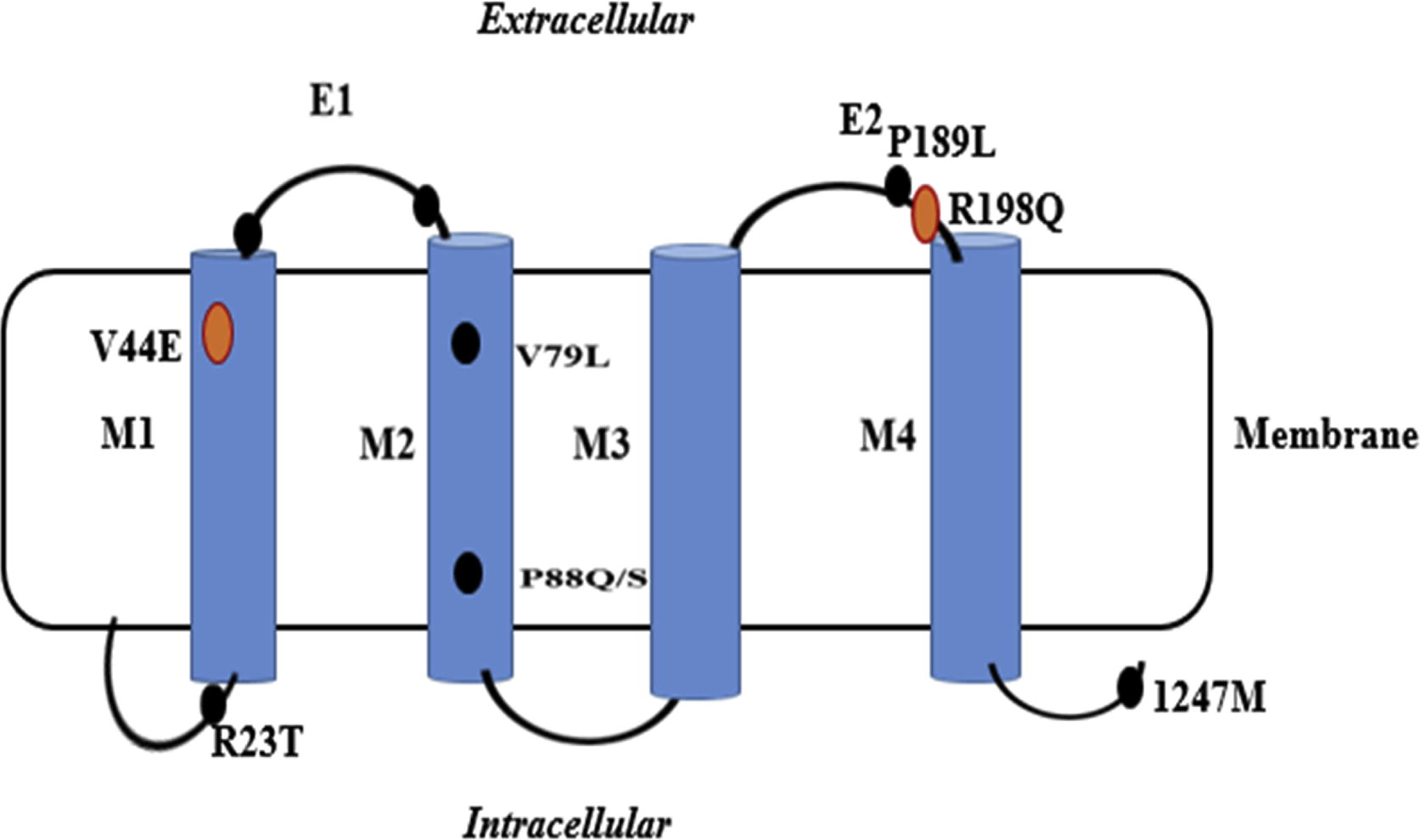
The GJA8 locus was characterised on mouse chromosome number 3. Mutations in GJA8 have been identified in the family with autosomal dominant cataract [Citation30]. First time measured the connection of GJA8 in autosomal recessive cataract in the family of southern Indian populations [Citation31]. The mutation analysis indicates a solitary base internal increasing of the frameshift at the codon 203 of the connexins 50 and shown to co-separate with the disorder in the family. The CX50 (GJA8) gene mutation can also result in dominant cataract. A novel G > C exchange at nucleotide position c.235 give rise to the exchange of very much conserved valine by leucine at the codon 79 (V79L) with cataract like “full moon” with the Y Sutural opaqueness in an Indian family population ().
