Abstract
One of the major problems of avian infectious bronchitis virus (IBV) is the frequent emergence of new variants. In the present study 205 tracheal swabs and organs were collected from broilers and layers chicken farms during January to August 2012 from 19 governorates all over Egypt. The chickens demonstrated respiratory signs and mortality. Out of the examined samples, 130 of which (about 64%) of suspected farms were positive for IBV with real time RT-PCR. 13 IBV-positive samples were selected for further isolation and characterization. Isolation in specific pathogen free (SPF) embryos was carried out after studies three blind successive passages and the hypervariable region of spike protein1 (SP1) was amplified by RT-PCR and sequenced to study the genetic diversity between the isolated viruses. Phylogenetic analysis of the obtained sequences of 13 isolates compared with other IBV strains from the Middle East and worldwide reveled that 11 out of the 13 isolates had close relationship the Israeli variants (IS/885 and IS/1494/06) with nucleotide homology reached up to 89.9% and 82.3%, respectively. Only two isolates had close relationship with CR/88121 and 4/91 viruses with identities of 95% and 96%, respectively. This study indicates existence of two variant groups of IBV circulating in Egypt during 2012. Group I was similar but distinguishable from Israeli variant IS/885 and group II was related to 4/91 and CR/88121 vaccine strains. There was no geographical link between the 2 groups as they were distributed all over the country. These findings necessitate the need to revise the vaccination programs and control measures for IBV.
1 Introduction
Infectious bronchitis (IB) is an acute and highly contagious respiratory disease of chickens. This disease is characterized by respiratory signs and in young chickens severe respiratory distress commonly occurs while in layers it causes decrease in egg production [Citation1]. Until recently, the chicken was considered the only natural host of IBV and in which the virus cause disease. Pheasants are the other avian species that is now considered as a second natural host for IBV [Citation2]. The disease is transmitted by the air-borne route, direct chicken to chicken contact and indirectly through mechanical spread [Citation3].
The IB virus is a member of the genus Coronavirus, family Coronaviridae, Order Nidovirales. IBV and other avian coronaviruses of turkeys and pheasants are classified as group 3 coronaviruses, with mammalian coronaviruses comprising groups 1, 2 and 4. Group 4 is the more recently identified severe acute respiratory syndrome (SARS) coronavirus [Citation4]. IBV is an envelope; positive sense single stranded RNA virus containing an un segmented genome approximately 27.6 kb in size. The virion has four structural proteins: nucleocapsid protein (N), membrane glycoprotein (M) small envelope protein (E), and glycosylation spike glycoprotein (SP) [Citation5].
Many IBV serotypes have been described probably due to the frequent point mutations that occur in RNA viruses and also and recombination events. For this reason, the characterization of virus isolates existing in the field is very important [Citation6]. The spike (SP) protein is one of the major structure proteins of IBV proteins that is cleaved into two smaller proteins namely SP1 and SP2. SP1 gene contains two hypervariable regions that are responsible for the induction of neutralizing and serotype specific antibodies [Citation7].
The existence of variable genotypes of IBV in Egypt was recognized and strains related to the Massachusetts D3128, D274, D-08880 and 4/91 genotypes have been detected at different poultry farms in Egypt [Citation4,Citation8–Citation10]. Accordingly, genotyping of IBV field strains is very important for screening the new variants as well as evaluating the vaccination programs. Avian IBV was isolated from broiler chickens showing respiratory and renal lesions and characterized as Beni-Suef/01 with 89% and 84% amino acid sequence identity and 88% nucleotide sequence identity to the IS/885 strains [Citation11].
The aim of this study aimed to survey the Egyptian chicken field for infectious bronchitis virus and study the genomic differentiation between isolated field samples seeking the new variant strain emerged in the Egyptian field.
2 Materials and methods
2.1 Sampling
In this study 205 samples were collected from chicken farms showing respiratory manifestations and mortalities during surveillance from Jan to Aug 2012, to study the prevalence of IBV in 19 Egyptian Governorates from 179 broiler farms and 26 layer farms. Pooled 10 tracheal swabs/each case and organs (trachea, kidney, and lung) were collected from dead birds.
From which thirteen samples were selected for virus isolation representing positive cases from different governorates (13 governorates were positive, 3 were positive by PCR but fail in isolation and 3 governorates were negative by PCR and isolation). As shown in .
