Abstract
An in vivo study was carried out to verify whether extra-virgin olive oil (EVOO) has the potential to modulate alterations resulted from exposure to hexavalent chromium (CrVI) as potassium dichromate in rats. For this purpose, CrVI was injected intraperitoneally (i.p.) at a dose of 0.4 mg/kg bw/day, EVOO was given orally at a dose of 300 μl daily either a lone or co-treated with CrVI at the same doses, routes and duration (26 days). At the end of the experiment, blood and spleen samples were collected. Genotoxicity, cytotoxicity and immunotoxicity biomarkers induced by CrVI were evaluated. Also, histopathological and immunohistochemical investigations of spleen tissue were conducted. A significant increase in genotoxicity and cytotoxicity biomarkers (micronucleus frequency, 8-hydroxy-2-deoxyguanosine level and lactate dehydrogenase activity) were recorded in CrVI treated rats. In addition, the immunotoxicity biomarkers showed a significant decrease in phagocytic%, stimulated nitric oxide production and decrease in the serum lysozyme activity. Histopathological and immunohistochemical studies support the cytotoxicity study. Oral administration of EVOO can ameliorate those effects but not restored to control level. Thus, authors recommend that regular consumption of this oil in the diet provides a constant supply of potential antioxidants that could reduce these alterations.
1 Introduction
Nowadays, there is considerable interest in the cyto-protective effects of dietary compounds against oxidative stress and in the defense mechanisms involved. Natural dietary antioxidants have been given attention as possible therapeutic and protective agents against free radicals as a tool to combat oxidative stress. The extra virgin olive oil (EVOO) obtained by mechanical pressing of mesocarps of olives. Olive oil is an integral ingredient in the Mediterranean diet. There is growing evidence that it may have great health benefits including the reduction of DNA oxidation and a favorable influence on cholesterol regulation and low-density lipoprotein oxidation, as well as antithrombotic, antihypertensive and vasodilatory effects [Citation1]. In addition, the prevention of some cancers and the modification of immune and inflammatory responses [Citation2]. The mechanism proposed to explain the beneficial effects of olive oil may be attributed to its contents of the monounsaturated fatty acids, oleic acid and polyphenolic constituents that vary greatly and unexpectedly depending on the variety, the soil, the climate, area of production, irrigation, degree of fruit maturation, oil extraction procedure and storage [Citation3].
In vivo and in vitro studies suggested that phenolic hydroxytyrosol (HTy) and oleuropein compounds in EVOO are effective antioxidants through the inhibition of lipid peroxidation and scavenging of free radicals [Citation4]. Considerable data on the polyphenols of olive oil, such as flavonoids, have been reported, but few studies have been published on olive oil antioxidant effects.
In 2003, the Agency for Toxic Substances and Disease Registry (ATSDR) of the United States of America published a list of 275 organic and inorganic substances hazardous for humans and the environment. Among the 20 most dangerous compounds, chromium was at 17th position. Chromium is one of the commonly used metals and its particulates enter a variety of environmental media including soils, sediments and water. It exists in two valence states, trivalent (CrIII) and hexavalent (CrVI) compounds. It has long been recognized that CrIII occurs naturally and ubiquitously in most environmental media, while CrVI has only recently been discovered to also occur naturally in groundwater [Citation5].
The potential health effects of CrVI have been a matter of concern because of the potential wide human exposure consequent to its widespread use. It is commonly used in numerous industrial processes including applications in metal plating, leather tanning, the manufacture of color pigments, and refractory materials and as emission or erosion byproducts of chromium-based catalytic converters, asbestos brake linings, cement dust, as well as in tobacco and food additives. Non-occupational sources of CrVI include contaminated soil, air and water [Citation6]. Inadequate treatment of effluents from these industries often leads to the large-scale contamination of water resources by chromium. Eventually, the metal exerts a great influence on the survival of fish and other aquatic biota. Several studies on chromium have reported it’s cytotoxic, immunological, hematological, histological and genotoxic effects to fish [Citation7–Citation10].
CrVI induced acute and chronic toxicity, neurotoxicity, dermatotoxicity, genotoxicity, carcinogenicity, hepatotoxicity in humans and experimental animals and general environmental toxicity [Citation11]. Intracellular reduction of CrVI to CrIII induces overproduction of reactive oxygen species (ROS), which is an important characteristic of CrVI-induced toxicity [Citation12].
