Abstract
Little information is available on the presence of chlamydia infection in wildlife. This study was conducted to assess the occurrence of chlamydiae in asymptomatic birds from two species of wild birds (Cattle Egret and Hoopoe) in Egypt. In the present study Chlamydiaceae was analyzed using Giemsa stain, Giménez stain, fluorescent antibody test (FAT) and PCR. The results of these techniques were compared with CFT for detecting Chlamydia psittaci antibodies among the examined birds. The results reveal that 96.4%, 81.8%, 89.1%, 80.0% and 58.2% of the examined samples were positive for chlamydiosis using PCR, Giemsa stain, Giménez stain, FA, and CFT respectively among Hoopoe. The percentages were 90.6%, 77.4%, 83.0%, 75.5% and 66.0% respectively for the previous tests among Cattle Egret birds. The results suggest that Cattle Egret and Hoopoe may be reservoir of Chlamydiaceae species and thus shed the organisms in their excreta. The shedding of chlamydiae by free living birds in Egypt may expose humans that come in contact with these birds to zoonotic risks.
1 Introduction
The free-living birds act as vectors for a wide range of microorganisms. They fly freely and cover long distances during flying, so they play an important role in the epidemiology of human associated zoonoses. Wild birds have been implicated in the transfer of enteric human pathogens; as Campylobacter, Salmonella and toxin-producing strains of Escherichia coli [Citation1]. Also free-living wild birds are important reservoirs of Chlamydia psittaci [Citation2]. Chlamydiosis is a zoonotic disease, and human cases must be reported by state health departments to the Centers for Disease Control and Prevention [Citation3]. Sachse et al. [Citation4] detect C. psittaci in urban pigeons. Clinical presentations of C. psittaci infection in birds range from asymptomatic to systemic illness with severe respiratory and enteric signs.
Chlamydiosis in humans is most commonly reported among people in close contact with domestic birds, such as pet owners, veterinarians and workers in pet shops or poultry processing plants [Citation5]. The disease was described in the United States in 1904. From the period between 1985 and 1995, about 1332 cases of psittacosis in humans were reported by the Centers for Disease Control [Citation6].
Despite the importance of C. psittaci for wild birds, poultry, animals and humans, little information is available on the role of wild birds as reservoirs of Chlamydiaceae [Citation7]. To our knowledge, there were no current/past available studies investigating the shedding of chlamydiae by wild birds throughout the Egyptian habitat. Thus, the present study aimed to detect chlamydiae in Cattle Egret (Bubulcus ibis) and Hoopoe (Upupa epops) in Egypt.
2 Materials and methods
2.1 Specimens
One hundred and sixty asymptomatic free living birds from two species of wild birds Cattle Egret (n = 89), and Hoopoe (n = 71), were collected from Aborawash, Giza governorate and investigated for the presence of chlamydiosis.
2.1.1 Internal organs
From each bird, internal organs (liver, heart, lung and spleen) were collected. Parts of the collected internal organs of each bird were used fresh for Giemsa stained impression smears. Other parts were kept in clean labeled plastic bags in deep freezer until used for egg inoculation.
2.1.2 Blood samples
Fifty-three representative blood samples were collected from 89 Cattle Egret and 55 representative blood samples were collected from 71 Hoopoe birds for serum separation. Sera were kept at −20 °C till examined by CFT.
2.2 Cytological examination of the impression smears
Giemsa stain was used for staining of impression smears from the internal organs of the examined birds to demonstrate the Chlamydia species inclusions [Citation8].