Table 1 IBV isolates identification.
2.2 IBV detection by real time RT-PCR
Detection of IBV in collected samples and confirmation of the presence of virus after isolation in SPF-ECE has been conducted using real-time reverse transcription-polymerase chain reaction (RT-PCR) for un-translated region of IBV. RNA for RT-PCR was extracted from the supernatants of 10% w/v sample suspensions and allantoic fluid. The extraction of viral RNA was performed using a QiaAmp viral RNA mini kit (Qiagen, Germany) according to the manufacturer’s instructions. Amplification of the specific target genome was conducted using the forward primer IBV5_GU391, 5′-ACGTATGACTACCCGCAGTATTCA-3′ and reverse primer IBV5_GL533, 5′-AGACCAGCCACCATGATTGC-3′ and probe IBV5_G, 5′-FAMCACCACCAGAACCTGTCACCTC-BHQ1-3′ [Citation12]. Real-time RT-PCR was performed using Qiagen one step RT-PCR Kit (Qiagen, GmbH, Hilden, Germany) RT-PCR reactions were performed on Stratagene thermal cycler machine.
2.3 Virus isolation of selected samples
For virus isolation, the supernatants of IBV-positive selected 13 samples determined by RT-PCR were inoculated into five specific pathogen free embryonated chicken eggs (KoumOshiem SPF chicken farm, Fayoum, Egypt) 10-day-old for each sample. The eggs were inoculated with 0.2 mL of the sample into the allantoic cavity then incubated at 37 °C with candling daily. Allantoic fluids were harvested at 96 h post inoculation. Three successive blind serial passages were performed. The allantoic fluids were harvested and stored at −70 °C with examination of embryo for curling and dwarfism [Citation13].
2.4 Genetic characterization of hypervariable region of Spike 1 gene
For the positive 13 isolates the HVR of Sp1 gene were amplified using conventional PCR by Qiagen one step RT-PCR Kit (Qiagen, GmbH, Hilden, Germany) using forward primer IBV-S1-F 5′-CACTGGTAATTTTTCAGATGG-3′ and reverse primer IBV-S1-R 5′-CAGATTGCTTACAACCACC-3′ [Citation14], the amplicons were purified using the QIA quick gel extraction kit (Qiagen, GmbH, and Hilden, Germany). The sequence reactions were performed using genetic analyzer Applied Biosystems 3130 (ABI, USA) by Big dye Terminator V3.1 cycle sequencing kit. (Perkin, Elmer, Foster city, CA) using forward and reverse primers as previously mentioned.
The sequence analysis of the Sp1 gene of the Egyptian IBV Sequences used for comparison in this study were from GenBank and were available from the National Center for Biotechnology Information (NCBI) Infectious bronchitis Viruses Resource (http://www.ncbi). Sequence identities were calculated using DNAstar software [Citation15] and the phylogenetic tree of the nucleotides sequence were constructed using Mega 5 [Citation16] the sequences from the GenBank used in this study included M41 (Acc no.http://ncbi-n:HF674411), Ma5 (Acc no.http://ncbi-n:AY561713), H120 (Acc no.http://ncbi-n:JN600610), Connecticut (Acc no.http://ncbi-n:AF094818), CR/88 (Acc no.http://ncbi-n:JN592567), QXIBV (Acc no.http://ncbi-n:GQ253481), 4/91 (Acc no.http://ncbi-n:AF093794), D41 (Acc no.http://ncbi-n:AF036937), D274 (Acc no.http://ncbi-n:X15832), Egypt-F-o3 (Acc no.http://ncbi-n:DQ987085), IR/4/2010-S1 (Acc no.http://ncbi-n:JN792558), IBV-Sul/01/09-S1 (Acc no.http://ncbi-n:GQ281656), IS/1366-Sp1 (Acc no.http://ncbi-n:EU350550), IS/236-S1 (Acc no.http://ncbi-n:AY135205), IBV-S1-1494 (Acc no.http://ncbi-n:HM131453) and IS-885 S1 (Acc no.http://ncbi-n:AY279533).
3 Results
3.1 Result of infectious bronchitis virus detection in field samples
There were 205 farms tested for IBV, from which 117 broiler and 13 layer farms were positive. Fayom, Ismailia and Beni-suif governorates had the highest number of positives. However, Port-Said, North Sinai, Cairo and Aswan were tested negative for IBV using real-time RT-PCR. The positive percent of the IBV in broiler were higher than layer farms as it reached up to 65.4% in broilers while it was 50% in layer farms. As shown in .
