Abstract
For a long time, plant secondary metabolites have been strongly examined for their antitumor and cytotoxic impacts. These days, there is another pattern of making utilization of the waste products of plants because of their extravagance of numerous phytochemical components and adequacy on human wellbeing. This research work is handling the effect of diversity of lipoidal and phenolic compounds found in the peels of two common edible plants in the Middle East; Pisum sativum and Vicia faba L. for their assesment as anticancer agents. The GC/MS of the n-hexane extract of both plant peels led to identification of twenty compounds (82.99%) and seventeen compounds (85.97%) of the total lipoidal contents from P. sativum and V. faba, respectively. While the HPLC analysis of the ethyl acetate fraction of the two plant peels resulted in recognition of 17 flavonoids and 18 phenolics from P. sativum and 16 flavonoids and 17 phenolics from V. faba. Moreover, four flavonoidal compounds were isolated to our knowledge for the first time from the peels and tested separately against different human cancer cell lines and the mode of action of the most potent compound has been determined. P. sativum ethyl acetate fraction possessed the highest scavenging activity (31.2%) as well as the most cytotoxic effect on breast carcinoma cell line. Apigenin proved to be the most potent tested compound on (MCF-7) and has no cytotoxic effect on normal human skin cell lines.
1 Introduction
Peels of vegetables and fruits are frequently tossed or used to feed the livestock and as fertilizers. These wastes are profoundly inclined to microbial spoilage and thusly create significant issues to the environment. So that, these wastes ought to be figured out how to be used advantageously. Nowadays, many studies are performed to utilize these wastes to reduce the environmental pollution and get some medicinal benefits [Citation1]. Where fruit peels are an important source of bioactive compounds mainly as anti-oxidant and anticancer agents against colon, prostate and breast cancers [Citation2]. Therefore, it is necessary to unveil the biological activities of these peels and take the benefits from their waste materials, in addition to investigate their chemical composition to encourage adequate reuse of these wastes for several applications in the medicine [Citation3].
It has turned out to be certain that tumor is the principle driving reason for death in developed countries as well as developing countries. Mankind has been struggling with great efforts to get improved and discover cheaper treatments with fewer drawbacks to decrease the commonness of this disease and its resulting mortality. Legumes are well known of being rich in many bioactive non-nutrient components (phytochemicals) alongside their nutritional valued compounds (protein, carbohydrates, dietary fibers, and vitamins). Both plants under study belong to family Fabaceae. Pisum sativum L. is commonly known as the green pea or garden pea. In 2002, Troszynhska et al. [Citation4] proved that the acetone extract of the seed coat has antibacterial, antidiabetic, antifungal, anti-inflammatory, antihypercholesterolemic, antioxidant and anticancer properties. The HPLC analysis of the phenolic compounds in the same study showed the presence of some phenolic acids (benzoic, cinnamic acids, and their derivatives as well as some flavonoids (apigenin-7-glucoside, quercetin-3-rhamnoside, kaempferol-3- glucoside as well as other flavonoids). On the other hand, Vicia faba L. is among the oldest plant in the world [Citation5], and considered an essential source of protein and energy as it is rich in a large amount of amino acids [Citation6], also a potent source of levodopa; a precursor of dopamine, so that, it can be used for the treatment of Parkinson’s disease [Citation5].
The present study focused on phytochemical evaluation of lipoidal and phenolic extracts in the peels of Pisum sativum L. and Vicia faba L. for their assesment as anticancer agents.
2 Materials and methods
2.1 Material for phytochemical study
2.1.1 Plant material
The fresh fruits of both Pisum sativum and Vicia faba were collected from local markets in Cairo, Egypt in February 2016. Each species was peeled off; the peels were dried in shades, then grinded and kept in sealed bags.
2.1.2 Preparation of the extracts
The dried powdered peels of P. sativum and V. faba were separately defatted with n-hexane then extracted with ethyl acetate several times.
The obtained four extracts were concentrated under reduced pressure at 45 °C using the rotary evaporator to 1/10th of the initial volume and kept in refrigerator for the further studies.
2.1.3 Chemicals and reagents
All the chemicals and reagents were of analytical grade and purchased from Merck.
2.1.4 GC/MS analysis of the lipoidal extracts
The n-hexane extract of both plant peels were analyzed using GC/MS technique for identification of sterols, terpenes and fatty acid methyl esters.
2.1.5 HPLC determination of phenolics and flavonoids
High-performance liquid chromatography (HPLC) using Agilent Technologies 1100 series liquid chromatograph coupled with an auto sampler and a diode-array detector was performed for the identification and quantification of flavonoids and phenolics in the ethyl acetate fraction of P. sativum and V. faba according to [Citation7] and [Citation8] .
2.1.6 Screening of phenolics and flavonoids
The initial screening for the ethyl acetate fractions of both plants was carried out separately with the basic qualitative test for flavonoids, where 0.5 ml of the extract was mixed with 2 ml of conc. H2SO4 and few magnesium turnings. Further, Thin Layer Chromatography (TLC) of the fraction was carried out using the solvent system chloroform–methanol (90: 10 v/v). In the TLC screening procedure, a thin strip of 3x10 cm of TLC Silica Plate (Silica gel 60 F254, Merck), was taken and impregnated with the fine drop of fraction. The plate was then air dried and kept for the development in chromatographic chamber containing10ml of the prepared solvent system. After the successful development, the plate was examined under the UVChamber at 254 and 366 nm. The presence of flavonoid constituents was confirmed by spraying with 1% ethanolic solution of AlCl3 [Citation9].
2.1.7 Isolation and purification of flavonoids
The developing of the ethyl acetate fractions of both species was carried out separately on preparative TLC using Chloroform–methanol (90:10 v/v) as developing system. The plates were examined under the UV light at 254 and 366 nm, resp. [Citation10] and [Citation11] , and subjected to AlCl3 solution. The selected bands marked and scratched then collected. The Rf values of the isolated compounds were recorded and co-chromatographed against the available authentic flavonoids for the confirmation of the isolated compounds at the same Rf. the isolated compounds were identified by different spectral analyses (UV, H1-NMR, IR and MS spectrometry).
Determination of melting point and different Spectral Analyses: Koffler’s heating stage microscope was used to determine the melting point, UV–Visible Spectrophotometer double beam UVD–3500 spectrophotometer, Labomed, Inc., Visible Spectrophotometer, Shimadzu UV 240 (PIN 204-58000) (Japane), Infrared spectrophotometer, Perkin-Elmer 283 (Germany), Nuclear Magnetic Resonance spectrometers JEOL EX-270 MHz, 300 MHz and 500 MHz for determination of H1-NMR, Mass spectrometer; Finnigan Model 3200 at 70 eV.
2.2 Material for antioxidant and cytotoxic study
2.2.1 Free radical scavenging activity
The four extracts were screened at 50 μg/ml using 0.1 mM DPPH•. The absorbance was measured at 517 nm, after 30 min incubation [Citation12]. Ascorbic acid was used as standard reference [Citation13].
2.2.2 Chemicals
DPPH• was obtained from Fluka. Vitamin C (ascorbic acid) obtained from Laboratory Rasayan. Methanol used was of analytical grade.
2.2.3 Cell lines
Human breast carcinoma (MCF-7 cell line) and colon carcinoma (HCT-116 cell line) were obtained from Karolinska Center, Department of Oncology and Pathology, Karolinska Institute and Hospital, Stockholm, Sweden.
