Abstract
This study was performed for evaluation of the effect of pre-operative and post-operative administration of tramadol and meloxicam combination on hyperalgesia and selected inflammatory responses after sciatic nerve ligation in rats. An eighty male Wistar rats (Rattus norvegicus) were divided into two groups; preemptive analgesic group, in which the rats received analgesic agents 30 min before nerve ligation. Also the nociceptive pain tests were detected before surgery, 120 and 150 min post-analgesics injection. The serum IL-6 and PGE-2 concentrations were detected at 150 min post-analgesics injection. The second group was post-operative analgesic group; in which the rats exposed for the nerve ligation then the rats received analgesic agents at 5 till 11 day’s post-operative. Behavioral tests were performed before surgery and on each even days post-operatively. The serum concentration of IL-6 and PGE-2 was determined at 5, 10 and 14 days post-operative. Tramadol and meloxicam combination had a statistically significant (P < 0.05) reverse hyperalgesia while preemptive administration of tramadol and meloxicam significantly decreased (P < 0.05) serum IL-6 production compared to post-operative treatment. In conclusion, the preemptive combination of tramadol and meloxicam produced a potent analgesic effect post nerve ligation in rats.
1 Introduction
Nerve injury often results in development of hyperalgesia characterized by spontaneous pain, increased responsiveness to painful stimuli and allodynia which is a pain perceived in response to normally non-noxious stimuli [Citation1,Citation2]. Chronic neuropathic pain is a physically and emotionally debilitating condition for which there is no adequate treatment to prevent the development predictably and specifically controls established neuropathic pain [Citation3]. Insults to the central and peripheral nervous system can range from traumatic injury to chemical insult and to immunologic challenge [Citation3,Citation4].
Pains evoked from tissue destruction as well as during wound healing, inflammatory reaction are elicited this leads to activation of nociceptors (pain receptors) which can cross-communicate with the inflammatory infiltrate [Citation2,Citation3]. Among cytokines involved in pathological states, an important role is assigned to interleukin-6 (IL-6); an inflammatory cytokine, involved in the physiology of nociception and the pathophysiology of pain [Citation5,Citation6]. Prostaglandin E2 (PGE-2) is a key mediator in the processes of peripheral and spinal sensitization [Citation7,Citation8].
Cytokines activation take place following a neuroma formation after axotomy and increases neuronal activity and hyperalgesia when administered spinally [Citation9,Citation10]. Research using rat models has mainly focused on injury to a peripheral nerve, usually the sciatic or spinal nerve, to consistently produce behaviors suggestive to neuropathic pain in humans [Citation11–Citation13].
The choices of a drug modulating the immune responses are beneficial in the peri-operative period [Citation14]. It has been demonstrated that tramadol can contribute to beneficial effects on immune functions in patients, namely, induce an improvement of post-operative immunosuppression [Citation15].
Meloxicam is a novel non-steroidal anti-inflammatory drug (NSAID) and cyclooxygenase COX-2 inhibitor. It has been shown to have a potent anti-inflammatory effect in rats at doses that only weakly affect prostaglandin synthesis in the stomach and kidneys [Citation16]. Tramadol is a synthetic, centrally acting, analgesic agent with opioid and non-opioid like properties [Citation18]. Its analgesic effect in experimental pain models in rats was shown in thermal hyperalgesia [Citation19]. It could be an effective and dose-dependent control for heat hyperalgesia in chronic nerve constriction injury in rats [Citation20].
The combination of tramadol and meloxicam has the potential to overcome tolerance, efficacy and time-to-onset limitations of the component drugs and increase their analgesic effect synergistically [Citation14]. Thus the aim of the current study was to evaluate the effect of pre- and post-treatment with tramadol and meloxicam combination on pain anti-nociceptive test (thermal pain tests) and its effect on serum level of IL-6 and PGE-2 in rats undergoing sciatic nerve constriction injury.