Table 2 Results of IBV detection.
3.2 Virus isolation
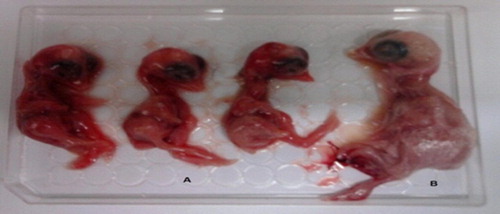
There were 13 positive isolates were identified after egg inoculation after 3 blind passages. The inoculated embryos showed curling and dwarfing with subcutaneous hemorrhages as shown in .
3.3 Results of genetic analysis
3.3.1 Result of conventional PCR
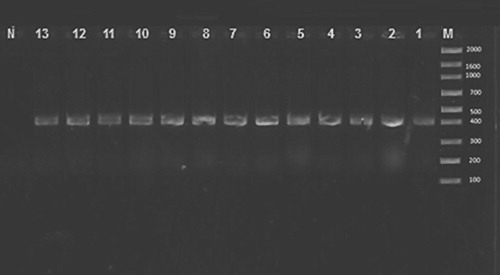
The allantoic fluids were collected and tested for confirmation of IBV by RT-PCR and they were positive as indicated in , where the 13 selected isolates showed specific 400 bp of SP1 gene.
3.3.2 Nucliotides similarity
We selected 6 isolates (numbered from 15 to 20 in for genetic analysis of SP1 gene. There were 4 isolates (#15, 16, 17, 18) had identity percent ranged from 96% to 100% among each other and about 89% in comparison with IS/885Israeli virus. While the 2 isolates (#19, 20) showed similarity reached up to 97% between each other and ranged from 93% to 96% in comparison to CR88121 and 4/91 strains. These 2 isolates also had a lower similarity with other Egyptian viruses (#15–18) with percentage of 77%. As shown in .
Table 3 Identity and diversity of IBV.
3.3.3 Genetic analysis of Spike 1 protein
The genetic analysis of 100 amino acids sequence from position 263–362 of SP1 gene for the selected 13 Egyptian viruses was done and the hypervariable region of SP1 gene showed multiple mutations as shown in in comparison with variant-2 strain. Group I had 10 amino acid substitutions in that part of hypervariable region of SP1 gene while group II had much higher genetic diversity where they were 15 amino acid substitutions in comparison to variant-2 Israeli strain .
Table 4 Amino acid substitution mutations in HVR of S1 gene of IBV.
3.3.4 Phylogenetic analysis
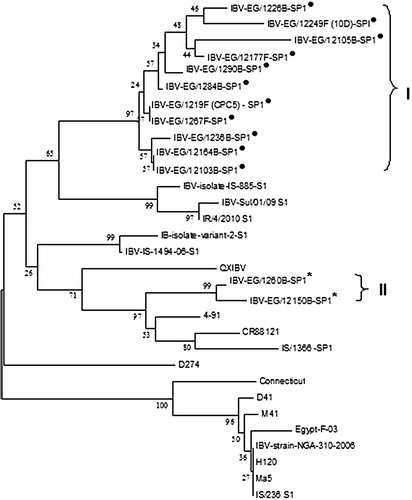
The phylogenetic analyses of the 13 selected isolates were constructed () and revealed that the Egyptian viruses in this study can be classified into two distinct groups. Group I consisted of eleven isolates which include EG/1219F, EG/1226B, EG/12164B, EG/12105B, EG/1290B, EG/12249F, EG/1284B, EG/1267F, EG/12103B, EG/12177F and EG/1236B, the isolates in group I belong to Israeli IBV (IS/885 strain). Group II consisted of two isolates EG/1260B and EG/12150B, which had a close relationship with, 4/91 and CR/88121 strains.
4 Discussion
In this study the prevalence of IBV infection in 205 examined farms by real-time RT-PCR indicated widespread distribution of infectious bronchitis virus all over Egypt. The positive percent in Beni-sueif, Ismailia and Fayoum Governorates showed the highest incidence (83%, 78% and 76%) respectively. The percent of positive broiler farms were higher than in layers Assuit was 65% in broilers while in layers it was 50%.