2.2.4 Determination of LC50 values
It was performed using SPSS computer program (SPSS for windows, statistical analysis software package/version 9/1989 SPSS Inc., Chicago, USA).
2.2.5 Cell culture
The procedure was done in laminar air flow cabinet biosafety class II level. Culturing and subculturing were carried out according to Thabrew et al. [Citation14]. Doxorubicin was used as a positive control. DMSO used as negative control.
2.2.6 Cell viability assay
This was done according to Mosmann et al. [Citation15]. The cells were seeded at concentration of 10x103cells per well in case of MCF-7, 20 × 103 cells/well in case of HCT-116 cell lines using 96-well plates at 37 °C. After 48 h’ incubation, the medium was aspirated and 40 μl MTT salt (2.5 mg/ml) were added and furtherincubated for 4 h. 200 μl 10% sodium dodecyl sulphate (SDS) was added. The absorbance was measured at 595 nm.
2.2.7 Measurement of Bcl-2 levels
BCL-2 in the samples and standards were estimated according to [Citation16]. A biotin-conjugated antibody was added followed by streptavidin-HRP. The reaction is then terminated by addition of acid and absorbance was measured at 450 nm.
2.2.8 Measurement of Bax levels
Bax protein level were evaluated according to [Citation17]. Monoclonal antibody specific to Bax captured on the plateis added. After incubation, Streptavidin conjugated to Horseradish peroxidase is added. The reaction is terminated by the addition of acid and optical density of the color produced measured at 450 nm.
2.2.9 Human CASP7 (Caspase 7) estimation
The micro ELISA plate provided in this kit pre-coated with CASP7 specific antibody. A biotinylated CASP7antibodyand Avidin-Horseradish Peroxidase (HRP) conjugate was added. Aspire the excess components. The substrate solution was added. wells that contain CASP7, biotinylated detection antibody and Avidin-HRP conjugate will appear blue in color. The color turns yellowfollowed the addition of sulphuric acid solution. The optical density (OD) was measured at a wavelength of 450 nm ± 2 nm. [Citation18].
3 Results
3.1 GC/MS analysis of the lipoidal extracts
GC/MS analysis of the n-hexane extracts of P. sativum and V. faba was performed, and identification of the constituents was carried out by comparison of their spectral fragmentation patterns with those of the available database libraries Wiley (Wiley Int.) USA and NIST (Nat. Inst. St. Technol., USA)] and/or published data in Adams [Citation19] using Aglient 6890, 70 eV with positive ion mode. Quantitative determination was carried out based on peak area integration. The identified components are compiled in and .Twenty compounds were identified from the lipoidal matter of P. sativum, representing 82.99% of the total content from which 2, 2-dimethyl-1-(2,4,6 trimethylphenyl) was the major compound (12.56%) followed by 6-Phenylundecane (12.18%). Nevertheless, seventeen compounds were identified from V. faba representing 85.97% of the total lipoidal content, 5-phenylundecane was identified as the main compound of V. faba (23.24%) followed by 2 (Benzoyloxy) cycloheptanone (12.74%).
Table 1 GC/MS analysis of the n-hexane extract of P. sativum:
Table 2 GC/MS analysis of the n-hexane extract of V. faba.
3.2 Identification and quantification of phenolics and flavonoids
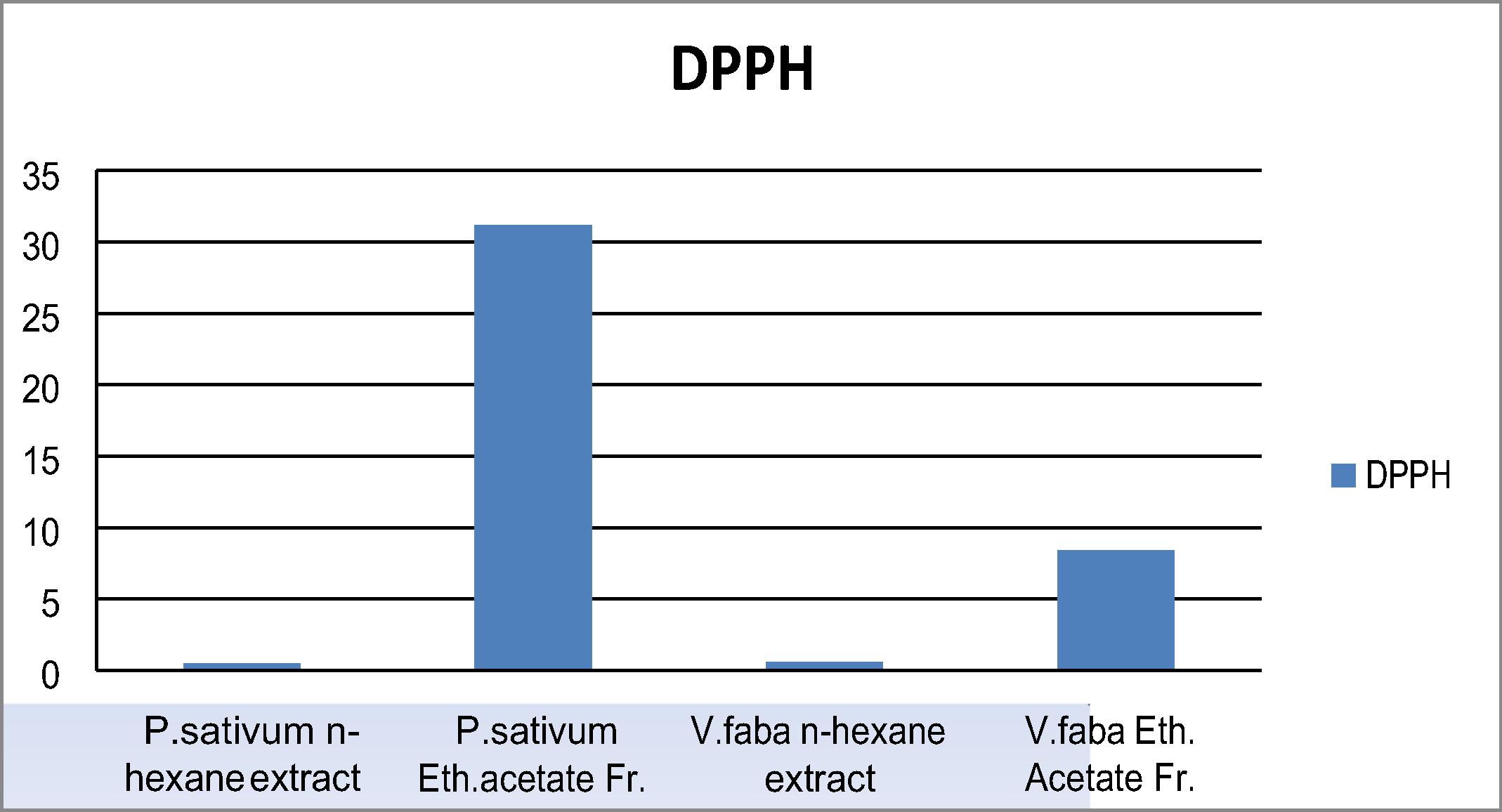
and summarize the results of HPLC analysis of the ethyl acetate fraction of both plant peels. The total identified flavonoids from P. Sativum were 17 compounds representing 19.31 mg/g of the total content, while 16 compounds were identified for V. faba representing 12.30 mg/g dry weight of the whole fraction. Hesperidin was found to be the major flavonoid in both plants recording (6.10 mg/g) for P. sativum and (2.46 mg/g)for V. faba. On the other hand, regarding the results of total identified phenolics, 18 compounds were identified from P. sativum (89.65 mg/g) of dry weight of which pyrogallol (8.61 mg/g) was the main phenolic followed by catechol (8.53 mg/g). Moreover, 17 phenolic compounds were identified from V.faba and the total phenolic content recorded (62.40 mg/g). Pyrogallol also was the main compound identified (8.50 mg/g) followed by ρ-hydroxy benzoic acid (8.10 mg/g). These recordable numbers of phenolics with reasonable amounts were very encouraging for the cytotoxic study.