Since there is a lack of knowledge on the effect of olive oil against the commonly occurring environmental contaminants like chromium. In addition, most experimental studies regarding chromium toxicity are applied to aquatic creatures (fish), therefore this study planned to monitor the possible hazardous effects of CrVI on rats (mammalian model) and the possible effects of EVOO against CrVI induced toxicity by studying the cellular alteration, DNA damage, immune function alterations and histopathological changes of rat spleen (light microscopy and immunohistochemistry).
2 Material and methods
2.1 Chemicals and reagents
Potassium dichromate; K2Cr2O7 (yellow crystals dissolved in distilled water just before use) was purchased from El-Gomhoria Chemical Company, Egypt. The extra virgin olive oil (EVOO; Angel Camacho Alimentacion, S.L., Spain), obtained directly from olives, was purchased from local market. All other chemicals were obtained from Sigma (St. Louis, MO, USA).
2.2 Animals and treatment
Twenty four healthy adult male Sprague–Dawley rats (average body weight of 150–175 g) were used in this study. They were obtained from the Laboratory Animal’s farm of Faculty of Vet. Medicine, Zagazig University and acclimated to the laboratory environment for 2 weeks prior to use. Animals were housed in stainless-steel cages, maintained on 12 h light–darkness cycle with controlled temperature (21–24 °C) and relative humidity (50–60%) and given standard diet and water ad libitum throughout the study. All animals were treated in accordance with the guidelines of the National Institutes of Health (NIH) for the Care and Use of Laboratory Animals, and were conformed by Ethics of animal use in research committee (EAURC), Cairo University.
The animals were assigned into four equal groups each containing 6 rats. The group I (control group) was injected with 0.1 ml distilled water/kg bw/day as vehicle for 26 days. Group II (K2Cr2O7 treated group) was i.p injected with K2Cr2O7 dissolved in sterile distilled water at a dose of 0.4 mg/kg bw/day for 26 days in a dose volume 0.1 ml [Citation13]. The group III (EVOO administered group) was gavaged with EVOO at a dose of 300 μl daily for 26 days [Citation14], while the group IV, rats were co-treated with K2Cr2O7 and EVOO at the same mentioned doses, routes and duration. At the end of the experiment, blood sampling and rats scarification were done under diethyl ether anaesthesia.
2.3 Blood sample collection and serum separation
The blood samples were collected from median canthus (orbital vessels) of control and treated rats, where it classified into 2 parts. First part allowed to clot overnight at room temperature, then centrifuged at 3000 rpm for 10 min for separation of serum which stored at −20 °C for biochemical analysis, while the other part was collected in heparinized tubes and immediately used for evaluation of phagocytosis assay and micronucleus assessment.
2.4 Genotoxicity and cytotoxicity biomarkers
2.4.1 Micronucleus assay
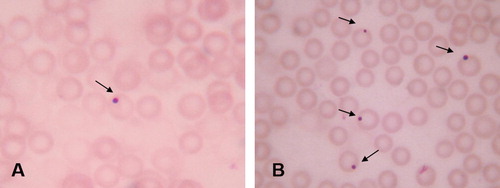
Drops of whole blood were directly smeared on slides. The slides were air-dried for 24 h, fixed in methanol for 10 min, followed by 10% Giemsa staining. To detect micronuclei in erythrocytes, the slides were analyzed using a 1000× oil-immersion lens. The mean frequency of micronuclei was evaluated per 1000 cells per group of rats [Citation15].
2.4.2 Estimation of serum 8-hydroxy-2-deoxyguanosine (8 OHdG) level
The OxiSelect™ Oxidative DNA Damage ELISA Kit (Cell Biolabs, Inc., USA) was used for quantitative detection of 8 OHdG according to [Citation16]. The quantity of 8 OHdG in unknown sample is determined by comparing its absorbance with that of a known 8 OHdG standard curve. The kit has a detection sensitivity range of 100 pg/mL to 20 ng/mL.
2.4.3 Lactate dehydrogenase (LDH) activity
Cellular damage induced by K2Cr2O7 was evaluated by LDH activity using a readymade reagent kit (Bio Med Diagnostic) according to the method described by Kachmar and Moss [Citation17].
2.5 Immunotoxicity biomarkers
2.5.1 Phagocytosis assay
To measure the phagocytic capacity, the white blood cells were separated from peripheral blood of rat in the different groups. Heat-inactivated Candida albicans (C. albicans) was used to determine the phagocytic capacity. The phagocytic activity was assessed according to [Citation18]. Macrophages containing C. albicans (phagocytosis%) as well as the number of C. albicans per one hundred phagocytes (phagocytic index) were counted.