Samples for virus isolation commonly are inoculated into embryo chicken eggs from a specific-pathogen-free source. Samples should receive at least 3 blind passages before being called negative based on failure to cause death or lesions in embryos [Citation17,Citation18]. As mentioned in , there were 13 cases successfully isolated in chicken eggs. The thirteen isolates showed curling and dwarfing of embryos after the third passage.
The sequencing of the SP1 gene is important for IBV genotyping. IBV identification and genotyping was performed by the sequencing of hyper variable region (HVR) of the SP1 gene [Citation13]. In this study, the 13 isolates were sequenced for HVR of SP1 and the similarity among isolates was addressed in (). The similarity of Egyptian isolates with others from the neighboring countries like Israel was ranged between 82% and 90% in comparison to stains IS/1494, variant-2 and IS/885.This comparison is important due to uncontrolled movement of inhabitants and smuggling through borders [Citation19,Citation20].
The IB variant (Delaware DE072) was first reported in USA in 1992 [Citation21] was found to show little genomic relatedness in the S1 region of the S gene to other US variants; however it was related to the Dutch variant, D1466. The DE072 increased in incidence causing major disease problems in vaccinated flocks in USA [Citation22].
IBV variants have been recognized in Egypt since the 1950s [Citation23] with the isolation of a variant shown by neutralization tests to be closely related to the Dutch variant D3128.
The emergence of Israeli Variant-2 and IS/1494/06strains and the information of their SP1 gene sequence (GenBank accession number: http://ncbi-n:EU780077) had been previously reported [Citation24]. The IS/1494 has been reported to be a major variant involved in IBV infections in the Middle East like in Israel, and possibly in turkey.[Citation25].
Currently, Ma5 and H120 IBV-based vaccination strategies have been applied for the control of IB in chickens in Egypt. In addition recent variant vaccines like CR/88 and 4/91 have also been available for vaccination. However, there is persistence in problems related to IB suspected cases in spite of the use of different vaccine seeds. There is also a report, indicating the persistence of the Israeli Variant isolate (IS/1494/06) related problems in spite of vaccinations of the broiler flocks with H120, which supports the argument of IS/1494/06 IBV presence in the Middle East countries. Also a nephropathogenic IBV related problem other than IS/1494/06 IBV has been reported in Iraq as a neighboring country to Turkey [Citation20].
In this study, the phylogenetic analysis of 13 Egyptian isolates in comparison with other variant and classic viruses classified Egyptian viruses into two groups. There were11 viruses placed in group I and other two isolates belong to group II. The circulating viruses in 19 Egyptian governorates widely distributed without geographical limitation as shown in . It was found that, group I present in samples from Luxor, Menia, Sharkia and Fayuom. Group II was found in samples from Beni-suif and Qaluobia.
Sp1 gene sequencing is used for distinguishing between different IBV serotypes. Diversity in S1 probably results from mutation, recombination and strong positive selection in vivo. The generation of genetic variants is thought to be resulted from few amino acid changes in the spike (S) glycoprotein of IBV [Citation8]. Analysis of the hypervariable region of the S1 spike glycoprotein gene of thirteen viruses in group I belong to Israeli IBV (IS/885) and group II consisted of two isolates which had a close relationship with variant vaccines like (4/91&CR/88).
5 Conclusion
In this study, the IBV circulating in Egypt during 2012 can be classified into two groups of viruses. Group I was clearly variant from IS/885 and group II was more closer to variant vaccine viruses like 4/91, CR/88121 that indicates independent evolution of IBV in Egypt and persistence of divergent stains currently circulating in the country. It is very critical to complete genetic characterization of circulating IBV viruses to study the genetic relatedness among viruses and vaccine strains. This will guide us for best vaccines selection and improve our effort to control the disease.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- Butcher GD, Shapiro DP, Miles RD. Infectious Bronchitis Virus: Classical and Variant Strains. One of a series of the Veterinary Medicine-Large Animal Clinical Sciences Department, Florida Cooperative Extension Service, IFAS 2011;VM127.
