Abstract
This work aimed to study the pathogenicity and to investigate the antimicrobial susceptibility and resistance patterns of Helicobacter pullorum (H. pullorum). The minimum inhibitory concentration (MIC) value of ciprofloxacin, ampicillin, gentamycin, erythromycin, colistin sulfate and tetracycline was determined for eight different H. pullorum isolates. H. pullorum resulted into 33.3% mortality of infected chickens with signs of diarrhea, stunted growth and poor conversion rate in survivors. All experimentally infected embryonated chicken eggs showed embryonic mortalities within 48-h post yolk sac inoculation. H. pullorum was re-isolated from cecum, liver, yolk sac and air-sacs of all dead and sacrificed infected chickens. H. pullorum was also re-isolated from dead embryos, embryonic membranes and fluids of infected embryonated chicken eggs. Polymerase Chain Reaction (PCR) assay was used to detect H. pullorum in experimentally infected chickens and embryonated chicken eggs. All tested H. pullorum isolates were resistant to ciprofloxacin, gentamycin and erythromycin, while 7 out of 8 isolates were resistant to tetracycline. All isolates were susceptible to colistin sulfate and ampicillin.
1 Introduction
Helicobacter species is a group of taxonomically related Gram-negative, microaerophilic bacteria, some of which are pathogenic and known to colonize the gastrointestinal and biliary tracts of many animal species. These pathogens are generally separated into two groups, gastric and enterohepatic, based on their preferred site of colonization [Citation19]. During the last decade, Enterohepatic Helicobacter Species (EHS) have gained recognition in the field of emerging infectious pathogens [Citation7]. Infection with this group of micro-organisms is generally characterized by colonization of the distal gastrointestinal tract and, in selected cases, the biliary tree. As reported for the gastric pathogen Helicobacter pylori, gastrointestinal colonization by EHS can be associated with chronic inflammation and neoplasia [Citation8,Citation10,Citation22].
Helicobacter pullorum is an EHS, which was first isolated by Stanley et al. [Citation19] from the feces of diarrheic humans and the intestinal contents and livers of chickens. The organism is suspected to cause vibrionic hepatitis in chickens. Infection with this organism is most often associated with farm raised birds, including chickens, Turkeys and Guinea fowl [Citation16,Citation19]. In one report, H. pullorum was isolated from human feces three months following the patients’ initial presentation with diarrhea [Citation20]. In another case, H. pullorum was isolated from the feces of a male with diarrhea and elevated liver enzymes [Citation3]. H. pullorum has also been identified by PCR in humans with inflammatory bowel disease, hepatitis, cholecystitis and hepatocellular carcinoma [Citation4,Citation9,Citation18,Citation21]. A recent report identified an association between EHS and Crohn’s disease, with H. pullorum being one of the most prevalent EHS identified [Citation13].
Despite the frequent occurrence of H. pullorum in chickens and its possible association with hepatoenteric disease, the interactions of H. pullorum with its natural host have not yet been studied [Citation6]. In addition, there is little information in the literature about H. pullorum antibiotic resistance [Citation5].
The aim of the present work is to study the pathogenicity of H. pullorum by experimental infection of one day-old chicks and embryonated chicken eggs and to investigate the antimicrobial susceptibility and resistance patterns of the organism by minimum inhibitory concentration technique.
2 Materials and methods
2.1 Experimental infection in one-day old chicks
2.1.1 Chickens and H. pullorum isolates
Fifty-two, one-day old chicks were kept separately and fed on antibiotic free ration in cleaned and disinfected isolation units. All chicks were examined clinically. Pooled cloacal swabs were collected from examined chicks and isolation trials of the pathogen were done with special reference to H. pullorum to ensure their freedom of infections.
H. pullorum isolates (broth cultures were adjusted to 1011 colony-forming units (CFU)/ml) biochemically identified and confirmed by PCR at the Laboratory of Poultry Diseases Diagnosis, Faculty of Veterinary Medicine, Assiut University [Citation11] using the method described by Stanley et al. [Citation19]. The standard plate count method technique [Citation2], with slight modification was used to adjust the number of H. pullorum per milliliter in the inoculated brain heart infusion (BHI) broth.
2.1.2 Experimental design
Fifty-two, one-day old chicks were randomly divided into two groups; first group was thirty-nine chicks, were inoculated with H. pullorum isolates via gavages (forced feeding). Each chick received a 200 μl of BHI broth containing 1011 CFU of H. pullorum organism/ml [Citation19]. The second group was thirteen chicks, were inoculated with sterile BHI broth via gavages and kept as a non-infected negative control. All infected and control chicks were observed daily for clinical signs. By the end of experiment (40th day of age), survived infected and control chickens were sacrificed and subjected for necropsy and bacteriological examination.
