Abstract
The first appearance, distribution and frequency of immunoglobulins (Igs)-positive lymphocytes were investigated in the lymphoid organs of native chicken’s embryos from embryonic day (ED) 8 to ED 20. The tissues from the lymphoid organs were dehydrated in alcohol, cleared in xylene, embedded in different grades of paraffin and 6-micron thick sections were immunostained by the indirect immunoperoxidase method using antichicken immunoglobulins. IgM-positive lymphocytes were first identified in the follicles of bursa of Fabricius at ED 10, in the white pulp of the spleen at ED 14 and in the lamina propria of the cecal tonsil at ED 20. Their frequencies of populations were statistically significant from ED 14 to ED 20. IgG-positive lymphocytes were first appeared in the bursa of Fabricius and spleen at ED 20. In the bursa IgG-positive lymphocytes were located in the medulla and cortical part of the follicles, whereas, in the spleen these immune cells were located around the white pulp. IgA-positive lymphocytes were not observed in any of the developing lymphoid organs of the present study. When the data for bursa of Fabricius, spleen, thymus and cecal tonsil were statistically compared, it was observed that both IgM- and IgG-positive lymphocytes were significantly higher in bursa of Fabricius.
1 Introduction
Immunological research has made available monoclonal antibodies (mAbs) that recognize B lymphocytes and immunoglobulins (Igs)-containing plasma cells in chicken [Citation1,Citation2]. Immunocytochemistry using these mAbs has allowed the detailed analysis of B lymphocytes and Igs-containing plasma cells in lymphoid tissues, and mucosa-associated lymphatic tissues (MALT) [Citation2–Citation6] and in the Harderian gland [Citation7] of chicken.
In the tissues and organs the B lymphocytes differentiate into plasma cells which secrete antibodies and participating in humoral immune response [Citation8]. Ontogenically B lymphocytes first synthesize IgM followed by IgG cells in the bursa of Fabricius [Citation9], and further these cells disseminate into the spleen, cecal tonsil and thymus during prenatal stages of development of chicken [Citation10]. Postnatally, the frequency and distribution of the immunoglobulin (Ig)-containing plasma cells have been studied in the major lymphoid organs [Citation1,Citation4], Harderian gland [Citation6,Citation7,Citation11,Citation12], lamina propria of digestive tract [Citation2,Citation13] and in oviduct [Citation3,Citation14] at the different postnatal stages of chickens. However, research on the embryonic lymphoid organs native chickens is lacking.
In Bangladesh, most of the farmers at village level rear native chickens (Gallus domesticus), which are scavenging in nature [Citation15], fed by green grasses, insects, and human refusal materials. Our previous study revealed that the immune cells in the lymphoid organs [Citation15], MALT [Citation13], and Harderian glands [Citation6,Citation7] of these native chickens were more than that of the hybrid chickens.
Literature review revealed limited work has been carried out regarding ontogenic development of Igs-positive lymphocytes in the hybrid chicken [Citation10,Citation16–Citation20]. Therefore, the present research has designed to understand the first appearance, distribution and development of the populations of Igs-positive lymphocytes in the embryonic stages of native chickens of Bangladesh.
2 Materials and methods
2.1 Embryos
The eggs were collected from the apparently healthy native chickens (Gallus domesticus) of Bangladesh and then incubated in a standard egg incubator at 37 °C and at 55–60% relative humidity [Citation21].
2.2 Experimental design
The lymphoid tissues (bursa of Fabricius, thymus, spleen and cecal tonsil) were dissected out from the embryos during their prenatal growth using a dissecting microscope. The lymphoid tissues were started to dissect out from the ED 8 which was followed by ED 10, ED 12, ED 14, ED 16, ED 18 and ED 20. A total of 42 embryos were used for the present study (six embryos from each group). Experiments were carried out in accordance with the guidelines laid down by the National Institute of Health (NIH) in the USA and in accordance with Bangladesh Veterinary Council (BVC) laws and regulations.