2.3 Preparation of samples for inoculation of chicken embryo [Citation9]
The internal organs of each bird were pooled and grinded in sterile mortar with sterile sand under aseptic conditions with the addition of PBS (pH 7.5) till complete soft paste was produced. Sufficient amount of PBS buffer was added to form a 20% suspension, and then centrifuged for 15 min at 3000 rpm. A clear supernatant fluid was transferred under aseptic conditions using a sterile pipette to another centrifuge tube. Centrifugation was repeated for 15 min. The clear supernatant fluid was recollected in screw capped and a stock solution of antibiotics (Sigma–Aldrich, MO, USA); streptomycin (1 mg/ml), vancomycin (1 mg/ml), and nystatin (100 units/ml) were added to inhibit micro-organisms other than chlamydiae [Citation10]. Further, the suspension was held for 1 h at room temperature and centrifuged for 2 times. The final supernatant was used for inoculation of the embryonated chicken egg through yolk sac route.
2.4 Chicken embryo inoculation and staining of yolk sac using Giménez stain
Six to seven days old Specific Pathogen Free (SPF) fertile chicken eggs from Koom Ousheem Al Fayyom Poultry Farm, Egypt were used for detection of chlamydiae. Two hundred μl of each sample was inoculated into the egg yolk sac and the inoculated eggs were incubated at 37 °C in a humidified incubator. Non inoculated control eggs were labeled and incubated beside the inoculated eggs. The eggs were candled on a daily basis and the eggs that died within 3 days post inoculation were discarded while those died after day 3 to day 10 are opened. The yolk sac membranes were harvested and stained by Giménez stain [Citation11]. Embryos of specific deaths were examined for pathological changes and lesions specific for chlamydial infection.
2.5 Direct detection of chlamydial inclusion bodies in the infected yolk sac using direct immunofluorescence kit
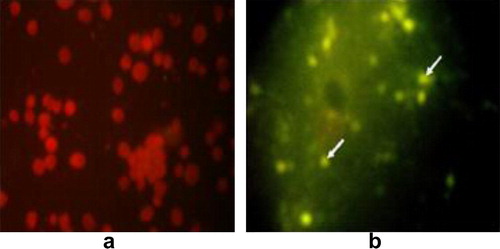
The kit (Ref 55311-Biomeriux) consisted of 2 monoclonal antibodies, one directed against the antigen of the genus Chlamydia, and the other against the species trachomatis. These antibodies were fluorescein conjugated. The kit was able to react with the 15 serotypes of Chlamydia trachomatis and the species C. psittaci. Direct fluorescence antibody test (FAT) according to Lecomte [Citation12], enabled the detection of Chlamydia in impression smears from the inoculated yolk sac membrane. A specimen was considered positive if there were at least 10 characteristic fluorescent chlamydial bodies (elementary or reticulate) over the whole surface of the smear.
2.6 Detection of C. psittaci antibodies in the collected serum samples by complement fixation test (CFT)
CFT was conducted according to Edwin and Nathalie [Citation13] using Amboceptor (Anti-sheep red blood cell); reference antiserum and antigen of Chlamydiae for CFT (C. psittaci CF test Reagent “Seiken”) supplied commercially from Denka Sieken Co., Ltd., Tokyo, Japan. Controls were included throughout the entire testing (complement control, positive known serum control and antigen control). The serum sample was considered positive if the titer was equal or above 16 unit/ml (1:16) [Citation14].
2.7 Identification of Chlamydia species using PCR
Chromosomal DNA was prepared and extracted from the infected yolk sac membranes according to McClenaghan et al. [Citation15]. The PCR procedures were performed using agarose (Prolabo, Italy); ethidium bromide solution, proteinase K, paraffin oil, Tris–EDTA buffer pH 8.0 from Sigma; phenol: chloroform, ice cold absolute ethanol from Merck & Co., Inc, NJ, USA; ice cold 70% ethanol (ADWIC, Cairo, Egypt); PCR Master Mix (DyNAzyme™ II) from Finnzymes, Vanta, Finland; and 100 bp ladder DNA marker (Invitrogen, CA, USA). Primer pairs 16S-IGF; 5′-GAT GAG GCA TGC AAG TCG AAC G-3′ and 16S-IGR; 5′-CCA GTG TTG GCG GTC AAT CTC TC-3′) specific for Chlamydiales were selected to amplify a 278-bp product according to Borel et al. [Citation16]. The PCR was run according to Everett et al. [Citation17]. Amplification was performed in 45 cycles with initial denaturation at 95 °C for 15 min, followed by denaturation at 94 °C for 30 s. and annealing at 51 °C for 30 s. then extension at 72 °C for 45 s. The gel was stained with ethidium bromide then specific bands were detected under the ultraviolet (UV) trans-illuminator. The detected bands were photographed on gel documentation system using Digital camera.