2.5.2 Lysozyme activity assay
Serum lysozyme activity was measured using the turbidometric method as described by Ellis [Citation19]. A 25 μl of each serum sample was added to the plate wells containing agarose gel diluted in 1% in phosphate buffer saline in which Micrococcus lysodeikticus cell had been dispersed. The diameter of clear zone formed around the wells after 24 h was measured. The lysozyme concentration levels were obtained from logarithmic curve using standard lysozyme.
2.5.3 Nitric oxide (NO) assay
The NO level in serum samples were measured using the method described by [Citation20] using Griess reagent. Fifty microliters of each serum sample was incubated with an equal volume of Griess reagent in a 96 flat-bottomed microtiter plate well and incubated for 10 min at 27 °C. After incubation, the optical density was recorded using an ELISA reader at wave length 570 nm. The nitrite concentration was calculated by using Na-nitrite standard curve.
2.6 Histopathological and immunohistochemical investigation
Spleen specimens were taken from rats of different groups, weighed and fixed in 10% buffered neutral formalin solution. Five-micron thick paraffin sections were prepared, stained by hematoxylin and eosin for histopathological examination [Citation21]. Another group of sections was also prepared for immunohistochemical purposes for detection of splenic CD3 and B220 (as a marker for T or B lymphocytes, respectively) positive cells by the avidine–biotin–peroxidase (ABC) method. The procedures were previously described [Citation22]. Negative control sections were applied by incubating with phosphate-buffered saline 0.01 M instead of the primary antibodies. The following primary monoclonal antibodies were used: YE2/36HLK mouse antibody for B220 lymphocyte detection (1:10, Serotec MCA71), KT3 mouse antibody for antigens CD3 (1:200, Serotec MCA5OOG), all tissue sections were then observed by light microscopy.
2.7 Statistical analysis
Data were expressed as mean ± standard error. The data were analyzed for a statistical significance between the control and treated groups with an analysis of variance (one-way ANOVA) with the SPSS 10.1 computer program (SPSS) followed by Duncan’s multiple range test (DMRT) [Citation23]. P-Values < 0.05 were considered statistically significant.
3 Results
There is neither mortalities nor clinical signs were observed in control and treated groups through out the experiments.
3.1 Genotoxicity and cytotoxicity biomarkers
The micronuclei were identified in intact cells with preserved cytoplasm as spherical cytoplasmic inclusions with sharp contour (). K2Cr2O7-treated group showed a significant increase in the frequency of micronucleus-containing erythrocytes (37.33 ± 2.9), while this frequency significantly decreased in EVOO plus K2Cr2O7 group (29.00 ± 1.73) but still higher than the frequency reported in both EVOO and control groups (11.66 ± 0.88 and 9.33 ± 1.45, respectively; p < 0.05) ().
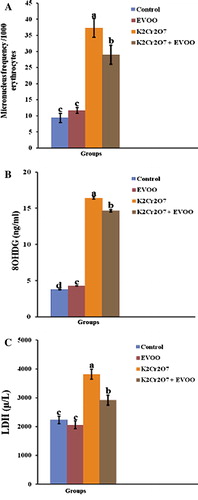
K2Cr2O7 significantly elevated 8 OHdG levels in serum (16.43 ± 0.16), while EVOO protected DNA from oxidative damage either alone or co-treated with K2Cr2O7 (4.26 ± 0.04 and 14.66 ± 0.13, respectively), where the level of it was decreased in both groups (). The serum LDH activity was elevated markedly in K2Cr2O7-treated rats (3813.33 ± 173.62). EVOO alone did not affect the activity of LDH and the simultaneous treatment with K2Cr2O7 significantly attenuated the activity of this enzyme (2920.66 ± 176.12) when compared to K2Cr2O7-treated group ().
3.2 Immunotoxicity biomarkers
The effect of successive K2Cr2O7 and EVOO exposure on immune status in rat for 26 days was illustrated in . Phagocytic% statistically decreased in rat macrophages treated with K2Cr2O7 (65.60 ± 1.17) when compared with control group (84.13 ± 0.93) (P < 0.05). Administration of EVOO to K2Cr2O7 treated rats showed non significant increase when compared with K2Cr2O7-treated group. The phagocytic index was significantly decreased in K2Cr2O7 treated group (0.67 ± 0.025) with respect to control group (1.16 ± 0.144). Oral administration of EVOO to K2Cr2O7 treated rats showed non significant increase when compared with K2Cr2O7 treated group (P < 0.05). Both phagocytic% and phagocytic index not restored to control level ( and ).