Table 3 HPLC analysis of flavonoids in the ethyl acetate fractions of P. sativum and V. faba.
Table 4 HPLC analysis of phenolics in the ethyl acetate fractions of P. sativum and V. faba.
3.3 Structure characterization of the isolated phenolics from P. Sativum
Apigenin: Yellow colored amorphous powder, melting point: 180–181 °C, UV: deep purple, UV/NH3: yellow green. Rf 0.87 using Chloroform–methanol (90:10 v/v) as developing system, UV λ MeOH max (nm) 267, 296 sh, 336; +NaOMe 275, 324, 392; +AlCl3 276, 301, 348, 384; +AlCl3 – HCl 276, 299, 340, 381 nm; + NaOAc 274, 301, 376; +NaOAc – H3BO3 268, 303sh, 338. IR data showed a broad intermolecular OH stretch vibrations band at 3333 cm−1, an aromatic C–H stretch appeared at 3040 cm−1, in addition to a vibration band at 1646 cm−1 characteristic for flavone of conjugation between the C=O and double bonded of C2–C3, also, 1801 cm−1 for lactone ring, in addition to three vibration bands at (1466, 1497, and 1578 cm−1) for the ring C=C, while 1466 cm−1 denotes the characteristic of C–O–H stretch. The intensive band at 1024 cm−1 was most probably the result of C–O–C stretch from the central heterocyclic ring. H1-NMR (400 MHz, CH3OH):δ7.75 (2H,d, J = 8.3 Hz, H-2′ and H-6′),6.86 (2H,d, J = 8.3 Hz, H-3 and H-5′), 6.79 (1H, d, J = 2.1 Hz, H-6), 6.68 (1H, d, J = 2.1 Hz, H-8), 6.65 (1H, s, H-3).
Mass spectrum confirmed the molecular formula of C15H10O5,EI- MS m/z: [M + H]+ 271, the base peak was observed at m/z 117, another significant peak at m/z 151 (31%). Apigenin can undergo sequential loss of C2H2O and CO2to give a fragment at m/z 183.
Apigenin-7-O-glucoside: Yellow crystal, melting point: 227 °C (223–226 °C) [Citation20], UV: dark purple, UV/NH3: yellow green. Rf 0.89, UVdata λ MeOH max (nm) 267, 334; +NaOMe244sh, 267, 300, 386; +AlCl3 276, 302, 349, 383; +AlCl3 – HCl278, 303, 343, 380 nm; +NaOAc 270, 350, 388; +NaOAc – H3BO3 270, 343.IR data: 1462, 1501, and 1580 cm−1 for the ring C=C, while 1466 cm−1 denotes the characteristic of C–O–H stretch, H1-NMR (400 MHz, CH3OH): δ ppm 7.81 (2H, d, J = 9.1 Hz, H-2′/6′), 7.36 (2H, d, J = 9.1 Hz, H-3′/5′), 6.65 (1H, s, H-3), 6.55 (1H, d, J = 2.6 Hz, H-6), 6.88 (1H, d, J = 2.6 Hz, H-8), δ 5.01 (1H, d, J = 7 Hz, H-1″). Mass spectrum gave M+at 433 for molecular formula C21H20O10 other fragments observed at 443 (48%), 473 (42%) and 503 (22%) especially indicate the presence of substituted pentose. From the spectral analyses, melting point [Citation21] (Neil et al., 2001), co-chromatography against authentic and comparing with the published data [Citation22], this compound was identified as apigenin-7-O-β-D-glucopyranoside.
3.4 Structure characterization of the isolated phenolics from V. faba
Quercetin: It was in the form of yellow amorphous powder, melting point: 315–316 °C, UV: yellow, UV/ NH3: yellow, Rf 0.55, UV data λ MeOH max (nm) 255, 269 sh, 301 sh, 370; +NaOMe 247, 321; +AlCl3 272, 304 sh, 333, 458; +AlCl3 – HCl 265, 301 sh, 359, 428 nm; +NaOAc 257 sh, 274, 329, 390; +NaOAc – H3BO3 261, 303sh, 388.IR data: at 3421.20 cm−1 for (–OH group), 2932.54 cm−1 representing (CH-stretching), 1067.25 cm−1 (C–O bond), peaks at 1612.0, 1561.0, 1421.6 cm−1 significant for (aromatic ring system), H1-NMR (400 MHz, CH3OH):H-8 appeared at δ 6.47 (d, J = 1.1, 1H) and H-6 at δ 6.21 (d, J = 1.5, 1H), H-2′ at δ 7.74 (d, J = 2.2, 1H-6′), H-5′ showed at δ 6.95 (d, J = 8.4, 1H-6′), H-6′ at 7.64 (d,d., J = 2.2, 1H-2′-J = 8.4, 1H-5), Mass Spectrum illustrated the [M] + at 302 (100%) for molecular formula C15H10O7, beside other main fragments at m/z with relative abundance: 301 (60%), 151 (58%).
Quercetrin (Quercetin-3-rhamnoside): Yellow powder, melting point: 181–182 °C, gave deep purple color under UV and yellow green with UV/NH3, Rf 0.60, UV data λ MeOH max (nm) 256, 265sh, 301 sh, 350; + NaOMe 270, 326, 393; +AlCl3 276, 304sh, 333, 430; +AlCl3 – HCl 272, 303sh, 353, 401 nm; +NaOAc 272, 322, 372; +NaOAc – H3BO3260, 300sh, 367. IR analysis: peak at 3425.00 cm−1 broad represents hydroxyl group, (–OH stretch), peak at 2980.02 cm−1 represents C–H stretching (–CH), peak at 1371.50 cm−1 represents CH3bending and peak at 1465.13 cm−1 represents bending of methylene and methyl group, peak between 1720 cm−1 to 1732 cm represents conjugated ketone (C=O), peak at 1430.00 cm−1 and 653.94 cm−1 represent aromatic ring system, peak at 1300.21 cm−1 C=C group, peak at 1078.21 cm−1 represents etherlink (C–O–C–). 1H-NMR (400 MHz, CH3OH) δ: 7.51 (1H, d, J = 1.4 Hz, H-2′), 7.61 (1H, dd, J = 8.3, 1.8 Hz, H-6′), 6.65 (1H, d, J = 8.1 Hz, H-5′), 6.32 (1H, d, J = 1.9 Hz, H-8), 6.20 (1H, d, J = 1.7 Hz, H-6), 5.10 (1H, d, J = 2.2 Hz, H-1″), 4.25–3.20 (sugar H), 0.99 (3H, d, J = 6.0 Hz, H-6″). Mass spectrum was confirmed the molecular formula of C21H20O11 [M+1] at m/z 448 (100%), in addition to another significant fragment was noticed at 107 (30%) for [M-H-C15H16O9]−.