2.2 Experimental infection in embryonated chicken eggs
2.2.1 Embryonated chicken eggs and H. pullorum isolates
Seventy, six-day-old embryonated chicken eggs were used. Five randomly selected different H. pullorum isolates (broth cultures were adjusted to 1010 CFU/ml) previously identified [Citation11].
2.2.2 Experimental design
Ten embryonated chicken eggs were randomly selected from the total number for bacteriological isolation with special attention for H. pullorum to ensure their freedom of pathogenic infections. The embryonated chicken eggs were classified into six groups; each one contained ten embryonated chicken eggs. Five groups were inoculated with five different H. pullorum isolates via yolk sac route of inoculation. Each embryonated chicken egg was inoculated with 0.2 ml of BHI broth culture containing 1010 CFU of H. pullorum organism/ml. The 6th group was inoculated with sterile brain heart infusion broth via yolk sac and kept as a negative control group. All infected and control embryonated chicken eggs were incubated at a temperature 37 °C and humidity 70% with manual turning twice per daily. All infected and control embryonated chicken eggs were daily observed by candling for embryonic mortality.
2.3 Isolation of H. pullorum
2.3.1 Sampling
Necropsy of dead/sacrificed, infected and control chickens was done and tissue samples of cecum, liver, yolk sac and air-sacs were collected for bacteriological isolation. In addition, dead embryos, embryonic fluids and sacs were collected from embryonated chicken eggs.
2.3.2 Isolation
Samples were inoculated into brain heart infusion (BHI) broth containing 10% sterile inactivated horse serum and Skirrow’s supplement (Oxoid LDT, Biolife, Sydney, Australia) then incubated in a microaerophilic condition (5% H2, 5% CO2, 5% O2, and 85% N2) in CampyPak II anaerobic system jar with CampyPak gas generating system envelopes (BBL Becton Dickinson Microbiology Systems, Sparks, USA) or in CO2 incubator with the same gases in same proportions at 42 °C for 24–48 h. Sub-culturing was carried out on (BHI) agar plates enriched with 5–10% sheep blood and containing Skirrow’s supplement and incubation at 42 °C for 48 h under a microaerophilic atmosphere. The cultured plates were examined for typical H. pullorum colonies.
2.4 PCR analysis and gel electrophoresis
DNA was extracted from randomly selected H. pullorum colonies retrieved from infected chickens and embryonated chicken eggs, using QIAamp DNA mini extraction kit (Qiagen, Germany) according to the manufacturer’s instructions. Species identification was confirmed using the H. pullorum species-specific 16S rRNA gene PCR assay [Citation19]. In brief, the primer sequences were: 5-ATG AAT GCT AGT TGT TGT CAG-3 (forward) and 5-GAT TGG CTC CAC CAC TTC ACA-3 (reverse) (Bioneer incorporation Daejaon 306-220, Korea). The parameters for all reactions were described in the following profile; initial denaturation at 94 °C for 3 min followed by 35 cycles of denaturation at 94 °C for 45 s, annealing at 56 °C for 30 s and extension for 1.5 min at 72 °C. The final extension took 10 min at 72 °C. The PCR product (448 bp) was seen by electrophoresis in a 1.5% agarose gel stained with ethidium bromide (Sigma–aldrich, Missouri, USA) for visualization performed in a horizontal gel chamber plate. The running buffer was 0.5× TBE (Tris borate EDTA (pH 8.3). The 1 kb plus DNA ladder (Invitrogen, California, U.S.A.) was used as a reference standard molecular weight marker. A gel documentation system (Biometra, Goettingen, Germany) with a digital camera was used for image capturing.
2.5 Determination of antimicrobial susceptibility and resistance patterns of H. pullorum using MIC
2.5.1 H. pullorum isolates and antimicrobial agents
Eight randomly selected different H. pullorum isolates (broth cultures were adjusted to 106 CFU/ml) previously identified [Citation11]. The standard plate count method technique [Citation2], with slight modification was used to adjust the number of H. pullorum per milliliter in the inoculated BHI broth.
The MIC value of ciprofloxacin, ampicillin, gentamycin, erythromycin, colistin sulfate and tetracycline was determined. All antimicrobial agents were purchased from Sigma (Missouri, USA), except for ciprofloxacin, which was obtained from Bayer AG (Leverkusen, Germany).