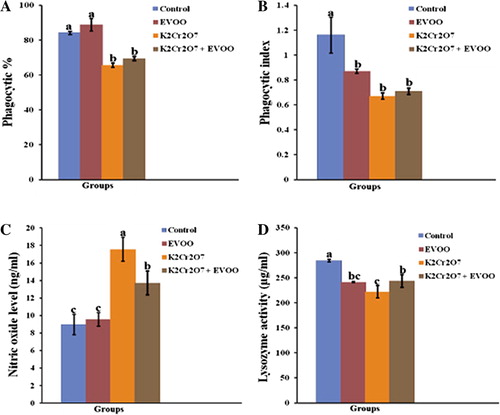
There was marked decrease in the serum lysozyme activity in K2Cr2O7 treated rats (222.13 ± 12.7). EVOO significantly restored the lysozyme activity in EVOO plus K2Cr2O7 group (244.06 ± 0.06) compared with K2Cr2O7 treated rats (P < 0.05). Also, CrVI significantly stimulated NO production (2-fold increase with respect to control; p < 0.05), whereas, co-treatment with EVOO, significantly decreased NO level (13.73 ± 0.52) ( and ).
3.3 Histopathological and immunohistochemical investigation
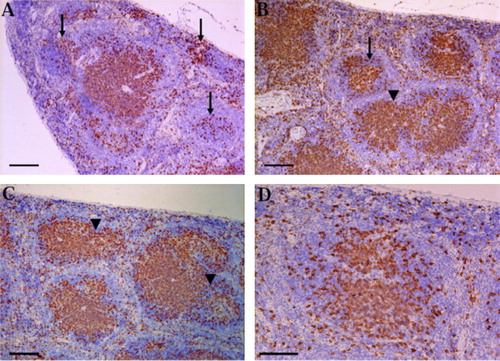
Macroscopically, the spleen of K2Cr2O7 treated rats was dark red and small in size, while in the other groups appeared an apparently normal.
Light microscopic investigation of the H&E paraffin stained spleen sections showed varied degrees of white pulp (follicular) depletion and hyperplasia among varied groups comparing to the control one. The control group showed normal splenic structure ( and ). EVOO compared to the control showed follicular hyperplasia of the most white pulp ( and ). On the contrary, the spleen in K2Cr2O7-treated groups revealed severe depletion and necrosis in the lymphocytes (B- and T-Cells) of most white pulp ( and ) Meanwhile, the red pulp revealed hyperplasia of reticuloendothelial cells and brown pigments of hemosiderosis. On the other hand, our results pointed out that the treatment of EVOO provide protection against the lymphoid depletion in rats given combination of K2Cr2O7 and EVOO, where the spleen showed hyperplasia of some white pulp and others showed depletion ( and ).
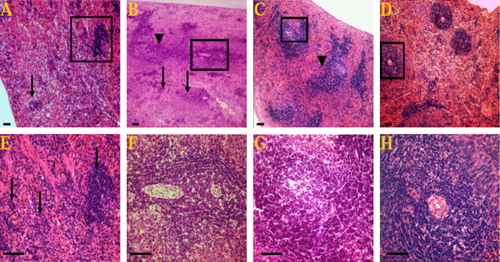
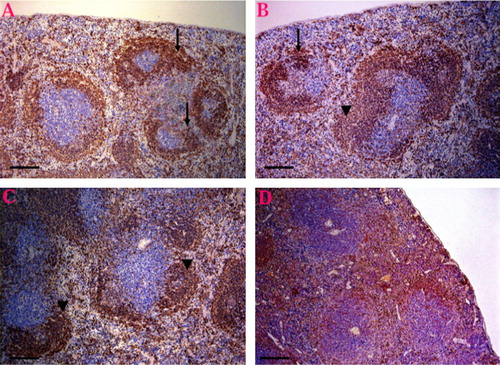
Immunohistochemical analysis revealed that, CD3 or B220 positive cells were mainly observed in the splenic lymphoid follicles. The CD3 and B220 positive areas were lower (showed severe loss) in K2Cr2O7-treated group ( and ), but more scattered in the EVOO administered group ( and ) than in the control group ( and ). and illustrate that the EVOO was ameliorate the immunosuppressive effect of K2Cr2O7 on the spleen where the CD3 and B220 cells showed intense expression and scattered throughout the white and red pulps.
4 Discussion
The micronucleus assay in erythrocytes was frequently used marker of cellular toxicity of environmental pollutants with clastogenic and aneugenic properties, where the presence of micronucleus in cells is a reflection of structural and/or numerical chromosomal aberrations a rising during mitosis. On our experiment, rats shows a significant elevation of micronucleus frequency which come on parallel with aquatic creatures results where elevated frequency of micronuclei was reported in goldfish after exposure to CrVI [Citation10]. Chorvatovicova and colleagues [Citation24] reported that micronucleus induction by CrVI is linked to its clastogenic activity, several chromium compounds induces micronucleus from both chromosome breakage and chromosome loss. De Flora et al. [Citation25] reported that CrVI do not increase the frequency of micronucleus in adult mice when introduced with the drinking water even at extremely high doses.