8 Eph2
The EPHA2 (ephrin receptor A2) gene encodes a transmembrane tyrosine kinase receptor (epithelial cell) which comprises an extracellular ligand-binding domain and is expressed in the human lens [Citation32]. Inhibition of EPHA2 induces apoptosis and repeals tumorigenic development of tumour cells [Citation33]. Actually, the downstream signalling of stimulated EPHA2 encourages the ant oxidative capability of lens epithelial cells to eliminate the overproduction of ROS [Citation34]. It is possible that the loss of EPHA2 role could affect the structural stability of the cell, cell-to-cell crosstalk, protein folding and transcriptional activation [Citation35]. Thus, the cytoprotective and ant apoptotic functions of EPHA2 in the lens specify the promising role of EPHA2 in evading lens opacity.
9 The use of the mouse models for studying cataract
Mouse models have made significant contributions to the area of cataract study. Primarily, they have confirmed the significance of numerous genes and proteins in the maintenance of lens transparency [Citation36]. Furthermore, the widespread synteny between the mouse and human genomes has aided the identification of novel candidate genes for human cataract development. Also, it is now clear that the lens acts a crucial role in regulating normal eye growth and the identification of certain mutations has facilitated this procedure to be dissected. The mouse mutants are shown here which are known human corollary () [Citation37].
Table 1 Mouse cataract models for which a human homologue is known.
10 Clinical diagnosis and treatment
Congenital cataract is the cause of visual failure, which can destroy the developing optical organization of a child; consequently, initial diagnosis and management are essential. It is put forward that all newborn be screened through red reflex investigation at the time of birth and suspected infant be referred to ophthalmic centres. Early surgery can be done at the age of 6 weeks, newborn health is important for the accomplishment of the best optical concerns predominantly in the case of unilateral cataract [Citation38]. In bilateral cases, surgery is more essential before the appearance of strabismus or nystagmus. The bilateral case should apply at the age of 10 weeks with no more than a one-week gap between the associated eyes. Cataract surgery is not an ending point but is essentially the starting point of treatment for a child. All new-borns offspring should have observed and passed through screening eye test, called red reflexes. The Red reflex check-up at birth is the easy way to screen for congenital cataract [Citation39]. The red reflex is the significant section of the examination that gives an assessment of size and position within the visual axis, even if the child is obstinate. The red reflex test is most effective if it is applying in a dark room and contain the shining of the straight ophthalmoscope into both of the eyes at the same time from a distance of 1 to 2 feet. Nurses, paediatricians, optometrists and family doctors can perform the test for routine. The most essential part of the operation is the complete ophthalmologic exam; it includes slit lamp check-up of both eyes, checking intraocular pressure, and an ultrasound of the posterior pole if undistinguishable. Not every congenital cataract needs operation. Phenylephrine or tropicamide with pharmacologic pupillary dilation can be useful. Atropine with dilation can be evaded, as it is amblyogenic. Casual obstruction may be crucial in unilateral cases, which are at risk for amblyopia. These measures can be late the persistence for operation until a point when the growth of eyes has strengthened and the IOL can be injected with less refractive uncertainty [Citation40]. These patients should be treated in an observant way. Typical to advance cataract which affects vision, or a cataract which affect one eye, should require surgical measures like cataract removal operation. Usually, the young child needs early surgery for removing the cataract because it will give rise to amblyopia. For ideal optical development in the innate and young child, an optically important unilateral congenital cataract would be identified and cured prior than the age of 6 weeks, while optically significant bilateral congenital cataracts would be operating before the age of 10 weeks [Citation41]. Usually, ophthalmologists choose operation is ideal when the patient‘s age is less than two months, so it can stop irreversible amblyopia and sensory nystagmus in bilateral congenital cataracts. The delay in surgery give rise to glaucoma [Citation42], most of the physicians delay the cataract surgery which leads to glaucoma since glaucoma happens in 10% of surgeries of congenital cataract [Citation20].