2.5.2 Antimicrobial susceptibility testing
It was carried out by broth micro-dilution method using micro-titer plates [Citation12]. The antibiotic concentrations ranged from 0.25 to 256 μg/ml. Since there are no break-points currently available for H. pullorum, we tentatively used Enterobacteriaceae break-points as described by the Clinical and Laboratory Standard Institute (CLSI, formerly NCCLS) for Campylobacter jejuni and related species [Citation15].
3 Results
3.1 Clinical signs, mortalities and necropsy findings
A total 13 out of 39 chickens were infected via gavages by H. pullorum isolates died from 4th to 9th -day post infection as summarized in and . Chickens died at 4th-day post infection did not show any clinical signs while signs of loss of appetite, depression, ruffled feathers, anorexia and yellowish-white diarrhea were observed in chickens found dead from other days post infection. Necropsy of dead chickens revealed distended abdomen, unabsorbed yolk sac with severe congestion and dark yellow to brown contents, enlarged and congested liver with streaks of hemorrhage, distended ceca with frothy yellowish exudates, mild fibrinous pericarditis and air-sacculitis, in some cases hydropericardium, perihepatitis and yellowish gelatinous exudates might be present in abdominal cavity. Survivor chickens were retarded in growth with a poor conversion rate. They expressed symptoms of weakness, depression, loss of appetite, diarrhea and mild respiratory signs. Necropsy of scarified survivors showed emaciated carcasses with prominent keel bone, enlarged, friable and hemorrhagic liver, mild fibrinous pericarditis and air-sacculitis, congested intestine with small patches of hemorrhage and ulcers on the intestinal mucosa, distended ceca with frothy exudates, and some chickens developed ascites; some chickens retained unabsorbed yolk sac, and pneumonia might be found in some cases. No mortalities, clinical signs or necropsy findings were observed in chickens of the negative control group.
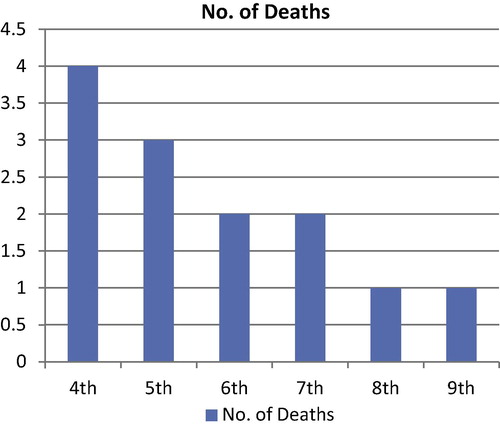
Table 1 Showing results of experimental infection of one-day old chicks with H. pullorum isolates.
All embryonated chicken eggs of the six experimentally infected groups showed embryonic mortalities 48-h post yolk sac inoculation, while no embryonic mortalities in the negative control group.
3.2 Isolation of H. pullorum
Randomly selected chicks for clinical and bacteriological examination were free from any infectious pathogen, including H. pullorum. Also, trails for isolation of pathogens from randomly selected embryonated chicken eggs were negative for H. pullorum and other pathogens. H. pullorum was re-isolated from cecum, liver, yolk sac and air-sacs of all infected dead chickens and scarified survivors. No H. pullorum was isolated from control chickens. H. pullorum was re-isolated from dead embryos, embryonic fluids and sacs. No H. pullorum was isolated from the control group of embryonated chicken eggs.
3.3 PCR analysis and gel electrophoresis
The results of PCR analysis of suspected H. pullorum isolated from experimentally infected chickens, and embryonated chicken eggs are shown in . Results revealed the appearance of 448 bp bands denoting the positive amplification of 16SrRNA for all tested isolates.
3.4 Antimicrobial susceptibility testing
Determination of antibacterial susceptibility and resistance patterns of H. pullorum using MIC for six antibiotics; ciprofloxacin, gentamycin, colistin, tetracycline, erythromycin and ampicillin against different eight H. pullorum isolates resulted in that all isolates were resistant to ciprofloxacin, gentamycin and erythromycin, while 7 out of 8 isolates were resistant to tetracycline. All isolates were susceptible to colistin sulfate and ampicillin, as summarized in and .
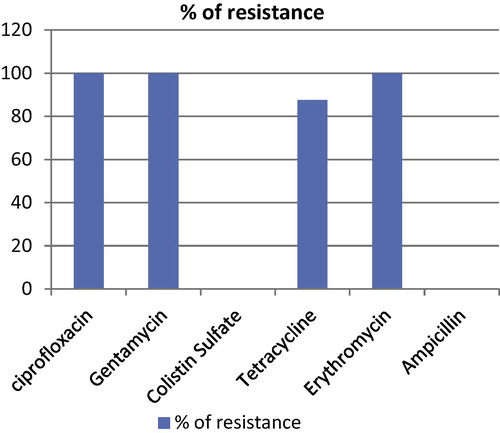
Table 2 Showing results of susceptibility of 8 chicken isolates of H. pullorum to antimicrobial agents.