3 Results
3.1 Detection of chlamydiae using Giemsa stain, Giménez stain and FAT
Out of 89 Cattle Egret and 71 Hoopoe organ samples, chlamydial inclusions were demonstrated in 67 (75.3%) and 54 (76.1%) organs impression smears respectively, using Giemsa stain (). The characteristic chlamydial inclusions demonstrated in smears of different organs (liver, lung, heart and spleen) stained with Giemsa appeared as small, rounded reddish purple inclusions ().
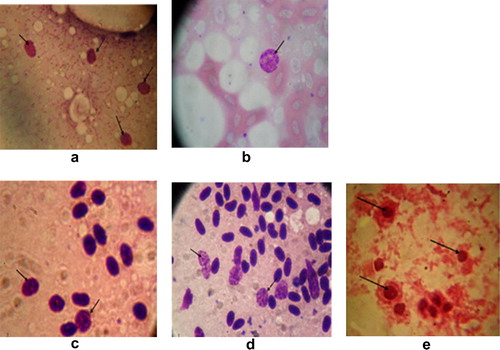
Table 1 Direct detection of chlamydial inclusion bodies within the examined samples.
Using Giménez staining, chlamydial inclusions appeared in the collected yolk sac membranes as small, rounded red dots (). The infected eggs embryos appeared dwarfed with presence of hemorrhagic spots in the head and toes ().
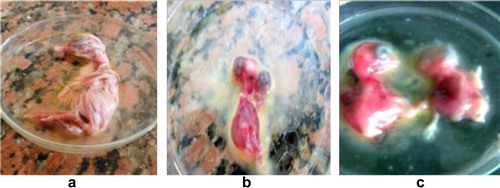
Obviously, out of 89 Cattle Egret samples (80.9%) and 63 out of 71 Hoopoe (88.7%) impression smears from the infected yolk sac membranes were positive by Giménez stain. On the other hand, chlamydial inclusions were demonstrated in impression smears from the infected yolk sac membranes using FAT technique with percentages of 73 and 80.3 from Cattle Egret and Hoopoe respectively ( and ). The pooled and grinded organs inoculated via intra yolk sac caused pathological lesions encountered in the embryonic membranes in the form of congestion and severe engorgement of the blood vessels.
3.2 Detection of C. psittaci antibodies in the collected serum samples by complement fixation test (CFT)
The data present in indicated that, 32 out of 55 (58.2%) and 35 out of 53 (66.0%) serum samples collected from Hoopoe and Cattle Egret respectively were positive for the presence of C. psittaci antibodies using CFT.
Table 2 Detection of Chlamydia psittaci antibodies in the collected serum samples of Hoopoe and Cattle Egret by complement fixation test (CFT).
3.3 Comparative study between serodiagnosis (CFT and FAT), molecular identification (PCR) and conventional methods (Giemsa stain and Giménez stain)
The results of CFT, FAT, PCR, Giemsa stain and Giménez stain were compared among the examined 55 Hoopoe and 53 Cattle Egret birds as shown in . The expected amplified product of 16S rRNA gene specific for family Chlamydiaceae at 278 bp was detected (). It is obvious that 53 (96.4%), 45 (81.8%), 49 (89.1%), 44 (80%) and 32 (58.2%) samples were positive for chlamydiosis using PCR, Giemsa stain, Giménez stain, FAT, and CFT respectively among Hoopoe. Chlamydial occurrences among Cattle Egret birds were 48 (90.6%), 41 (77.4%), 44 (83.0%), 40 (75.5%) and 35 (66.0%) respectively for the previous tests.