The cataract intraocular lens technique should be done only in recognised centres. If the physicians notice the cataract, then they recommend clinical involvement. For optical development, the time of surgery is critical [Citation43]. Abnormalities such as ocular hypoplasia and early or late clinical difficulties for example inflammation and glaucoma have an adverse effect on vision in children experiencing congenital cataract surgery. Late surgery is supposed to be one of the particular of reduced best-corrected visual acuity (BCVA), and only 53% of cases with a history of late congenital cataract surgery achieved BCVA of 0.60 Log MAR or better [Citation44]. There are two types of operations that can be performed to treat congenital cataract.
11 Extra-capsular cataract extraction (ECCE)
In this technique, the removal of the lens of the eye takes place, while the flexible capsule, which covers the eye lens, is left half sustained to the insertion of an intraocular lens (IOL) [Citation45]. This procedure is changed from an older procedure known as intracapsular cataract extraction (ICCE) in which the lens was entirely removed from its capsule while leaving the eye aphakic. The patient's vision was put right by contact lenses or by extremely thick eyeglasses after intracapsular cataract extraction [Citation46]. ECCE has two types, manual expression, the removal of lens occurs by an incision that made in the sclera or in the cornea, and phacoemulsification, in which the lens is broken into segments inside the capsule by ultrasound and removed by aspiration [Citation47]. Therefore, the clear vision will be brought back by removing of the affected lens and replacing that lens with an IOL. The anterior capsule of a child is much flexible as compared to an adult lens. This makes continuous curvilinear capsulorhexis (CCC) stronger. Many more choices are there to open the anterior capsule in cataracts. The best anterior capsulectomy procedure has outcomes in the low occurrence of radial tears and is easily achieved. While in dense cataract cases, the anterior capsule can be stained by the use of the dye that can make this step much easier and safer. A manual continuous curvilinear capsulorhexis (CCC), which is the distinctive method in the eyes of adults, can be tough in the cases of paediatric capsule because of the plasticity of the paediatric capsule. However, when it can be finalised and controlled, makes an edge, which has a low manifestation of radial tears [Citation48]. The rhexis would be retained small (4–5 mm) as the lens matter can easily be articulated with a Simcoe cannulae. A primary capsulotomy is done by some doctor at the end of the ECCE. However, this needs re-evaluation, particularly in children.
12 Intraocular lenses (IOLs)
The most common impairments related specifically with IOL implantation in children are secondary opacification of the visual axis due to cortex reproliferation and irregular deviations in refractive error as the kid matures [Citation49]. In newborn children, it is important to put right Aphakia after an operation [Citation50]. Another option is to insert an IOL after the removal of the cataract. The lens of the child‘s eye is more spherical as compare to the lens of the adults [Citation51]. That has a power of around 30D, which repay for the petite axial length of the child’s eye. This decreases to roughly 20–22D at the age of five years. It means that an IOL will lead to significant myopia to a child at the older age, as it gives normal vision to new-borns. It is further constituted by changes in the strength of the cornea and axial enlargement of the ball. These discrepancies are much fast within the first few years of life and make it almost uncertain to estimate the right strength of lens for any inborn. The IOL implantation is easy for grown-up children but in the children, it is still very conflictive, predominantly under the age of two years. The IOL implantation in children is supposed to be harmless and acceptable at the age of one year [Citation52].
13 Conclusion
Inherited cataracts typically are symmetric in affected individuals. The maximum awareness of congenital cataract is attained from linkage study and by the analysis of candidate-mutated genes. The candidate genes cannot have linked with some mapped cataract mutants and the same situation also happens in the mouse model. Studies and research related to gene function are essential to clear the condition within the genes implicated inocular lens cataractogenesis. However, the medical use of some agents like antioxidant, vitamin increment, carotenoids, nutrition and gene therapy will prevent opacification. Next-generation sequencing (NGS) technologies have revolutionised the approach to the study and diagnosis of human disease. Targeted-NGS approaches for the diagnosis of paediatric cataract have proven highly successful and are providing a strong model for the advantageous use of detailed phenotypic data and genomic information to dissect the molecular mechanisms and pathways underpinning ocular development and disease.