It is to be noted that the forementioned flavonoidal compounds were isolated to our knowledge for the first time from these peels, while some of them or their derivatives were previously isolated from some edible parts [Citation23].
4 Results of antioxidant and cytotoxic study
4.1 Free radical scavenging activity
The four extracts showed weak scavenging activity of DPPH• where P. sativum ethyl acetate fraction possessed the highest scavenging activity (31.2%) followed by V. faba ethyl acetate fraction (9.8%) as showed in .
4.2 Cytotoxic study of the four extracts
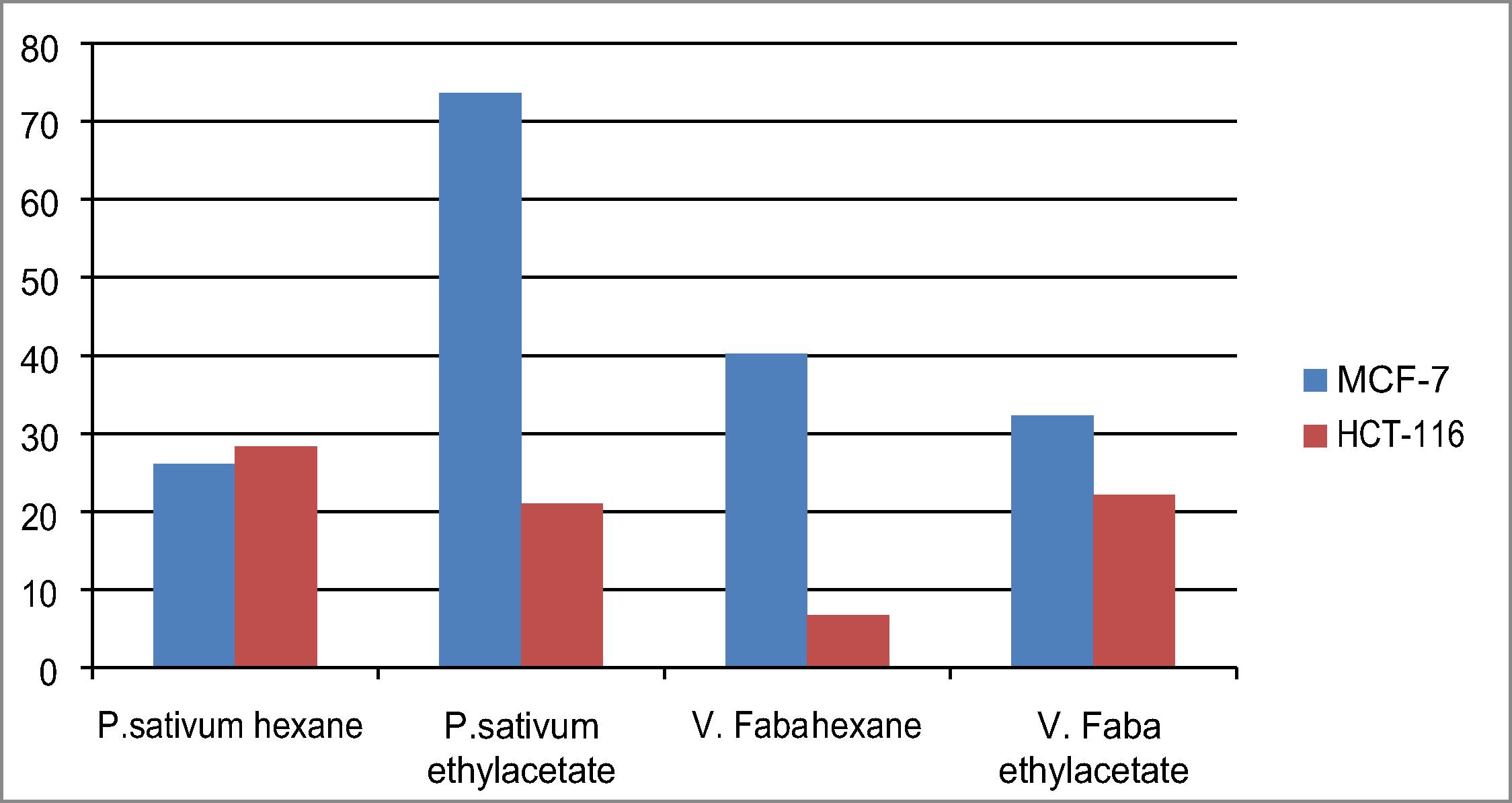
The four extracts were preliminary screening at 100 ppm for their antiproliferative effect using two human tumor cell lines [human breast carcinoma (MCF-7), human colon carcinoma (HCT-116)]. P. sativum ethyl acetate fraction showed high activity over breast cancer cell line with 73.6% and low activity over colon tumor cell line (HCT-116) with 21% while P. sativum n-hexane extract exhibited 26.1, 28.3 on both cell lines, resp. On the other hand V. faba n-hexane extract gave 40.2 and 6.7, while V. faba ethyl acetate fraction 32.3, 22.1 over both MCF-7 and HCT-116, respectively, . The P. sativum ethyl acetate fraction which possessed the highest activity over breast carcinoma cell line was further assayed at lower concentrations to calculate its LC50 which was calculated as 73.4 ± 1.7 on Breast carcinoma cell line.