In the present study, DNA damage was assessed by evaluation of 8 OHdG level. The DNA damage could be due to the elevated levels of hydroproxides in the tissues; induction of ROS under metallic stress could attack the DNA and damage its integrity. The significantly higher 8 OHdG level in the K2Cr2O7 treated group clearly showed that CrVI induced DNA breakage, revealed that the CrVI is genotoxic. The incidence of DNA damage is totally in agreement with the result of micronucleus assay of the present study, indicates a possible relation between micronucleus formation and DNA damage which results in formation of smaller fragments of DNA, the cellular mechanism could form smaller nuclei with these fragments. The DNA damage could have originated from DNA breaks, DNA interstrand crosslinks, DNA protein crosslinks, chromium–DNA adducts, severe base alteration, deoxyribosephosphate backbone breakage resulting from the interaction of chromium with DNA or through the interaction with oxygen radicals, or as a consequence of the excision repair enzymes [Citation12]. Our results are corroborated with the previous report who suggested that 8 OHdG has shown to be formed upon reaction of CrVI with DNA [Citation26].
The extent of cellular injury due to CrVI toxicity was assessed by monitoring the value of LDH. K2Cr2O7 significantly elevated LDH activity in serum which considered as a presumptive marker of necrotic lesions. Cell necrosis leads to rise in LDH enzyme concentration in serum and tissue, provides an index of cell death and membrane permeability to LDH, and an increase in its activity in the serum occurs as a result of cell membrane disintegration and enzyme leakage [Citation27]. Thus, it is obvious that the increase of degenerative effects of CrVI become more prominent with the increase in the LDH activity in serum.
The results are similar with the earlier observations recorded by Kalayarasan et al. [Citation28] who found an increase in LDH activity in the serum of K2Cr2O7-treated group, while Vasylkiv et al. [Citation10] found that LDH activity in brain and plasma decreased under chromium exposure by 24% and 34%, respectively. Lipid peroxidative damage that occurs mainly in the cell membrane, caused by CrVI may be the reason for increased release of LDH in to the systemic circulation, where the earlier studies observed that Cr induce an elevation of lipid peroxides and ROS level in different tissues [Citation12].
Chromium-induced intoxication could be attributed to an induction of oxidative stress, which induces the production of ROS, Which implicated in the toxicity of CrVI. According to this hypothesis, CrVI itself is not a cytotoxic or genotoxic agent but rather an oxygen free radical generator through cellular reduction division of CrVI by the cellular reductants to generate different reactive chromium reduction intermediates, such as CrV and CrIV. During CrVI reduction process, molecular oxygen is reduced to superoxide radical (O2−), which subsequently forms H2O2. Both CrV and CrIV are able to react with H2O2 to generate hydroxyl radical (OH−). Superoxide rad ical (O2−), H2O2 and OH− collectively form ROS, which may finally at tack proteins, DNA, and membranes lipids thereby disrupting cellular functions and integrity. These species can interact with DNA and proteins leading to a variety of alterations and cause chromosomal abnormalities [Citation29].
In the present study, EVOO was succeeded for minimizing the cytotoxicity and genotoxicity of K2Cr2O7 but still more than control level, therefore limits the damages caused by K2Cr2O7. This role of EVOO may be attributed to 3,4-dihydroxyphenylethanol–elenolic acid dialdehyde and hydroxytyrosol (HTy), a natural polyphenols found abundantly in olive oil which able to preserve more efficiently the integrity of the biological membranes. This phenolic fractions has proved to have antioxidant activity in vitro, scavenging peroxyl radicals, other free radicals and reactive nitrogen species, or breaking peroxidative chain reactions and preventing metal ion catalyzed production of ROS, and modulation of signalling pathways involved in antioxidant/detoxifying enzymes regulation [Citation1]. These mechanisms of action confer olive oil a great chemo-protective potential to prevent oxidative stress-associated cell damage for its antioxidant properties.
The obtained data are consistent with previous reports on decreased nuclear DNA damage detected in various cells pretreated with olive oil phenolic extract before being exposed to H2O2 [Citation30]. Guo and colleagues [Citation31] observed that HTy reduced 8 OHdG and DNA strand breaks in HaCaT cells and prevents oxidative damages in intestinal Caco-2 cells. Besides, HTy radical scavenger effects, it modulates important signaling proteins involved in the induction of cytoprotective enzymes, thereby fortifying the inherent cellular defense capacity as an additional mechanism of cell protection [Citation1].