2.3 Antibodies
For the study of Igs-positive lymphocytes normal rabbit serum was obtained from Biosource, Camarillo, California, USA, and goat anti-chicken IgA, -IgG, -IgM and HRP-conjugated rabbit anti-goat IgG were obtained from Bethyl Lab, Montgomery, TX, USA. The specificity of these antibodies was validated by other groups [Citation22,Citation23].
2.4 Immunohistochemical staining method
The tissues (bursa of Fabricius, thymus, spleen and cecal tonsil) were fixed in ice-cold periodate-lysine-paraformaldehyde, dehydrated in a series of graded alcohol, cleared in xylene, and embedded in paraffin. The sections were cut at 6 μm thickness using sliding microtome (MIC 509, Euromex, Tokyo, Japan) were immunostained by the indirect immunoperoxidase method as described earlier in our previous studies [Citation6,Citation7,Citation13]. In brief, after endogenous peroxidase was inhibited with methanol and H2O2, the sections were overlayed with 2% normal rabbit serum diluted with 0.01 M phosphate-buffered saline (PBS) for 2 h, followed by incubation with goat anti-chicken IgA (1:1000), goat anti-chicken IgG (1:1000) or goat anti-chicken IgM (1:1000) for 18 h at 4 °C. After brief washing with PBS, sections were treated with peroxidase-conjugated rabbit anti-goat IgG (1:500) diluted in PBS for 1 h at room temperature. Positive reactions for different classes of Igs were revealed by treating the sections with 0.2 mg 3,3′-diamino-benzidine-tetrahydrochloride dehydrate (ApliChem, Darmstadt.) per ml of Tris–hydrochloride (0.05 M, pH 7.6) containing 0.03% H2O2, and then counterstained slightly with hematoxylin. As control, normal goat serum was used instead of primary antibodies to IgM, IgG or IgA. No corresponding immunoreaction was detected in control studies (data not shown).
2.5 Histoplanimetry
The Igs-positive lymphocytes in the lymphatic tissues (bursa of Fabricius, thymus, spleen and cecal tonsil) were counted in 20 microscopic fields, each consisting of an area of 0.158 mm2 at a magnification of 40 and their relative frequency per 0.1 mm2 was calculated using an ocular micrometer [Citation24].
2.6 Statistical analysis
All values were expressed as mean ± SEM. Statistical significance of difference was evaluated by using paired sample t-test. The statistical analyses were performed by using the SPSS windows package (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Igs-positive lymphocytes in the bursa of Fabricius
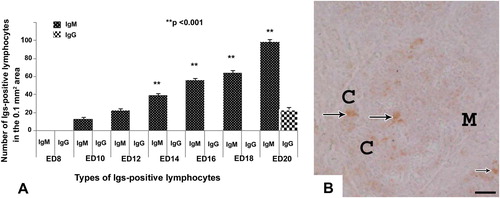
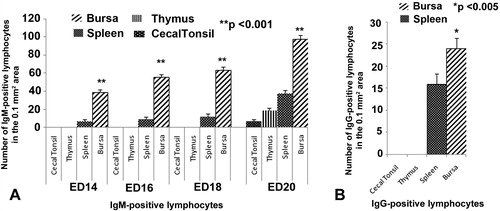
In the bursa of Fabricius, the IgM-positive lymphocytes were first appeared at ED 10 and their number increased significantly (p < 0.001) in the bursa from ED 14 to ED 20 of the embryonic ages (). These immune cells were distributed homogenously in the cortex and medulla of the bursal follicle (). At ED 20, few IgG-positive lymphocytes were stained in the bursal follicles ().
3.2 Igs-positive lymphocytes in the spleen
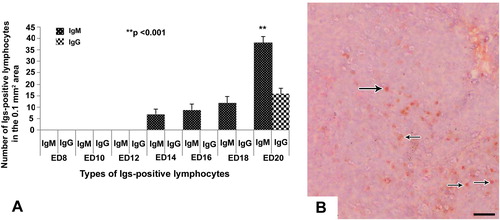
In the spleen, the IgM-positive lymphocytes first appeared at ED 14. A steady progress of appearance of IgM-positive lymphocytes was observed in this organ from ED 16 to ED 18 (). The number of these immune cells was found increased significantly (p < 0.001) at ED 20 comparing to ED 14 (). The Igs-positive lymphocytes in the embryonic spleen were located principally around the white pulps (), subcapsular tissue and around the trabeculae. Very few IgG-positive lymphocytes appeared at ED 20 ().