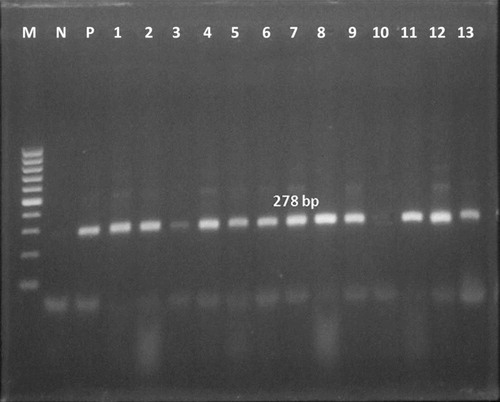
Table 3 Comparison of the percentages of positives yielded by different diagnostic methods used for detection of chlamydiae in Hoopoe and Cattle Egret samples.
4 Discussion
Wild birds: parrot, budgerigar, pigeon, dove, canary, turkey, duck, pheasant, water birds, and shore birds are known as natural hosts for C. psittaci infection [Citation18]. Chlamydiosis can be transmitted to humans by asymptomatic birds or by birds that are obviously sick. In the present study asymptomatic birds from two species of wild birds (Cattle Egret and Hoopoe) were investigated for chlamydiosis. To the best of our knowledge, it is the first study in Egypt to detect chlamydiae among Cattle Egret and Hoopoe.
Using Giemsa stain 67 (75.3%), out of 89 Cattle Egret organ samples, chlamydial inclusions were demonstrated in one or more organs from the same bird. For Hoopoe, 54 (76.1%) out of 71 birds, chlamydial inclusions were demonstrated in one or more organs from the same bird. Clinical chlamydiosis was reported in Common Kestrels (F. tinnunculus) and Lesser Kestrels (Falco naumanni) sampled from three different geographical locations in Spain [Citation19]. The pet birds could be major factors through their close contact with families in spreading chlamydiosis, which is a zoonotic infection [Citation20].
Culture of chlamydiae is difficult and infrequent because of the obligate intracellular nature of the bacteria and the hazard exposed to researchers [Citation21]. Cell culture or egg inoculation, is the gold standard for diagnosis of chlamydiae. Isolation of viable chlamydiae requires infection of embryonic egg or cell culture [Citation22]. In this study positive cases were confirmed by pathological lesions encountered in the embryonic membranes of the infected embryonated chicken eggs in the form of congestion and severe engorgement of the blood vessels. Embryos appeared dwarfed with presence of hemorrhagic spots in the head and toes. Chlamydial inclusions were demonstrated in the impression smears of collected yolk sac membranes stained with Giménez stain. Out of 89 Cattle Egret organ samples, chlamydial inclusions were demonstrated in 72 (80.9%) birds. For Hoopoe, chlamydial inclusions were demonstrated in 63 (88.7%) out of 71birds. Also, chlamydial inclusions were demonstrated in the impression smears of the collected yolk sac membranes using commercial reagents available for the detection of Chlamydiaceae by direct immunofluorescence test. The researchers conducted FAT after the inoculation to the L929 cell culture and found the positive ratios as 11.8% of cockatoos, 20.8% of African grey parrots, 21.8% of Amazon parrots, 17.4% of other parrots, 30.1% of parakeets, 9.8% of cockatiels, 8.6% of budgerigars, 11.8% of lovebirds, 5.1% of canaries, 25% of doves and pigeons, and 7.5% of finches [Citation20].
The World Organization for Animal Health and the Australian Standard Diagnostic Techniques for Animal Diseases [Citation23] recommend isolation procedures and/or serological tests. In previous study, using complement fixation test, the presence of C. psittaci antibodies (47.70%) in pigeon was confirmed by Pavlak et al. [Citation24]. In the present study, Out of 55 Hoopoe serum samples examined for the presence of C. psittaci antibodies, 32 (58.2%) were positive in CFT. While in Cattle Egret, Out of 53 serum samples examined for the presence of C. psittaci antibodies, 35 (66.0%) were positive in CFT. Complement fixation test is the most commonly used serological method for detection of antibodies against C. psittaci. Positive results were recorded by Osman et al. [Citation25] in 29.91% of chickens’ sera.