4 Discussion
H. pullorum is an enterohepatic pathogen with a powerful ability to colonize the distal intestinal tract, liver of poultry and human beings. This species has been associated with diarrhea in gastrointestinal patients and enteritis and hepatitis in chickens [Citation1,Citation9,Citation19,Citation20].
Ceelen et al. [Citation6], who were the first to study the pathogenicity of H. pullorum, and they concluded that H. pullorum can colonize broiler chickens and additionally is excreted in their feces until the age of slaughter. The preferred colonization site is the cecum wherein the bacterium shows close association with the surface epithelium. Experimentally infected chickens did not reveal overt clinical signs, although mild lesions in the ceca were present. This study is only a first step in the investigation of the interaction of H. pullorum with its chicken host and stipulates further research [Citation6]. The pathogenicity of chicken H. pullorum isolates in this work was evaluated by oral inoculation of one day-old chicks, our results revealed 33.3% mortality with signs of diarrhea, retardation of growth and poor conversion rate in survivors. The differences in results of mortalities, clinical signs and autopsy findings may be attributed to many factors, like the difference in the age at which infection performed, dose of inoculation and period of experimental infection.
The present study was the first one that evaluated the pathogenicity of H. pullorum using the embryonated chicken eggs as an experimental model, and it is found that all infected embryonated chicken eggs showed embryonic mortalities within 48-h post yolk sac inoculation. This means that there is no variation in the pathogenicity of different H. pullorum chicken isolates.
H. pullorum was re-isolated from cecum and colon of experimental chickens with different bacteriological titrations, but it could not be isolated from liver tissue of experimentally infected chickens [Citation6]. In the current study, H. pullorum was re-isolated from cecum, liver, yolk sac and air-sacs of all experimental dead and sacrificed chickens. H. pullorum was also re-isolated from dead embryos as well as from embryonic membranes and embryonic fluids. The differences in results may be due to using different methods of isolation and age of infection.
PCR analysis was used to detect H. pullorum from liver, jejunum, cecum and colon of experimentally infected chickens [Citation6]. In our study, PCR assay was used to detect H. pullorum from H. pullorum colonies retrieved from experimentally infected chickens and embryonated chicken eggs.
Despite the increasing number of reports emphasizing the significance of H. pullorum, hardly any data about the antibiotic sensitivity of H. pullorum are available in the literature [Citation5]. They mentioned that different resistance percentages exhibited by H. pullorum to nalidixic acid were encountered by several research groups. On et al. [Citation17] and Atabay et al. [Citation1] reported 6% and 28% in vitro resistance respectively, while antimicrobial susceptibility assays showed 55% resistance to this antimicrobial agent among the tested strains in a study of Melito et al. [Citation14]. Thus far, no susceptibility studies comprising widely used antibiotics with H. pullorum strains have been reported [Citation5]. It was reported that H. pullorum is resistant to cephalothin and cefoperazone [Citation17,Citation19]. H. pullorum is naturally sensitive to polymyxin B, which is a phenotypic characteristic distinguishing this species from the other Helicobacter species [Citation1]. Zanoni et al. [Citation23] concluded that all the tested isolates of H. pullorum were resistant to cephalothin and all but one susceptible to nalidixic acid [Citation23]. The results of the present study proved that H. pullorum isolates were resistant to most antibacterial drugs used in that study. It could be inferred that ampicillin and/or colistin sulfate is the drugs of choice that can help in prevention and control of H. pullorum infection in chickens. Variation in results of determination of antimicrobial susceptibility and resistance patterns of H. pullorum may be due to several factors, from which; method used for determination, types of antimicrobial drugs used, types and doses of prophylactic antimicrobial drugs used in poultry farms.
In conclusion, H. pullorum resulted into 33.3% mortality with signs of diarrhea, stunted growth and poor conversion rate in survivors. All experimentally infected embryonated chicken eggs showed embryonic mortalities within 48-h post yolk sac inoculation; this means that there is no variation in the pathogenicity of H. pullorum isolates. H. pullorum was re-isolated from cecum, liver, yolk sac and air-sacs of all dead with sacrificed chickens with dead embryos, embryonic membranes and fluids. PCR assay was used to detect H. pullorum from experimentally infected chickens and embryonated chicken eggs. All tested isolates of H. pullorum were resistant to ciprofloxacin, gentamycin and erythromycin, while 7 out of 8 isolates were resistant to tetracycline. All isolates were susceptible to colistin sulfate and ampicillin.