3.3 Igs-positive lymphocytes in the thymus and cecal tonsil
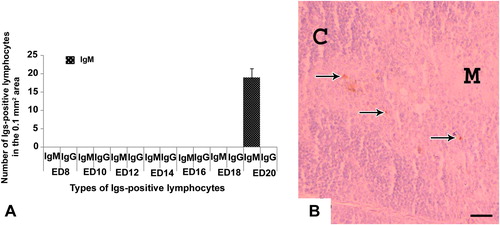
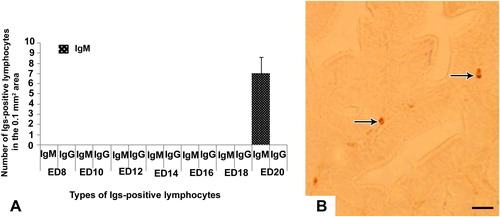
In the embryonic thymus and cecal tonsil, IgM-positive lymphocytes were immunohistochemically located first at the late embryonic stage at ED 20 ( and ). These immune cells were distributed in the cortex and medulla of thymus () and in the lamina propria of cecal tonsil (). In the thymus, IgM-positive cells were more in the cortex than the medulla (). IgG- and IgA-positive lymphocytes were not appeared in the thymus and cecal tonsil of the native chicken’s embryo in the present study ( and ).
3.4 Comparative frequency of Igs-positive lymphocytes in cecal tonsil, thymus, spleen and bursa of Fabricius
In the present study, statistical comparison of the frequency of Igs-positive lymphocytes was carried out comparing cecal tonsil, thymus, spleen and bursa of Fabricius. From ED 10 to ED 12 IgM-positive lymphocytes were present only in bursa of Fabricius. From ED 14 to ED 18 IgM-positive lymphocytes were present in bursa of Fabricius and spleen, whereas, on ED 20 these immune cells were present in all the lymphoid organs of the present study. The IgM-positive lymphocytes were significantly (p < 0.001) higher in bursa of Fabricius than that of other lymphoid organs at all stages from ED14 to ED20 (). IgG-positive lymphocytes were detected only in bursa of Fabricius and spleen on ED 20. When comparing statistically, IgG-positive lymphocytes were also significantly (p < 0.005) higher in bursa of Fabricius than that of spleen ().
4 Discussion
In the present study, we used embryo of the native chickens of Bangladesh. In the villages of Bangladesh most of the farmer rear native chickens (Gallus domesticus) and these are scavenging in nature [Citation13,Citation15]. We examined three classes of Immunoglobulins (Igs)-positive lymphocytes (IgM, IgG, and IgA) in the embryonic lymphoid organs of native chickens of Bangladesh. IgA-positive lymphocytes were not detected in any of the lymphoid organs of the present study. Possibly IgA cells appear during early postnatal period of life in this strain of chicken. In the present study we were able to stain IgM- and IgG-positive lymphocytes only in the embryonic lymphoid organs on native chickens. These findings were consistent with the report about the presence of Igs-positive lymphocytes in the major lymphoid organs of hybrid chicken embryos [Citation10,Citation17,Citation20]. They noticed only IgM- and IgG-positive cells in the embryonic lymphoid organs of chickens. This indicates that the ontogeny of Igs-positive lymphocytes in hybrid and native chicken of Bangladesh are alike.