The 16S rRNA gene was investigated as a target DNA sequence among Chlamydiaceae [Citation26]. Most of the examined samples showed the expected amplified product specific for chlamydiae (278 bp). Out of the examined 53 yolk sac samples for Cattle Egret, chlamydial 16sRNA gene was demonstrated in 48 (90.6%) samples. For Hoopoe, chlamydial 16sRNA gene was demonstrated in 53 (96.4%) samples out of the examined 55 yolk sac samples. C. psittaci DNA was detected in 58% of the common Kestrels and in 47% of the Lesser Kestrels sample [Citation27].
The results of CFT, FAT, PCR, Giemsa stain and Giménez stain were compared among the examined 55 Hoopoe and 53 Cattle Egret birds. It is clear that 53 (96.4%), 45 (81.8%), 49 (89.1%), 44 (80%) and 32 (58.2%) samples were positive for chlamydiosis using PCR, Giemsa stain, Giménez stain, FAT, and CFT respectively among Hoopoe. Among Cattle Egret birds the occurrence were 48 (90.6%), 41 (77.4%), 44 (83.0%), 40 (75.5%) and 35 (66.0%) respectively for the previous tests. Previous research indicated that the Giménez stain is more practical than others [Citation28]. While FAT reported by Vanrompay et al. [Citation29] to be more sensitive than Giménez stain in diagnosing chlamydiosis. The sensitivity and the specificity between FAT and Giménez stain were 59% and 94%, respectively [Citation20]. Polymerase chain reaction (PCR) would constitute an ideal alternative for the detection of Chlamydiaceae species because it offers advantages in terms of sensitivity and reduces the processing time compared to conventional serological techniques [Citation27]. The existence of vaginal Chlamydia infection in symptomatic gynecologically diseased women in Egypt was analyzed by PCR, Chlamydia trachomatis (15.2%), C. psittaci (50.0%), and Chlamydia abortus (35.7%) were identified [Citation30].
The results suggest that Cattle Egret and Hoopoe are an ideal reservoir of chlamydiae species and thus shed the organisms in their excreta. Thus, the shedding of chlamydiae by wild birds throughout the Egyptian habitat may trigger another zoonotic potential to humans existing at their vicinity.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- H.H.AbulreeshR.GoulderG.W.ScottWild birds and human pathogens in the context of ringing and migrationRing Migrat232007193200
- C.J.BrandChlamydial infections in free living birdsJ Am Vet Med Assoc195198915311535
- Centers for Disease Control and PreventionCase definitions for infectious conditions under public health surveillanceMMWR46 (RR-10)199727
- K.SachseS.KuehlewindA.RuettgerE.SchubertG.RohdeMore than classical Chlamydia psittaci in urban pigeonsVet Microbiol1573–42012476480
- D.SclossbergChlamydia psittaci (psittacosis)G.MandelPrinciples and Practice of Infectious Diseases4th ed.1995Churchill LivingstoneNew York16931696
- Centers for Disease Control and PreventionSummaries of notifiable diseasesUnited States MMWR445319957477
- D.ZweifelR.HoopK.SachseA.PospischilN.BorelPrevalence of Chlamydophila psittaci in wild birds—potential risk for domestic poultry, pet birds, and public health?Eur J Wildlife Res5562009575581
- D.W.BusbyW.HouseJ.R.MacdonaldVirology Techniques1964London J and AC Girchill, Ltd.