Fabiani and colleagues [Citation30] suggested that decreased DNA damage is associated with olive oil phenolics’s metal ion chelating properties and/or the endogenous antioxidant defenses and DNA repair systems by protection of APEX1, a DNA repair gene of cells. It may also be related to the repair oxidative damage and enhance antioxidant defense either by induction of phase II enzymes or by stimulating mitochondrial biogenesis.
It was clearly demonstrated that HTy reduced the micronucleus frequencies induced in HepG2 cell [Citation32], the biomarker of DNA damage (urinary 8 OHdG) levels was reduced in association with the intake of EVOO, regardless of the phenolic content [Citation1]. Besides, the ameliorated LDH activity resulted from EVOO administration indicates that the cellular integrity is protected, which might be due to the prevention of ROS accumulation, offered potent protection against cytotoxicity.
The results obtained in this work indicate that CrVI can affect immune parameters in rats. Significant decreases in lysozyme activity and phagocytic activity and increases in NO production were observed. This results confirmed the previous findings indicate that immunologic functions were stimulated or inhibited depending on dose and time of CrVI, spleen and head kidney macrophages phagocytic activity was altered following exposure of Asian catfish to chromium for 28 days [Citation7]. Chronic exposure of Oreochromis mossambicus to CrVI exerted suppressive effects on lymphocytes, decreased serum lysozyme activity, phagocytic killing mechanisms and disease resistance at high concentrations [Citation9]. Also, the effects of CrVI on immune parameters were confirmed by of in vitro and in vivo experiments, indicate that common effects of CrVI were decrease in lysosomal membrane stability, inhibition of lysozyme activity and phagocytosis, and stimulation of NO production [Citation33].
The study of Boscolo and colleagues [Citation34] reported an increase chromium levels in blood and urine of a group of workers exposed to lead chromate dust by inhalation at work they had reduced levels of circulating CD4+ helper, activated B and natural killer cells. On the other hand, Hanovcova and colleagues [Citation35] found higher counts of T-lymphocytes, CD4+ and CD8+ lymphocytes in their examined group of workers.
The mechanism whereby metals can alter health had been explained through modulation of immune homeostasis via inadequate or excessive production of inflammatory cytokines, direct effects of the metal, as well as protein nitration. Peroxynitrite formed from O2− and NO decreased lysozyme catalytic activity, and enhanced its susceptibility to proteasomal degradation [Citation36].
Concurrent treatment with EVOO significantly attenuated the increased serum nitrite levels. Earlier it is reported that HTy has been shown to have direct inhibitory effect on the expression of iNOS and COX-2 protein and mRNA levels after ROS generation. It has also been shown that HTy blocks the activation transcription factors such as NF-kB, which are essential in the expression of pro-inflammatory genes as cytokine, TNF-α, which leads to a pathologic loop, whereby oxidative stress and TNF-α production intermittently overplay each other [Citation37].
The reduction of spleen weight observed in this study was confirmed by histopathological alterations in the spleen architecture, as manifested by severe depletion and necrosis of lymphocyte in white pulp, which supported the previous studies [Citation7,Citation8] who reported that the Asian catfish and O. mossambicus fish exposed to chromium have lower spleen weight, reduced splenocyte number and the percentage of blood lymphocytes. Also, the immunohistochemical investigation of immune system markers (CD3 and B220) confirmed the previous findings as it showed slight expression in spleen tissue.
Altogether these results suggest toxic alterations within the spleen induced by K2Cr2O7, indicating that the immune system may be hampered and so interfering in the body mechanisms of defense. The administration of EVOO ameliorate the immunosuppressive effect of K2Cr2O7 through the protection against the lymphoid depletion in rats given combination of K2Cr2O7 and EVOO, where the spleen showed hyperplasia of some white pulp and others showed depletion. Beside, the B and T cells showed intense expression and scattered throughout the white and red pulps.