5 Cytotoxic study of the isolated compounds
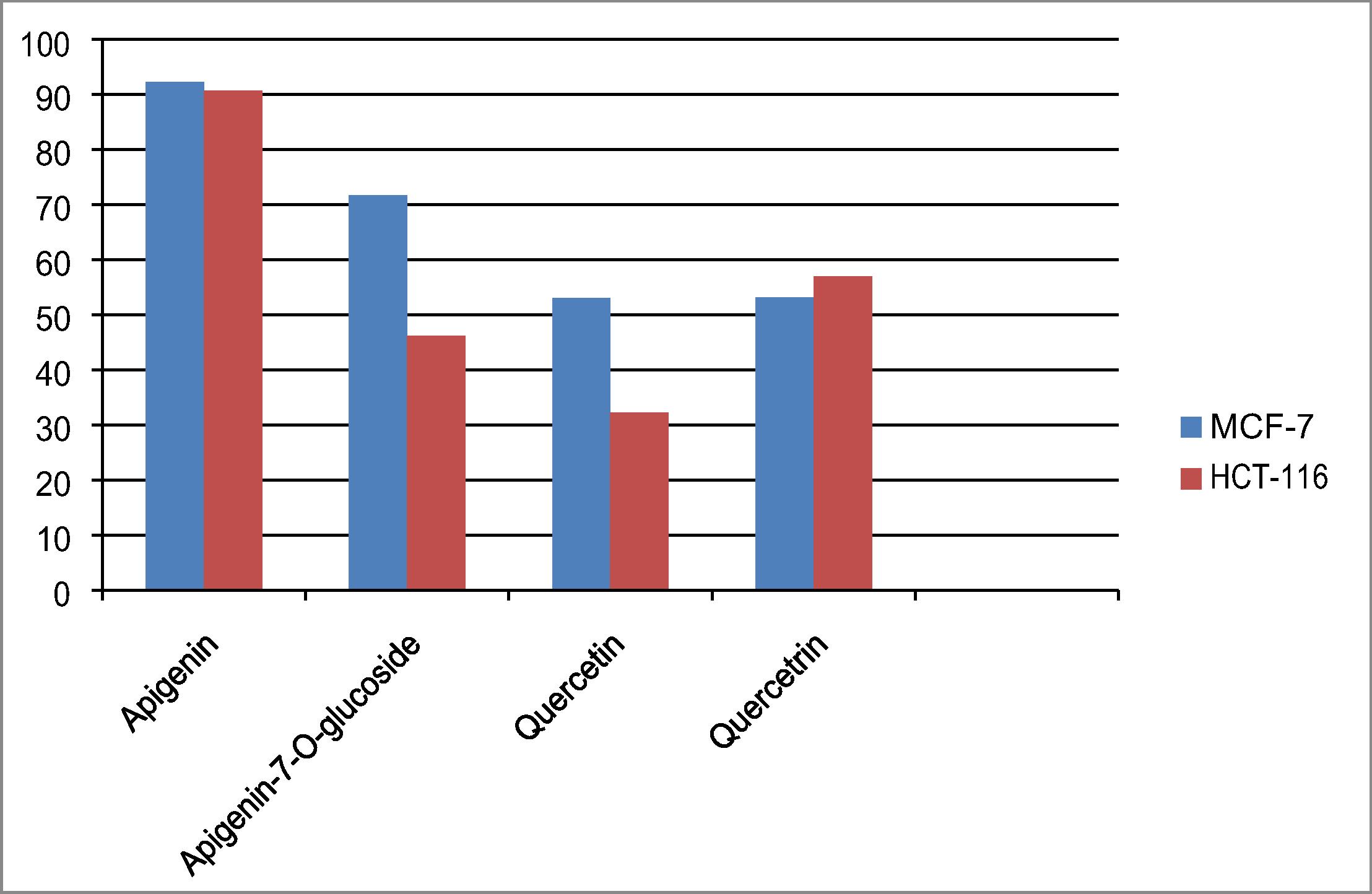
The four compounds were tested for their cytotoxic activity over [(MCF-7) and (HCT-116)] as shown in . Apigenin possessed promising cytotoxic effect on breast cancer and colon cell line, so it is further screened over the cell lines to calculate its LC50 value where it recorded the following LC50 values; 44.8, 60.8 over breast and colon tumor cell line, respectively. Apigenin proved to be the most potent tested compound on (MCF-7) and has no cytotoxic effect on normal human skin cell lines, so it is directed to explore its apoptotic mode of action.
6 Apoptotic mechanism of Apigenin
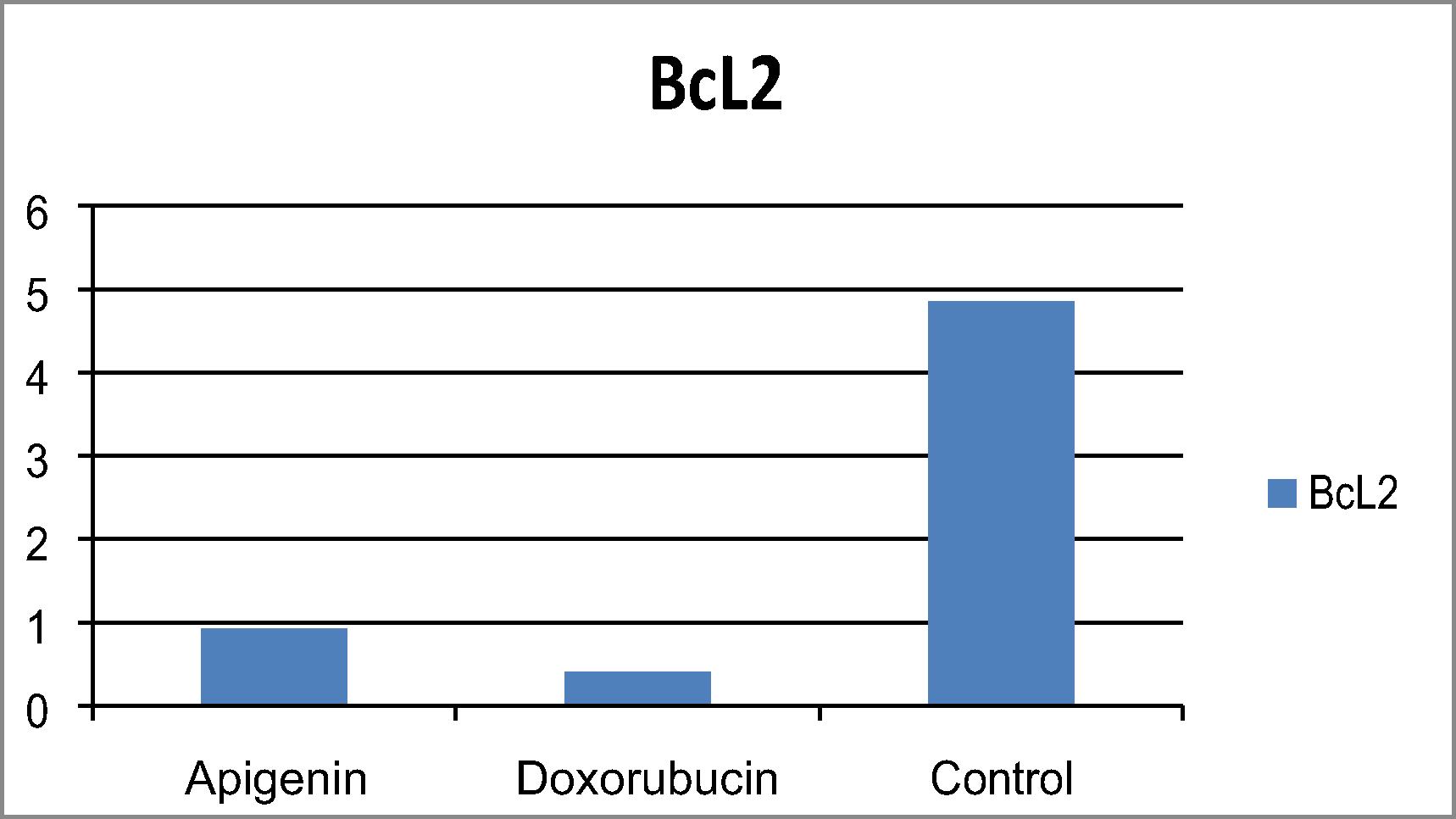
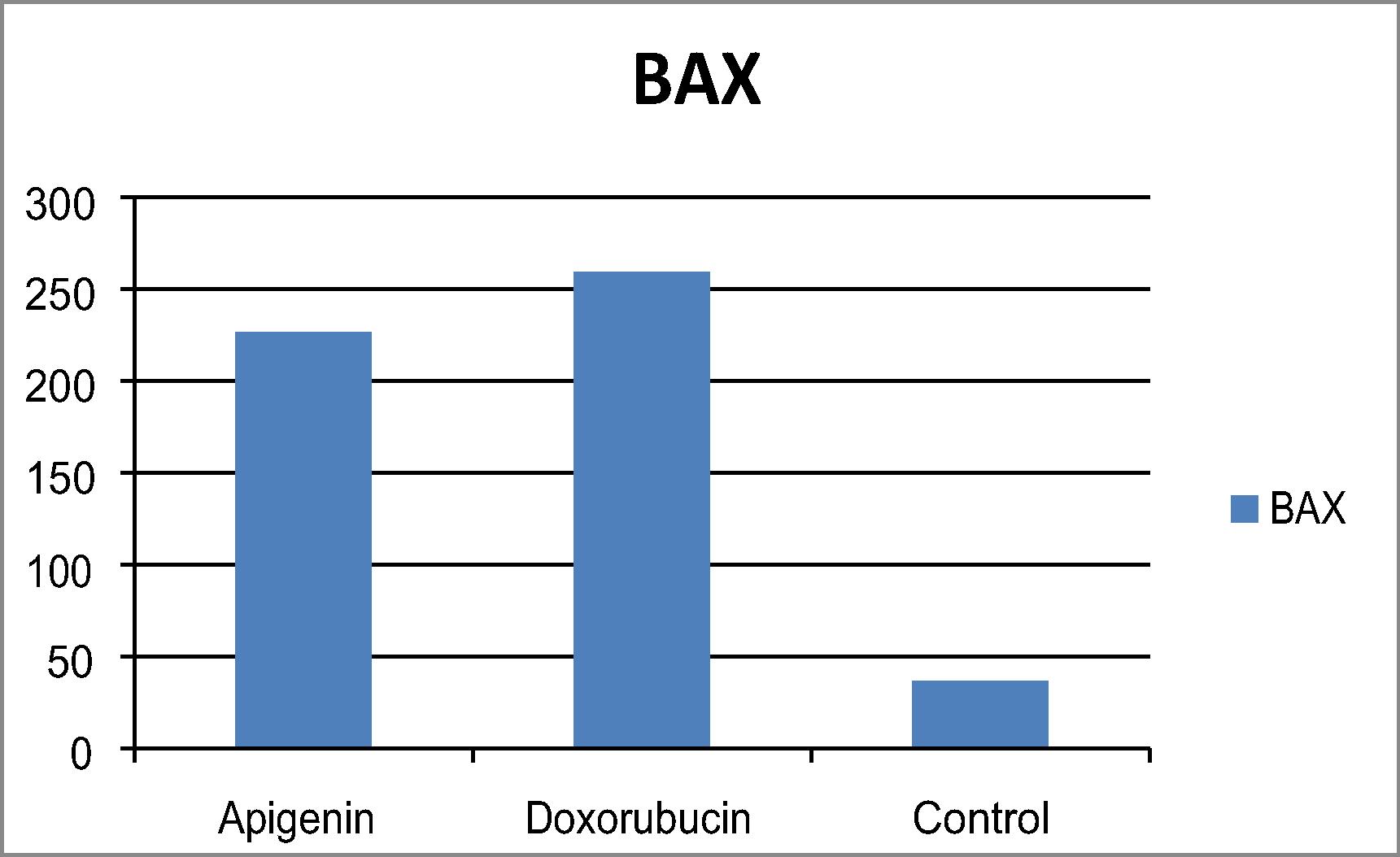
Apoptosis, or programmed cell death, takes place in all living organisms. Disruption of apoptotic mechanisms could lead to the deregulation of cell proliferation. Targeting the process of apoptosis is an appropriate strategy for prevention and treatment of cancer. Based upon that apigenin effectively suppressed the growth of both colon and breast tumor cell line with LC50 on breast less than colon, changes in expression of apoptosis- (apoptosis-related genes) of apigenin over (MCF-7) were investigated. Quantitative estimation of BcL2, Bax, Bax/ Bcl2 ratio and caspase 7 were determined. Bcl-2 family members are associated with the intrinsic pathway of poptosis comprised of both pro-apoptotic (e.g. Bax, Bid) and anti-apoptotic (e.g. Bcl-2, Bcl-XL) members. Expression of the anti-apoptotic Bcl-2 in breast cancer cell line treated with apigenin was significantly decreased as compared with control “untreated breast cancer cells “as shown in . Apigenin resulted in an increase in Bax protein levels in MCF‐7 cells chart .