2 Materials and methods
2.1 Animals and housing
Eighty apparently healthy male Wistar rats (Rattus norvegicus) (aged 2–3 years; average body weight, 250–300 g) were used. Rats were housed under standard laboratory conditions (temperature: 23 ± 2 °C, with a 12-h/12-h light/dark cycle). They had free access to standard laboratory feed and water up to the time of the experiment. All experiments were conducted in accordance with the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals [Citation21]. In addition, the protocol was approved by the Mansoura Medical Research Ethics Committee (MMREC).
Tramadol hydrochloride (15 mg/kg; Tramal 5%, Minpharm, Grünenthal, Germany; T15), meloxicam, (6 mg/kg; Anti-CoxII 0.5%, Adwia, Cairo, Egypt; M6), medetomidine hydrochloride (0.4 mg/kg; Domitor 0.1%, Pfizer, Seixal, Portugal) and ketamine hydrochloride (60 mg/kg; Ketamax 5%, Troikaa, Gujarat, India).
2.2 Experimental procedure and treatments
Rats had randomly assigned into two main groups; preemptive group: which include preemptive T15, M6, T15 + M6 or saline (5 rats for each treatment) and post-operative group: which include post-operative T15, M6, T15 + M6 or saline (15 rats for each treatment).
The rats were received 1 ml saline IM in control group or T15, M6 or combination between each other at 30 min before injection of anesthetics in preemptive groups while in post-operative groups they received the same drugs from the 5th till 11th days post-operative.
In the preemptive analgesic treatment, thermal pain tests were assessed before surgery (base line) and 120 and 150 min post-injection of analgesics, whereas in the post-operative analgesic groups, thermal pain tests were assessed at base line then at 2, 4, 6, 8, 10, 12 and 14 days post-operatively.
2.3 Neuropathic pain (sciatic nerve ligation)
The model of sciatic nerve injury was performed at the mid-thigh level of the left hind leg [Citation22]. Briefly, animals were anesthetized with combination of medetomidine hydrochloride and ketamine hydrochloride intra-peritoneum and the surgery were aseptically performed. One sterile chromic gut suture (4-0 chromic gut, Ethicon, USA) was loosely ligated around the gently isolated sciatic nerve proximal to its trifurcation. The muscles overlying the nerve were sutured with chromic gut ligature (3-0) (Sharpoint®, USA).
2.4 Thermal pain latency tests [Citation12]
2.4.1 Tail withdrawal latency test (TWL)
The water was maintained at 50 °C in a constant-temperature water bath. Rats were wrapped in a breathable cloth cone, and the distal third of the rat’s tail was immersed in the bath. The time required for the rat to remove its tail was measured by use of a stopwatch, and the tail-flick latency score was calculated as the mean of the last 2 of 3 trials. Trials were separated by 30 s intervals. Between trials, the rat’s tail was dried with a tissue (one swipe beginning at the mid-tail region) and was terminated at 30 s if no withdrawal response occurred.
2.4.2 Hot plate test
All animals were placed on a hot plate apparatus maintained at a temperature of 50 °C. The anti-nociceptive effect was quantified by measuring latency times for kicking, licking of hind paw or jumping. Each rat had just been exposed to the hot plate before analgesic drug injection and after 30 and 60 min. The time required for the rat was measured by use of a stopwatch, and the latency times were calculated as the mean of the last 2 of 3 trials. A cut off time of 15 s was considered if no response.
2.5 Sample collection and analysis
Rats were quickly decapitated to collect trunk blood for assessment of serum IL-6 and PGE-2 level. Blood samples were centrifuged at 3000 rpm for 10 min, and the separated sera were stored at −80 °C until being used in the assay. Serum PGE-2 was detected by using Rat PGE-2 commercial ELISA kits (Pierce, USA) and serum interleukin-6 by using Rat IL-6 ELISA kit (Pierce, USA) at 2 h post-operatively in preemptive group and at 5, 10 and 14 days in post-operative group.