The time of synthesis of Igs-positive lymphocytes were varied among the lymphoid organs. IgM-positive lymphocytes first appeared at ED 10 in the bursa of Fabricius and then possibly via circulation reached to other lymphoid organs and appeared in the spleen at ED 14 and in the cecal tonsil and thymus at ED 20 in the present study. In contrast, IgG-positive lymphocytes were detected at ED 20 in the bursa of Fabricius and spleen. We did not notice any IgG-positive lymphocytes in cecal tonsil and thymus. Concerning the time of visualization of Igs-positive lymphocytes in the embryonic lymphoid organs, various conflicting findings have been reported in chickens. Yamamoto et al. reported that Igs-containing cells were not found in bursa of Fabricius, spleen or thymus of the chicken embryo. He added that bursa of Fabricius synthesized IgM-, IgG- and IgA-containing cells after hatching of the embryo. Kincade and Cooper reported that, IgM-containing cells were first appeared at ED 14 in the bursa of Fabricius and at ED 20 in the spleen and thymus; IgG-containing cells at ED 21 in the bursa of Fabricius of chicken embryo. Moral et al. stated that a few IgM-bearing cells were present in the lamina propria of cecal tonsil at the end of embryonic life of white leghorn chickens. However, for the first time, we have reported immunohistochemically that Igs-positive lymphocytes were appearing at ED 10 in the embryonic lymphoid organs of native chickens. The data of our present study were in agreement with the reports on the ontogeny of Igs-positive lymphocytes in embryonic lymphoid organs of chicken [Citation16,Citation25]. They mentioned that plasma cell differentiation started with the migration of stem cells from the yolk sac to the bursa of Fabricius by 10–12 days of embryonic life and within one or 2 days the stem cell differentiated into bursa lymphocytes which synthesized and incorporated IgM in their cell surface. They also added that IgG-producing cells originated from IgM-producing cells in the bursa.
In the present study, we analyzed the number of Igs-positive lymphocytes in all the stages of chicken’s embryo. We compared the frequency of the IgM- and IgG-positive lymphocytes in cecal tonsil, thymus, spleen and bursa of Fabricius. Both the IgM- and IgG-positive lymphocytes were significantly higher in bursa of Fabricius than that of other embryonic lymphoid organs. In the available literature we could not find any reports regarding the comparison of Igs-positive lymphocytes in embryonic lymphoid organs. It is interesting that we have detected more Igs-positive lymphocytes in the prenatal lymphoid organs of native chickens than that of high yielding strains of chickens [Citation10,Citation17–Citation20]. Our previous data also showed statistically abundant Igs-containing plasma cells in the postnatal lymphoid organs, Harderian gland, and MALT of native chickens of Bangladesh than that of hybrid chickens [Citation7,Citation13,Citation15] indicating that the native chickens of Bangladesh containing more Igs-positive lymphocytes during both embryonic and posthatching life. This may be due to genetic differences between native breed of Bangladesh and other breed of chickens.
In conclusion, variation in the first appearances, distribution and frequency of different classes of Igs-positive lymphocytes in the lymphoid organs of native chicken embryo was due to the sequential development of the embryo.
Acknowledgements
This project was supported financially by the BAURES (Bangladesh Agricultural University Research System). We cordially thank Dr. Mahbub Hossain (Associate Professor, Department of Hygiene, Graduate School of Medicine, Yamaguchi University) for his advice on statistical analysis. We are also grateful to Mr. Asghar Ali (English Language Instructor, Yamaguchi, Japan) for his careful English proof checking of this manuscript.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- S.H.JeurissenE.M.JanseS.EkinoP.NieuwenhuisG.KochG.F.De BoerMonoclonal antibodies as probes for defining cellular subsets in the bone marrow, thymus, bursa of Fabricius, and spleen of the chickenVet Immunol Immunopathol191988225238