It is postulated that K2Cr2O7 caused damage to lymphoid organs involved in immune responses and ultimately led to immunosuppression. The chromium ions accumulated largely in liver, kidney, and spleen and thus interfered with the immune functions. The results reported for salmonids and other fish species also indicate that chromium is concentrated and strongly retained in liver, kidney and spleen and exert pathological effects on tissues [Citation7,Citation38]. Chromium causes injury to spleen lymphocytes and impairs the humoral and cell-mediated immune functions. In vitro studies showed signifcantly inhibited T-lymphocyte responses of spleen and pronephros of S. fossilis at all concentrations tested. The explanation for these results is that chromium ions react with splenic lymphocyte cell surfaces proteins, thereby altering the responses of the cells to various stimuli. Furthermore, chromium may become internalized within the cell and react with proteins responsible for replication. Lawrence [Citation39] also observed the immunosuppressive effects of heavy metals including chromium on mammalian lymphocyte proliferation, which explained by the reduction in splenic weight and the depletion of splenocytes observed in the present study.
5 Conclusion
Administration of CrVI at a dose of 0.4 mg/kg bw/day for 26 days induced genotoxic, cytotoxic, immunotoxic and histopathological effects on rats which mainly due to production of ROS which attack macromolecules, protein and membrane lipids disrupting cell viability and function which monitored by alterations of various studied biomarkers which counteracted by EVOO but not restored to control level. The results underline the important role of dietary antioxidants such as olive oil. Also we recommended other studies using several dose levels and durations of EVOO which may give a full protection against CrVI toxicity. We conclude that EVOO consumption may offer a new approach in preventing oxidative stress deterioration induced by environmental contaminants like CrVI.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- A.MachowetzH.E.PoulsenS.GruendelA.WiemannM.FitoJ.MarrugatEffect of olive oil on biomarkers of oxidative DNA stress in northern and southern EuropeansFASEB J21120074552
- A.H.StarkZ.MaderOlive oil as a functional food: epidemiology and nutritional approachesNutr Rev6062002170176
- A.F.VinhaF.FerreresB.M.SilvaP.ValentãoA.GonçalvesJ.A.PereiraPhenolic profiles of Portuguese olive fruits (Olea europaea L.): influences of cultivar and geographical originFood Chem892005561568
- C.L.LegerN.Kadri-HassaniB.DescompsDecreased superoxide anion production in cultured human promonocyte cells (THP-1) due to polyphenol mixtures from olive oil processing wastewatersJ Agric Food Chem48200050615067
- C.OzeD.K.BirdS.FendorfGenesis of hexavalent chromium from natural sources in soil and ground waterProc Natl Acad Sci U S A104200765446549
- T.J.O’BrienS.CeryakS.R.PatiernoComplexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanismsMutat Res5332003336
- B.S.KhangarotR.S.RathoreD.M.TripathiEffects of chromium on humoral and cell-mediated immune responses and host resistance to disease in a freshwater catfish, Saccobranchus fossilis (Bloch)Ecotoxicol Environ Saf4319991120
- R.I.ArunkumarP.RajasekaranR.D.MichaelDifferential effect of chromium compounds on the immune response of the African mouth breeder Oreochromis mossambicus (Peters)Fish Shellfish Immunol102000667676
- M.PrabakaranC.BinurameshD.SteinhagenR.D.MichaelImmune response and disease resistance of Oreochromis mossambicus to Aeromonas hydrophila after exposure to hexavalent chromiumDis Aquat Org682006189196
- O.VasylkivO.KubrakK.StoreyV.LushchakCytotoxicity of chromium ions may be connected with induction of oxidative stressChemosphere80201010441049
- Z.H.LiP.LiT.RandakEvaluating the toxicity of environmental concentrations of waterborne chromium (VI) to a model teleost, oncorhynchus mykiss: a comparative study of in vivo and in vitroComp Biochem Physiol C2011402407
- S.J.StohsD.BagchiE.HassounM.BagchiOxidative mechanisms in the toxicity of chromium and cadmium ionsJ Environ Pathol Toxicol Oncol192000201213
- A.K.ChandraA.ChatterjeeR.GhoshM.SarkarEffect of curcumin on chromium-induced oxidative damage in male reproductive systemEnviron Toxicol Pharmacol2007160166
- A.NakbiW.TayebA.GrissaM.IssaouS.DabbouI.CharguiEffects of olive oil and its fractions on oxidative stress and the liver’s fatty acid composition in 2,4-dichlorophenoxyacetic acid-treated ratsNutr Metabol7201080
- E.H.HenryB.M.JennessS.DebbieA direct comparison of mouse and rat bone marrow and blood as target tissues in the micronucleus assayMutat Res/Genet Toxicol Environ Mutagen3911–219978789
- J.BretonE.SichelF.BianchiniV.PrevostMeasurment of 8-hydroxy-2-deoxyguanosine by a commercial ELISA test: comparison with HPLC/electrochemical detection in calf thymus DNA and determination in human serumAnal Lett36200312321234
- J.F.KachmarD.W.MossN.W.TiezEnzymes in fundamentals of clinical chemistry1976SaundersPhilladelphia652660
- P.C.WilkinsonR.A.ThompsonTechniques in clinical immunology1977Blackwell Scientific PublicationsOxford, USA201218
- A.E.EllisLysozyme assaysJ.S.StolenT.C.FletcherD.P.AndersonB.S.RobersonVan W.B.MuiswinkelTechniques in fish immunologyvol. 11990SOS PublicationsFair Haven, NJ101103
- V.RajaramanB.J.NonneckeS.T.FranklinD.C.HammellR.L.HorstEffect of vitamins A and E on nitric oxide production by blood mononuclear leukocytes from neonatal calves fed milk replaced milk replacerJ Dairy Sci81199832783285
- Bancroft JD, Stevens A. Theory and practice of histological technique. 4th ed. Churchill, Livingston, New York, London, San Francisco, Tokyo; 1996.