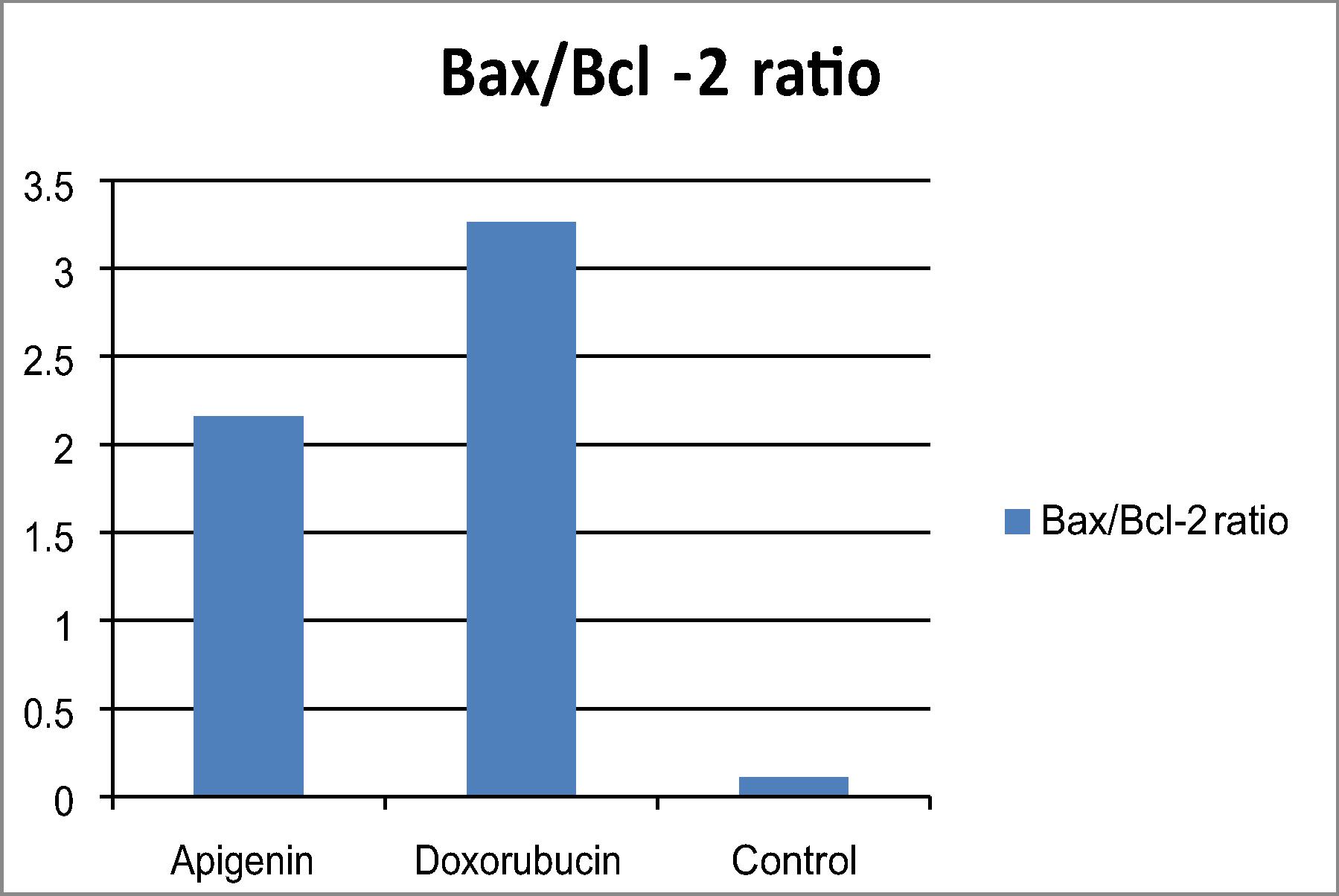
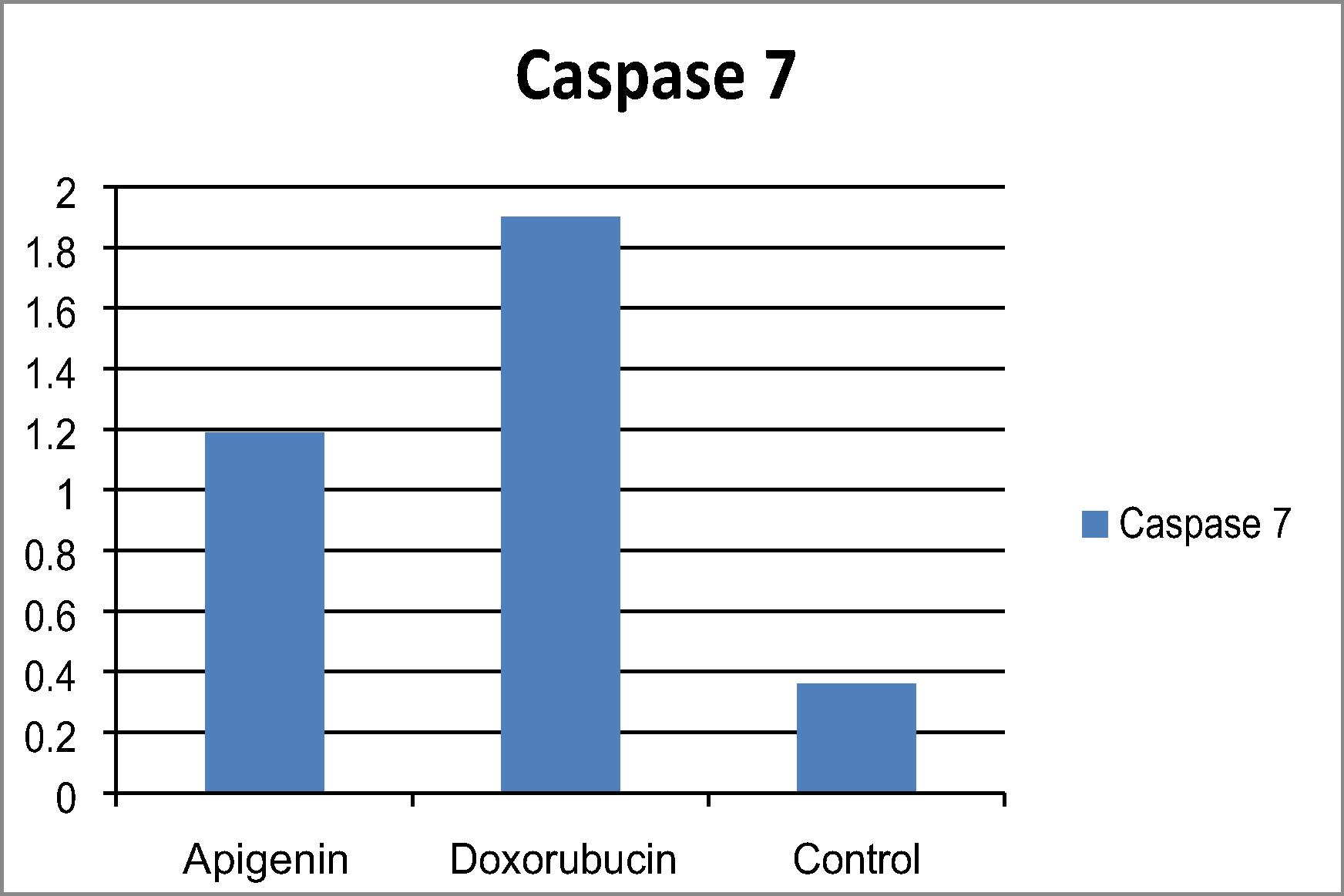
The ratio between pro- and anti-apoptotic levels an important determinant of cell survival. The Bax/Bcl-2 ratio in cells treated with apigenin increased drastically, indicating that apoptosis induced in breast cancer cells might be mediated by the mitochondrial pathway (). Caspase-7 is a member of the caspase family of proteins plays a central role in the apoptotic machinery. Apigenin showed increased in caspase 7 levels in treated breast cancer cell line in comparison to untreated breast cancer cell line as shown in .
7 Discussion
In our work we attempted to discover the correlation of the lipoidal and phenolic compounds and their impact on some human cell line carcinoma. An extensive number of hydrocarbons and triterpenoids with different groups are known to exhibit chemoprevention and cytotoxicity against many tumor cells as well as anticancer remedy both in vitro and in vivo [Citation24Citation[25]–Citation26] . Phenolics with its assorted variety of classes are famous of inducing apoptosis and cytotoxic activities on various cancer cell lines. The ability of scavenging of radicals and antioxidant properties are principally responsible for the antitumor activities of phenolic compounds. Quantitative structure–activity relationship studies on the cytotoxic effect of phenolic compounds have been examined in the recent period by many studies [Citation27Citation[28]–Citation29] .