References
- L.KhanM.AliM.QasimF.JabeenB.HussainMolecular basis of glaucoma and its therapeutical analysis in Pakistan: an overviewBiomed Res Ther403201712101227
- A.ChurchillJ.GrawClinical and experimental advances in congenital and paediatric cataractsPhilos Trans R Soc Lond B: Biol Sci3661568201112341249
- S.JavadiyanJ.E.CraigS.SharmaK.M.LowerT.CaseyE.Haanet al.Novel missense mutation in the bZIP transcription factor, MAF, associated with congenital cataract, developmental delay, seizures and hearing loss (Aymé-Gripp syndrome)BMC Med Genet181201752
- R.L.GillespieI.C.LloydG.C.BlackThe use of autozygosity mapping and next-generation sequencing in understanding anterior segment defects caused by an abnormal development of the lensHuman Heredity771–42014118137
- L.HansenA.MikkelsenP.NürnbergG.NürnbergI.AnjumH.Eiberget al.Comprehensive mutational screening in a cohort of Danish families with hereditary congenital cataractInvest Ophthalmol Vis Sci507200932913303
- T.W.WhiteD.L.PaulGenetic diseases and gene knockouts reveal diverse connexin functionsAnn Rev Physiol6111999283310
- M.EcksteinP.VijayalakshmiM.KilledarC.GilbertA.FosterAetiology of childhood cataract in south IndiaBr J Ophthalmol8071996628632
- Addison PKF. Clinical, molecular, genetic and functional studies on inherited human cataracts: University of London; 2006.
- R.L.GillespieJ.O’SullivanJ.AshworthS.BhaskarS.WilliamsS.Biswaset al.Personalized diagnosis and management of congenital cataract by next-generation sequencingOphthalmology121112014 2124–37. e2
- J.GrawGenetics of crystallins: cataract and beyondExp Eye Res8822009173189
- P.FrancisV.BerryS.BhattacharyaA.MooreThe genetics of childhood cataractJ Med Genet3772000481488
- X.-Y.LengS.WangN.-Q.CaoL.-B.QiY.-B.YanThe N-terminal extension of βB1-crystallin chaperones β-crystallin folding and cooperates with αA-crystallinBiochemistry5315201424642473
- S.NadeemM.AyubH.FawadCongenital cataract: morphology and managementPak J Ophthalmol2932013151
- A.O.KhanM.A.AldahmeshF.S.AlkurayaPhenotypes of recessive pediatric cataract in a cohort of children with identified homozygous gene mutations (An American Ophthalmological Society Thesis)Trans Am Ophthalmol Soc1132015
- A.SantanaM.WaiswoThe genetic and molecular basis of congenital cataractArquivos Brasileiros de Oftalmologia7422011136142
- A.R.ClarkN.H.LubsenC.SlingsbysHSP in the eye lens: crystallin mutations, cataract and proteostasisInt J Biochem Cell Biol4410201216871697
- U.P.AndleyCrystallins in the eye: function and pathologyProg Retinal Eye Res26120077898
- A.ShielsJ.HejtmancikGenetics of human cataractClin Genet8422013120127
- Hayat M. Autophagy: cancer, other pathologies, inflammation, immunity, infection, and aging: volume 4-mitophagy: Academic Press; 2014.
- E.A.AinsburyS.BarnardS.BrightC.DalkeM.JarrinS.Kunzeet al.Ionizing radiation induced cataracts: recent biological and mechanistic developments and perspectives for future researchMut Res7702016238261
- Rijk AFV. There is more about a-crystallin than meets the eye [Sl: sn]. 2002.
- Brook Johnson A, Fort Sam Houston AMC. TX objective: 1. Describe the range of eye trauma experienced in the deployed environment 2. Identify techniques to triage and implement initial management of severe eye. Practical advice for the management of ocular trauma subspecialty sessions 2016:16.
- A.ShielsT.M.BennettJ.F.HejtmancikCat-Map: putting cataract on the mapMol Vis1620102007
- Vaccarelli G, Miccoli MC, Antonacci R, Pesole G, Ciccarese S. BMC genomics Volume: 9 ISSN: 1471-2164 ISO Abbreviation: BMC Genomics Publication Date: 2008. Detail.