- S.H.JeurissenE.M.JanseG.KochG.F.De BoerPostnatal development of mucosa-associated lymphoid tissues in chickensCell Tissue Res2581989119124
- M.Z.I.KhanY.HashimotoY.IwamiT.IwanagaPostnatal development of B lymphocytes and immunoglobulin-containing plasma cells in the chicken oviduct: studies on cellular distribution and influence of sex hormonesVet Immunol Immunopathol561997329338
- M.Z.I.KhanS.H.AkterM.N.IslamM.R.KarimM.R.IslamY.KonThe effect of selenium and vitamin E on the lymphocytes and immunoglobulin-containing plasma cells in the lymphoid organ and mucosa-associated lymphatic tissues of broiler chickensAnat Histol Embryol3720085259
- T.KimijimaY.HashimotoH.KitagawaY.KonM.SugimuraLocalization of immunoglobulins in the chicken oviductJpn J Vet Sci521990299305
- M.NasrinM.Z.I.KhanM.N.H.SiddiqiM.A.MasumMobilization of immunoglobulin (Ig)-containing plasma cells in Harderian gland, cecal tonsil and trachea of broilers vaccinated with Newcastle disease vaccineTissue Cell452013191197
- M.Z.I.KhanM.R.JahanM.N.IslamZ.HaqueM.R.IslamY.KonImmunoglobulin (Ig)-containing plasma cells in the Harderian gland in broiler and native chickens of BangladeshTissue Cell392007141149
- A.M.MowatJ.L.VineyThe anatomical basis of intestinal immunityImmunol Rev1561997145166
- P.J.Van AltenH.J.MeuwissenProduction of specific antibody by lymphocytes of the bursa of FabriciusScience17619724547
- P.W.KincadeM.D.CooperDevelopment and distribution of immunoglobulin-containing cells in the chicken. An immunofluorescent analysis using purified antibodies to mu, gamma and light chainsJ Immunol1061971371382
- K.OhshimaK.HiramatsuImmunohistochemical localization of three different immunoglobulin classes in the Harderian gland of young chickensTissue Cell342002129133
- T.R.ScottM.L.SavageI.OlahPlasma cells of the chicken Harderian glandPoult Sci72199312731279
- M.N.IslamM.Z.I.KhanM.R.JahanM.R.KarimY.KonComparative studies of mucosa and immunoglobulin-containing plasma cells in the gastrointestinal tract of broiler and native chickens of BangladeshJ Poult Sci452008125131
- W.M.ZhengY.YoshimuraT.TamuraEffects of sexual maturation and gonadal steroids on the localization of IgG-, IgM- and IgA-positive cells in the chicken oviductJ Reprod Fertil1111997277284
- M.L.RahmanM.R.IslamM.AsaduzzamanM.Z.I.KhanLymphoid tissues in the digestive tract of deshi chicken (Gallus domesticus) in BangladeshPak J Biol6200311451150
- M.D.CooperA.R.LawtonP.W.KincadeA two-stage model for development of antibody-producing cellsClin Exp Immunol111972143149
- G.D.M.MoralJ.FonfriaA.VarasE.JimenezJ.MorenoA.G.ZapataAppearance and development of lymphoid cells in the chicken (Gallus gallus) caecal tonsilAnat Rec2501998182189
- E.C.LawrenceF.Arnaud-BattandierJ.GraysonI.R.KoskiN.J.DooleyA.V.MuchmoreR.M.BlaeseOntogeny of humoral immune function in normal chickens: a comparison of immunoglobulin-secreting cells in bone marrow, spleen, lungs and intestineClin Exp Immunol431981450457
- A.M.LebacqM.A.RitterB-cell precursors in early chicken embryosImmunology371979123134
- H.YamamotoH.WatanabeT.MikamiDistribution of immunoglobulin and secretory component containing cells in chickensAm J Vet Res3819772271230
- M.N.IslamM.Z.I.KhanM.R.JahanR.FujinagaA.YanaiK.KokubuK.ShinodaHistomorphological study on prenatal development of the lymphoid organs of native chickens of BangladeshPak Vet J322012175178
- M.OnoK.OkudaS.YazawaY.ImaiI.ShibataS.SatoK.OkadaAdenoviral gizzard erosion in commercial broiler chickensVet Pathol402003294303
- T.PogonkaC.KlotzF.KovácsR.LuciusA single dose of recombinant Salmonella typhimurium induces specific humoral immune responses against heterologous Eimeria tenella antigens in chickenInt J Parasitol3320038188
- E.R.WeibelStereological principles for morphometry in electron microscopic cytologyInt Rev Cytol261969235302
- D.K.HammerThe immune system in chickensAvian Pathol319746567