2.6 Statistical analysis
Data were analyzed using one-way ANOVA showed significant differences among groups. Analysis was performed with the software SPSS version 16.0 (SPSS Inc, USA). To determine which groups are different, the data were analyzed by Dunnett’s test was used to compare the experimental groups versus respective saline treatment using Statistical program. The data in thermal pain tests after surgical experiment were expressed as mean of TWL (second) ± stander deviation. While in biochemical analysis data were presented as mean of production of IL-6 (pg/ml), PGE-2 (pg/ml) ± stander deviation.
3 Results
Prior to nerve ligation, thermal withdrawal latency showed no significant difference in all rats in both acute preemptive and chronic post-operative treatments.
3.1 Acute preemptive group
In this group, rats treated with normal saline exhibited little increase of TWL after 120 and 150 min post-treatment compared to base line while T15 mg/kg induced a significant decrease (P < 0.05) heat hyperalgesia in thermal pain tests post analgesic injection compared to M 6 mg/kg and saline one. The administration of M6 mg/kg showed a mild significant increase of WLT post analgesic injection compared to saline. On the other hand, the rats which were treated with T15 + M6 showed statistical significance decreased (P < 0.05) of thermal hyperalgesia and increased of withdrawal latency time ().
Table 1 Mean values ± standard error of withdrawal latency (s) of anti-nociceptive tests (tail withdrawal latency test and hot plate test) baseline and after 120 and 150 min from preemptive acute analgesic groups in albino rats.
An acute preemptive anti-nociceptive effect of (tramadol 15 mg/kg plus meloxicam 6 mg/kg) in sciatic nerve injury was accompanied by a smooth anesthetic induction, short induction of anesthesia and prolong recovery period.
3.2 Chronic post-operative group
In this group, thermal hyperalgesia was detected 2 days post-operative and noticed 4 days post-operative as well as decreased withdrawal latency time of thermal tests compared to base line ().
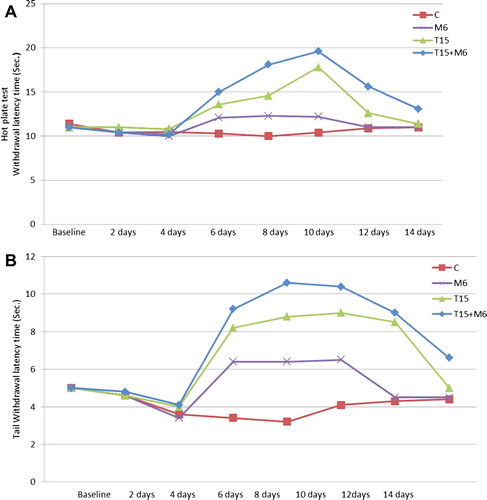
In control group thermal hyperalgesia appeared at day 4 post-operative and reached to maximum value at 8 day following the surgery and then fluctuated in the second week after operation.
Chronic post-operative intramuscular injection of analgesic drugs (T15 mg/kg, M6 mg/kg or T15 mg/kg + M6 mg/kg) showed significant decrease (P < 0.05) of thermal hyperalgesia post-injection. This was indicated by increase of TWL compared to saline ().
Chronic post-operative administration of T15 mg/kg post-injection induced a significant reduction (P < 0.05) of thermal hyperalgesia when compared to the saline one at 6, 8, 10 and 12 days post-operative. The highest TWL index in T15 mg/kg treatment was reached at day 10 post-operative and was significantly different (P < 0.05) from the pre-operative baseline value ().
Post-operative chronic injection of M6 mg/kg into operated rats produced a partial relief of thermal hyperalgesia compared to saline injected operated rats at day 6, 8, and 10 post-operative. Whereas, at day 12 and 14 post-operative, thermal hyperalgesia was rebound and showed no difference between saline-treated rats and rats treated with a meloxicam.
The combination of 15 mg/kg dose of tramadol and 6 mg/kg of meloxicam, in chronic post-operative injection, was completely reverse neurologic inflammatory thermal hyperalgesia, and produced a significantly higher (P < 0.05) thermal withdrawal latency compared to control group. At 12 and 14 days post-operative, the withdrawal latency time had significant differences (P < 0.05) than saline injected rats.