Hesperidin which is a major flavonoid in the ethyl acetate fractions of both plants under study, has demonstrated to protectively affect CCl4-induced oxidative stress and resultant dysfunction of rat liver which has been correlated to its antioxidant property [Citation30]. Another study investigated hesperidin impact on the proliferation of MCF-7 human breast cancer cells, and prostate cancer cells [Citation31]. On the other hand, pyrogallol, a major phenolic compound identified from V. faba exhibited a moderate effect against prostate cancer cell in a previous study Chew et al. [Citation32] Catechol which is another significant phenolic identified from P. sativum apparently turned out to have a sensible effect on two breast cancer cell lines (MCF-7 and MDA-MB-231[Citation33]. ρ-Hydroxy benzoic acid, a phenolic acid present in P. sativum and V. faba with great amounts was previously proven to have strong cytotoxic activity on both colon (HCT116) and liver (HEPG2) carcinoma cell line [Citation34]. While pyrogallol exhibited a potential anticancer activities against renal cell carcinoma cell lines and no activity was observed with normal healthy cells [Citation35].
8 Conclusion
In the present investigation, it can be clearly noticed that both plant peels are wealthy in phenolics and flavonoids which assumed an important role as antioxidant and anticancer as well. Our outcomes affirmed that apigenin isolated from P. sativum ethyl acetate fraction caused the loss of cell viability of MCF-7 breast cancer cells and induces mitochondrial-dependent apoptosis through Bax activation, Bcl-2 down regulation and imbalance between Bcl-2 and Bax expressions which prompt mitochondria-mediated caspases pathways including activation of caspase-7. These results urge to make the best utilization of plant wastes and reconsider their advantageous use as a promising and effective remedy against many cancer diseases.