- G.YangS.ZhongX.ZhangB.PengJ.LiT.Keet al.Molecular genetic analysis of autosomal dominant late-onset cataract in a Chinese FamilyJ Huazhong Univ Sci Technol Med Sci3062010792797
- A.M.TerrellD.AnandS.F.SmithC.A.DangS.M.WatersM.Pathaniaet al.Molecular characterization of mouse lens epithelial cell lines and their suitability to study RNA granules and cataract associated genesExp Eye Res13120154255
- J.BjörkRole and Regulatory Mechanisms of heat shock Factor 22011Department of Biosciences, Åbo Akademi UniversityFinland
- R.OuvrierN.GeevasinghaM.M.RyanAutosomal-recessive and X-linked forms of hereditary motor and sensory neuropathy in childhoodMuscle Nerve3622007131143
- X.CuiP.P.XieP.P.JiaQ.LouG.DunS.Liet al.Hsf4 counteracts Hsf1 transcription activities and increases lens epithelial cell survival in vitroBiochim Biophys Acta185332015746755
- N.SajjadI.GoebelN.KakarA.M.CheemaC.KubischJ.AhmadA novel HSF4 gene mutation (p. R405X) causing autosomal recessive congenital cataracts in a large consanguineous family from PakistanBMC Med Genet91200899
- D.A.GeridoC.SellittoL.LiT.W.WhiteGenetic background influences cataractogenesis, but not lens growth deficiency, in Cx50-knockout miceInvest Ophthalmol Vis Sci446200326692674
- E.B.PasqualeEph receptors and ephrins in cancer: bidirectional signalling and beyondNat Rev Cancer1032010165
- K.R.AmatoS.WangA.K.HastingsV.M.YoungbloodP.R.SantapuramH.Chenet al.Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLCJ Clin Invest1245201420372049
- J.YangD.LiQ.FanL.CaiX.QiuP.Zhouet al.The polymorphisms with cataract susceptibility impair the EPHA2 receptor stability and its cytoprotective functionJ Ophthalmol20152015
- J.E.ParkA.I.SonR.ZhouRoles of EphA2 in development and diseaseGenes432013334357
- X.GongE.LiG.KlierQ.HuangY.WuH.Leiet al.Disruption of α 3 connexin gene leads to proteolysis and cataractogenesis in miceCell9161997833843
- J.GrawCataract mutations and lens developmentProg Retinal Eye Res1821999235267
- A.MedsingeK.K.NischalPediatric cataract: challenges and future directionsClin Ophthalmol (Auckland, NZ)9201577
- S.KerscherR.L.ChurchY.BoydM.F.LyonMapping of four mouse genes encoding eye lens-specific structural, gap junction, and integral membrane proteins: Cryba1 (CrystallinβA3/A1), Crybb2 (CrystallinβB2), Gja8 (MP70), andLim2 (MP19)Genomics2921995445450
- D.TaylorK.WrightL.AmayaL.CassidyK.NischalI.Russell-EggittShould we aggressively treat unilateral congenital cataracts?Br J Ophthalmol85920011120
- P.VashistB.TalwarM.GogoiG.MarainiM.CampariniR.D.Ravindranet al.Prevalence of cataract in an older population in India: the India study of age-related eye diseaseOphthalmology11822011 272–8. e2
- Rajavi Z, MOEZI GH, Mohammad RH, Mohseni BAM. Glaucoma after congenital cataract surgery. 2004.
- N.Saraygord-AfshariH.Naderi-ManeshM.NaderiIncreasing proteome coverage for gel-based human tear proteome maps: towards a more comprehensive profilingBiomed Chromatogr297201510561067
- S.K.PandeyM.E.WilsonR.H.TrivediA.M.IzakT.A.MackyL.Werneret al.Pediatric cataract surgery and intraocular lens implantation: current techniques, complications, and managementInt Ophthalmol Clin4132001175196
- P.M.GogateSmall incision cataract surgery: complications and mini-reviewIndian J Ophthalmol571200945
- R.C.DrewsRisk benefit analysis of anterior chamber intraocular lenses for the correction of myopia in phakic patientsEur J Implant Refractive Surg331991171194
- M.AngJ.R.EvansJ.S.MehtaManual small incision cataract surgery (MSICS) with posterior chamber intraocular lens versus extracapsular cataract extraction (ECCE) with posterior chamber intraocular lens for age-related cataractCochrane Library2014
- B.T.BarrettA.BradleyT.R.CandyThe relationship between anisometropia and amblyopiaProg Retinal Eye Res362013120158
- D.A.PlagerComplications following congenital cataract surgery. Congenital cataract2017Springer173179
- M.M.ParksD.A.JohnsonG.W.ReedLong-term visual results and complications in children with aphakia: a function of cataract typeOphthalmology10061993826841
- C.ZetterströmA.LundvallM.KugelbergCataracts in childrenJ Cataract Refract Surg3142005824840
- R.H.TrivediM.E.WilsonPediatric cataract surgery with an intraocular lens implantExpert Rev Ophthalmol252007819832