- J.IgnjatovicS.SapatsAvian infectious bronchitis virusRev Sci Off Int Epiz192000493508
- T.PohuangN.ChansiripornchaiA.TawatsinJ.SasipreeyajanDetection and molecular characterization of infectious bronchitis virus isolated from recent outbreaks in broiler flocks in ThailandJ Vet Sci102009219223
- D.CavanaghSevere acute respiratory syndrome vaccine development experiences of vaccination against avian infectious bronchitis coronavirusAvian Pathol322003567582
- Jin-LingSuZu-TaoZhouZi-ShengGuoQing-RongXuYun-CaiXiaoZi-LiLiIdentification of five bronchitis virus (IBV) strains isolated in China and phylogenetic analysis of the S1geneAfr J Microbiol Res69201121942201
- N.V.VakhariaA.AmmayappanC.UpadhyayJ.GelbJrComplete genomic sequence analysis of infectious bronchitis virus Ark DPI, strain and its evolution by recombinationVirol J15720085
- G.HaqshenasK.AssasiH.AkramiIsolation and molecular characterization of infectious bronchitis virus isolate Shiraz IBV, by RT PCR and restriction enzyme analysisIranian J Vet Res622005 [Ser. No 12]
- A.S.Abdel-MoneimM.F.El-KadyB.S.LadmanJ.GelbS1 gene sequence analysis of a nephro-pathogenic strain of avian infectious bronchitis virus in EgyptVirol J3200678
- M.F.El-KadyA.A.E.El-SawahH.M.MadboulyB.T.MohamedStudies on infectious bronchitis in broiler chickens in El-Menia GovernorateVet Med J Giza5422006343358
- A.ShebleM.Z.SabryF.G.DavelaarA.R.BurgerA.K.KhafagyM.M.MoustafaPresent status of infectious bronchitis in EgyptJ Egypt Vet Med Assoc6441986393411
- A.S.Abdel-MoneimM.A.AfifiM.F.El-KadyEmergence of a novel genotype of avian infectious bronchitis virus in EgyptArch Virol2012http://dx.doi.org/10.1007/s00705-012-1445-1
- S.A.CallisonM.W.JackwoodD.A.HiltMolecular characterization of infectious bronchitis virus isolates foreign to the United States and comparison with United States isolatesAvian Dis4522001492499
- OIE Terrestrial Manual. Avian Infectious Bronchitis, 2008; p. 443–455 [Chapter 2.3.2].
- A.K.AdznarR.E.CoughD.HaydonK.ShawP.BrittonD.CavanghMolecular analysis of the 793/B serotype of IBV in great BritainAvian Pathol262007625640
- J.D.ThompsonD.G.HigginsT.J.GibsonCLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choiceNucleic Acids Res2222199446734680
- K.TamuraD.PetersonN.PetersonG.StecherM.NeiS.KumarMEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methodsMol Biol Evol2810201127312739
- J.J.De WitDetection of infectious bronchitisAvian Pathol2920007193
- J.GelbJrM.W.JackwoodLaboratory manual for the isolation and identification of avian pathogens4th ed.1989American Association of Avian PathologistsKennett Square, PA [p. 169–174]
- Y.A.BochkovG.V.BatchenkoL.O.ShcherbakovaA.V.BorisovV.V.DryginMolecular epizootiology of avian infectious bronchitis in RussiaAvian Pathol352006379393
- Z.H.MahmoodR.R.SlemanA.U.UthmanIsolation and molecular characterization of Sul/01/09avian infectious bronchitis virus, indicates the emergence of a new genotype in the Middle EastVet Microbiol15020112127
- J.GelbC.L.KeelerJrW.A.NixJ.K.RosenbergerS.S.CloudAntigenic and S-1 genomic characterization of the Delaware variant serotype of infectious bronchitis virusAvian Dis411997661669
- C.W.LeeM.W.JackwoodSpike gene analysis of the DE072 strain of infectious bronchitis virus: origin and evolutionVirus Genes2220018591
- A.BallalA.E.KarrarA.M.El-HusseinIsolation and characterization of infectious bronchitis virus strain 4/91 from commercial layer chickens in the SudanJ Anim Vet Adv4112005910912
- R.MeirE.RosenblutS.PerlN.KassG.AyaliS.PerkIdentification of a novel nephropathogenic infectious bronchitis virus in IsraelAvian Dis4832004635641
- S.KahyaF.CovenS.TemelliA.EyigorK.T.CarliPresence of IS/1494/06 genotype-related infectious bronchitis virus in breeder and broiler flocks in TurkeyAnkara Üniv Vet Fak Derg6020132731