- A.A.AndersenJ.P.TappeGenetic, immunologic, and pathologic characterization of avian chlamydial strainsJ Am Vet Med Assoc19511198915121516
- B.J.BevanC.D.BracewellChlamydiosis in birds in Great Britain. Isolation of Chlamydia psittaci from birds sampled between 1976 and 1984J Hyg961986453458
- D.F.GiménezStaining Rickettsiae in yolk sac cultureStain Technol391964135140
- J.LecomteImmunofluorescenceP.PaymentM.TrudelMethods and Techniques in Virologyvol. 131993Marcel DekkerNew York, NY125131
- H.L.EdwinJ.S.NathalieDiagnostic Procedure for Viral, Rickettsial and Chlamydial Infection5th ed.1979American Public Health AssociationWashington
- I.D.AitkenD.LongbottomEnzootic abortion of ewes (ovine chlamydiosis)OIE Biological Standards CommissionManual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammels, Birds and Bees)2004Office International des EpizootiesParis635641
- M.McClenaghanA.J.HeringI.D.AitkenComparison of Chlamydia psittaci isolates by DNA restriction endonuclease analysisInfect Immun4521984384389
- N.BorelE.KempfH.HotzelE.SchubertP.TorgersonP.SlickersR.EhrichtT.TasaraA.PospischilK.SachseDirect identification of chlamydiae from clinical samples using a DNA microarray assay—a validation studyMol Cell Probes2220085564
- K.D.EverettR.M.BushA.A.AndersenEmended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organismsInt J Syst Bacteriol4921999415440
- P.BougiouklisN.PapaioannouI.GeorgopoulouP.IordanidisI.VlemmasS.LekkasV.SiarkouChlamydia-induced bilateral ectropion of the inferior eyelids in pigeonsAvian Dis442000372378
- J.A.LemusJ.A.FargalloP.VergaraD.ParejoE.BandaNatural cross chlamydial infection between livestock and free-living bird speciesPLos One510201018
- B.S.ÇelebiS.AKA comparative study of detecting Chlamydophila psittaci in pet birds using isolation in embryonated egg and polymerase chain reactionAvian Dis5042006489493
- T.MessmerT.N.TullyB.W.RitchieJ.F.MoroneyA tale of discrimination: differentiation of Chlamydiaceae by polymerase chain reactionSemin Avian Exot Pet Med9120003642
- K.CondonJ.OakeyDetection of Chlamydiaceae DNA in veterinary specimens using a family-specific PCRLett Appl Microbiol4522007121127
- P.TimmsChlamydiosis in birds, wild and domestic animals: pathology, serology, microbiology, DNA and antigen detectionL.A.CornerT.J.BagustAustralian Standard Diagnostic Techniques for Animal Diseases1993CSIRO for the Standing Committee on Agriculture and Resource ManagementEast Melbourne
- M.PavlakK.VlahovićJ.GregurićŽ.ŽupaničićJ.JerčićJ.BožikovAn epidemiologic study of Chlamydia sp. in feral pigeons (Columba liviavar.domestica)Z Jagdwiss46220008495
- W.A.OsmanA.L.El-NaggarA.S.A.GoodaM.A.MahmoudDetection of Chlamydophila psittaci in chickens by complement fixation test and polymerase chain reactionBs Vet Med J17120073538
- K.D.EverettA.A.AndersenIdentification of nine species of Chlamydiaceae using PCR-RFLPInt J Syst Bacteriol4921999803813
- N.OrtegaD.ApazaF.GonzalezJ.SalinasM.R.CaroOccurrence of Chlamydiaceae in non-symptomatic free-living raptors in SpainEur J Wildlife Res5812012351355
- F.ArizmendiJ.E.GrimesComparison of the Giménez staining method and antigen detection ELISA with culture for detecting chlamydiae in birdsJ Vet Diagn Invest71995400401
- D.VanrompayR.DucatelleF.HaesebrouckDiagnosis of avian chlamydiosis: specificity of the modified Giménez staining on smears and comparison of the sensitivity of isolation in eggs and three different cell culturesZentralbl Vet Med B3921992105112
- K.M.OsmanH.A.AliJ.A.EljakeeM.M.GaafarH.M.GalalAntimicrobial susceptibility and molecular typing of multiple Chlamydiaceae species isolated from genital infection of women in EgyptMicrob Drug Resist1842012440445