- S.R.ShiM.E.KeyK.L.KalraAntigen retrival in formalin-fixed paraffin embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sectionsJ Histochem Cytochem3961991741748
- D.B.DuncanMultiple range and multiple F-testBiometrics111955142
- D.ChorvatovicovaZ.KovácikováJ.SandulaJ.NavarováProtective effect of sulfoethylglucan against hexavalent chromiumMutat Res3021993207211
- S.De FloraI.MariettaM.B.RoumenOral chromium(VI) does not affect the frequency of micronuclei in hematopoietic cells of adult mice and of transplacentally exposed fetusesMutat Res61020063847
- A.IzzottiM.BagnascoA.CamoiranoM.OrlandoS.De FloraDNA fragmentation, DNA-protein crosslinks, 32P postlabeled nucleotidic modifications, and 8-hydroxy-2X-deoxyguanosine in the lung but not in the liver of rats receiving intratracheal instillations of chromium_VI/. Chemoprevention by oral N-acetylcysteineMutat Res4001998233244
- A.AminA.H.HamzaOxidative stress mediates drug induced hepatotoxicity in rats: a possible role of DNA fragmentationToxicology2082005367375
- S.KalayarasanN.SriramA.SureshkumarG.SudhandiranChromium (VI)-induced oxidative stress and apoptosis is reduced by garlic and its derivative S-allylcysteine through the activation of Nrf2 in the hepatocytes of Wistar ratsJ Appl Toxicol282008908919
- O.V.LushchakO.I.KubrakM.Z.NykorakK.B.StoreyV.I.LushchakThe effect of potassium dichromate on free radical processes in goldfish: possible protective role of glutathioneAquat Toxicol872008108114
- R.FabianiP.RosignoliA.De BartolomeoR.FuccelliM.ServiliG.F.MontedoroOxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cellsJ Nutr138200814111416
- W.GuoY.AnL.JiangC.GengL.ZhongThe protective effects of hyrodxytyrosol against UVB-induced DNA damage in HaCaT cellsPhytother Res242010352359
- X.ZhangJ.CaoL.JiangC.GengL.ZhongProtective effect of hydroxytyrosol against acrylamide-induced cytotoxicity and DNA damage in HepG2 cellsMutat Res66420096468
- C.CiacciC.BarmoR.FabbriB.CanonicoG.GalloL.CanesiImmunomodulation in mytilus galloprovincialis by non-toxic doses of hexavalent chromiumFish Shellfish Immunol31201110261033
- P.BoscoloM.Di GioacchinoP.BavazzanoM.WhiteE.SabbioniEffects of chromium on lymphocyte subsets and immunoglobulin from normal population and exposed workersLife Sci60199713191325
- I.HanovcovaV.ChylkovaJ.TejralC.AndrysJ.ProchazkovaM.TurkovaLong-term monitoring of the immune reactivity of stainless steel weldersCent Eur J Public Health6119985156
- T.V.Curry-McCoyN.A.OsnaT.M.DonohueModulation of lysozyme function and degradation after nitration with peroxynitriteBiochim Biophys Acta17902009778786
- M.C.MaiuriD.De StefanoP.Di MeglioC.IraceM.SavareseR.SacchiHydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activationNaunyn Schmiedebergs Arch Pharmacol3712005457465
- D.R.BuhlerR.M.StokesR.S.CulawellTissue accumulation and enzymatic effects of hexavalent chromium in rainbow trout (Salmo gairdneri)J Fish Res Board Can341977918
- D.A.LawrenceHeavy metal modulation of lymphocyte activities. In vitro effects of heavy metals on primary humoral responseToxicol Appl Pharmacol571981439451