We certify that we handled the chickens and chicken embryonated eggs during our experimental work in accordance with The Code of Ethics of the World Medical Association for experiments.
Acknowledgments
The authors would like to thank the staff members of laboratory of Poultry Diseases, Faculty of Veterinary Medicine, Assiut University for their help and support to finish this work. This research work is funded by Assiut University, Ministry of High Education, Egypt.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- H.I.AtabayJ.E.L.CorryS.L.W.OnIdentification of unusual Campylobacter-like isolates from poultry products as Helicobacter pullorumJ Appl Microbiol84199810171024
- J.G.BlackMicrobiology: principles and applications3rd ed.1996Prentice HallUpper Saddle River (NJ)p.140–4
- A.P.BurnensJ.StanleyR.MorgensternJ.NicoletGastroenteritis associated with Helicobacter pullorumLancet344199415691570
- L.CasteraA.PedeboscqM.RochaRelationship between the severity of hepatitis C virus-related liver disease and the presence of Helicobacter species in the liver: a prospective studyWorld J Gastroenterol12200672787284
- L.M.CeelenA.DecostereL.A.DevrieseR.DucatelleF.HaesebrouckIn vitro susceptibility of Helicobacter pullorum strains to different antimicrobial agentsMicrob Drug Resist1122005122126
- L.M.CeelenA.DecostereK.ChiersPathogenesis of Helicobacter pullorum infections in broilersInt J Food Microbiol1162007207213
- J.G.FoxThe non-H. pylori helicobacters: their expanding role in gastrointestinal and systemic diseasesGut502002273283
- J.G.FoxL.YanB.ShamesPersistent hepatitis and enterocolitis in germfree mice infected with Helicobacter hepaticusInfect Immun64199636733681
- J.G.FoxF.E.DewhirstZ.ShenHepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitisGastroenterology1141998755763
- A.GarcıaM.M.IhrigR.C.FryGenetic susceptibility to chronic hepatitis is inherited codominantly in Helicobacter hepaticus-infected AB6F1 and B6AF1 hybrid male mice, and progression to hepatocellular carcinoma is linked to hepatic expression of lipogenic genes and immune function-associated networksInfect Immun76200818661876
- Hassan AK, Shahata MA, Refaie EM, Ibrahim RS. Preliminary studies on Helicobacter pullorum infection in domesticated birds, MVSc Thesis 2009; Faculty of Veterinary Medicine, Assiut University, Egypt.
- M.A.JenniferDetermination of minimum inhibitory concentrationsJ Antimicrob Chemother48Suppl. S12001516
- D.LaharieC.AsencioJ.AsselineauAssociation between entero-hepatic Helicobacter species and Crohn’s disease: a prospective cross-sectional studyAliment Pharmacol Ther302009283293
- P.L.MelitoD.L.WoodwardK.A.BernadDifferentiation of clinical Helicobacter pullorum isolates from related Helicobacter and Campylobacter speciesHelicobacter532000142147
- National Committee for Clinical Laboratory Standards (NCCLS). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals, 2nd ed. NCCLS, Wayne, Approved Standard M31-A2, 2002: 80.
- P.NebbiaC.TramutaM.OrtoffiE.BertS.Cerruti SolaP.RobinoIdentification of enteric Helicobacter in avian speciesSchweiz Arch Tierheilkd1492007403407
- S.L.W.OnB.HolmesM.J.SackinA probability matrix for the identification of campylobacters, helicobacters and allied taxaJ Appl Bacteriol811996425432
- M.RochaP.AvenaudA.MenardAssociation of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinomaGut542005396401
- L.D.StanleyA.P.BurnensF.E.DewhirstHelicobacter pullorum sp. nov. – genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritisMicrobiology140199434413449
- B.SteinbruecknerG.HearterK.PelzIsolation of Helicobacter pullorum from patients with enteritisScand J Infect Dis291997315318
- L.VeijolaI.NilssonL.HalmeDetection of Helicobacter species in chronic liver disease and chronic inflammatory bowel diseaseAnn Med392007554560
- J.M.WardJ.G.FoxM.R.AnverChronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter speciesJ Natl Cancer Inst86199412221227
- R.G.ZanoniM.RossiD.GiacomucciV.SanguinettiG.ManfredaOccurrence and antibiotic susceptibility of Helicobacter pullorum from broiler chickens and commercial laying hens in ItalyInt J Food Microbiol1162007168173