Acknowledgements
There hasn't been any financial support from NRC as this research is a totally independent from NRC Funding.
References
- K.F.KhattakT.U.RahmanAnalysis of vegetable’s peels as a natural source of vitamins and mineralsInt Food Res J242017292297
- P.BatraA.K.SharmaAnti-cancer potential of flavonoids: recent trends and future perspectivesBiotech32013439459
- A.ManiyanR.JohnA.MathewEvaluation of fruit peels for some selected nutritional and anti-nutritional factorsEmer Life Sci Res120151319
- A.TroszynskaI.EstrellaM.L.Lohpez-AmohresT.HernahndezAntioxidant activity of Pea (Pisumsativum) seed coat acetone extractLebensm-Wiss u Technol352002158164
- A.K.SinghR.C.BharatiN.C.ManibhushanA.PedpatiAn assessment of faba bean (Vicia faba L.) current status and future prospectAfr J Agric Res8201366346641
- I.A.EmiolaR.M.GousNutritional evaluation of dehulled faba bean (Vicia faba cv. Fiord) in feeds for weaner pigs. South AfricanJ Anim Sci4120117986
- P.GoupyM.HuguesP.BiovinM.J.AamiotAntioxidant composition and activity of Barly (Hordeumvulgare) and malt extracts and of isolated phenolic compoundsJ. Sci. Food Agric79199916251634
- P.MatillaJ.AstolaJ.KumpulanienDetermination of flavonoids in plant material by HPLC with diode-array and electro-array detectionsJ Food Chem48200058345841
- G.J.MishraM.N.ReddyJ.S.RanaIsolation of flavonoid constituent from Launaea procumbens Roxb. by preparative HPTLC methodIOSR J Pharmacy22012511
- K.HostettmanA.MarstonM.HostettmanPreparative chromatographic techniques: applications in natural product isolation2nd1998Springer-VerlagBerlin
- K.MarkhamIsolation techniques for flavonoidsJ.B.HarborneT.J.MabryH.MabryThe flavonoids1975Academic PressNew York
- C.ShenH.JunS.ChoiY.KimE.JungG.Ohet al.Evaluation of antioxidant activities and active compounds separated from water soluble extracts of Korean black pine barksBull Korean Chem Soc31201035673572
- S.M.MoustafaB.M.MenshawiG.M.WasselK.MahmoudM.M.MounierScreening of some wild and cultivated Egyptian plants for their free radical scavenging activityIntern J PharmTech Res4201412711278
- M.ThabrewR.D.HughesI.G.McfarlaneScreening of hepatoprotective plant components using a HepG2 cell cytotoxicity assayJ Pharm Pharmacol49199711321135
- T.MosmannRapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assaysJ Immunol Methods6519835563
- M.BarbareschiO.CaffoS.VeroneseR.D.LeekP.FinaS.Foxet al.Bcl-2 and p53 expression in node-negative breast carcinoma: a study with long- term follow-upHum Pathol27199611491155
- R.OnurA.SemerciözI.OrhanH.YekelerThe effects of melatonin and the antioxidant defense system on apoptosis regulator proteins (Bax and Bcl-2) in experimentally induced varicoceleUrol Res322004204208
- J.B.DenaultG.S.SalvesenHuman caspase-7 activity and regulation by its N-terminal peptideJ Biol Chem27820033404234050
- Adams P. Identification of essential oils by ion trap mass spectroscopy. New York: Academic Press, INC; 1989. Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005; 81: 317S-325S.
- J.PatoraB.KlimekFlavonoids from Lemon Balm (Mellissa officinalis L., LamiaceaeActa Poloniac Pharmaceutica-Drug Res592002139143
- Neil MJO, Smith A, Heckelman PE, Obenchain JR, Gallipeau JAR, Arecca MA & Budavari S. The Merck Index. 13th edition Published by Merck Research Laboratories division of Merck and Co. INC. White house Station; 2001.
- B.WeberM.HerrmannB.HartmannH.JoppeC.O.SchmidtH.J.BertramHPLC/MS and HPLC/NMR as hyphenated techniques for accelerated characterization of the main constituents in chamomile (Chamomilla recutita L. Rauschert)Eur Food Res Technol2262008755760
- A.TroszynskaI.EstrellaM.L.Lopez-AmoresT.HernandezAntioxidant Activity of Pea (Pisum sativum L.) Seed Coat Acetone ExtractLebensm-Wiss u-Technol352000158164
- A.BishayeeS.AhmedN.BrankovM.PerloffTriterpenoids as potential agents for the chemoprevention and therapy of breast cancerFront Biosci162011980996
- S.KhamsanB.LiawruangrathS.LiawruangrathA.TeerawutkulragS.G.PyneM.J.GarsonAntimalarial, anticancer, antimicrobial activities and chemical constituents of essential oil from the aerial parts of Cyperuskyllingia EndlRec Nat Prod52011324327
- M.M.ElbatanonyAmal M.El-FekyA.BahaaM.HemdanAzabEl-LiethyAssessment of the antimicrobial activity of the lipoidal and pigment extracts of Punica granatum L. leavesActa Ecologica Sinica201810.1016/j.chnaes.2018.05.003
- G.W.BurtonT.DobaE.J.GabeL.HughesF.L.LeeL.Prasadandet al.Autoxidation of biological molecules: 4. Maximizing the antioxidant activity of phenolsJ Am Chem Soc107198570537065
- L.ZhangH.GaoC.HanschC.D.SelassieMolecular orbital parameters and comparative QSAR in the analysis of phenol toxicity to leukemia cellsJ ChemSoc Perkin Trans2199825532556
- S.NandiM.VrackoManish C.BagchiAnticancer activity of selected phenolic compounds: QSAR studies using ridge regression and neural networksChem Biol Drug Des702007424436
- N.TirkeyS.PilkhwalA.KuhadK.ChopraHesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidneyBMC Pharmacol5200518
- C.J.LeeL.WilsonM.A.JordanV.NguyenJ.TangG.SmiyunHesperidin suppressed proliferations of both human breast cancer and androgen-dependent prostate cancer cellsPhytother Res242010S15S19
- Y.L.ChewY.Y.LimJ.StanslasG.C.Lian EeJ.K.GohBioactivity-guided isolation of anticancer agents from bauhinia kockianakorthTradit Complement Altern Med112014291299
- A.M.VillegasL.E.CatalánI.M.VenegasJ.V.GarcíaH.C.AltamiranoNew Catechol Derivatives of Safrole and their antiproliferative activity towards breast cancer cellsMolecules16201146324641
- M.H.M.Abd El-AzimA.A.M.AbdelgawadM.El-GerbyS.AliA.M.D.El-MesallamyPhenolic compounds and cytotoxic activities of methanol extract of basil (Ocimum basilicum L.)J Microb Biochem Technol72015182185
- K.CormierR.D.CurryM.P.BetschJ.A.GoguenC.M.VogelsA.Deckenet al.Synthesis, characterization, and anticancer activities of pyrogallol-based arylspiroboratesJ Heterocyclic Chem53201618071812