3.3 Biochemical analysis
3.3.1 In acute preemptive group
Serum IL-6 levels were significantly increased (P < 0.05) at 2 h after nerve injury in saline one (). T15 mg/kg + M 6 mg/kg had a minimal significant decrease (P < 0.05) of the serum IL-6 after 2 h post nerve injury compared to other treatments. In T15 mg/kg treatment, the IL-6 showed significant decrease (P < 0.05) compared to M6 mg/kg and saline one ().
Table 2 Mean values ± standard error of serum IL-6 (pg/ml) and PGE-2 (pg/ml) level 2 h post-operation in preemptive acute analgesics group.
Serum PGE-2 level remained unchanged in rats which received saline but in rats received T15 mg/kg showed a little significant decrease (P < 0.05) of PGE-2 than saline treatment. In M6 mg/kg treatment, it was reduced significantly (P < 0.05) than saline and T15 mg/kg. On the other hand, T15 mg/kg + M6 mg/kg treatment had a maximum reduction of serum PGE-2 than saline, T15 mg/kg or M6 mg/kg alone after 2 h post nerve injury ().
3.3.2 In chronic post-operative group
On 5th day post-operation, the serum IL-6 and PGE-2 post-operation had no significant difference between different treatments. Whereas on 10 and 14 days post-operation, the serum IL-6 significant increase (P < 0.05) in both treated rats and control group. Further, in post-operative treatment with M6 mg/kg plus T15 mg/kg, there was a maximum significant decrease (P < 0.05) of serum PGE-2 compared to using of M6 mg/kg or T15 mg/kg alone. In M6 mg/kg or T15 mg/kg, there was significant decrease (P < 0.05) of serum PGE-2 compared to control ().
Table 3 Mean values ± standard error of serum IL-6 (pg/ml) and PGE-2 (pg/ml) level 5, 10 and 14 days post-operative in chronic groups.
4 Discussion
In the current study, the combination of tramadol and meloxicam produced a potent anti-nociception effect in the neuropathic pain model in rats. Moreover, our data indicated that, there is a functional synergistic interaction between tramadol and meloxicam.
Evidences suggest that nerve injury and immune responses in injured nerves contribute to the initiation and maintenance of neuropathic pain that is manifested by hyperalgesia as well as increase of IL-6 and PGE-2 as pro-inflammatory cytokines [Citation23].
Although a mechanism of neuropathic pain are not well understood and considered difficult to manage. Neuropathic pain is a form of chronic pain that arises from functional changes in the pain sensory system after peripheral nerve injury that is manifested by hyperalgesia and allodynia [Citation24,Citation25]. Thus, in the current study, the using of neuropathic pain rat model is a major significant to understanding of the underlying mechanism, developing regimen of analgesics and treatment.
Experimental peripheral neuropathy was induced by loose ligation of rat’s sciatic nerve with chromic gut suture that was enough to constrict the nerve and retard epineural blood flow and result in hyperalgesia. The chromic cat gut has an inflammatory response, which lead to the nociceptive hypersensitivity associated with the neuropathic pain model and elicits an inflammatory response after the tensile strength is lost that result in increasing activity on the neutral and hot plate [Citation29]. In this study using of one ligature around the sciatic nerve with chromic cat gut was sufficient to produce hyperalgesia.
Meloxicam acts through inhibition of PGE-2 synthesis and its effects were demonstrated in supraspinal analgesia models [Citation26], while tramadol produces its analgesic effect through inhibition of noradrenaline reuptake [Citation27]. Similarly, in the present study the synergistic interaction between tramadol and meloxicam in rat after sciatic nerve ligation lead to attenuation of hyperalgesia. This could be explained by the fact that, tramadol increased the ability of meloxicam to inhibit of PGE-2 synthesis via the blockage of cyclooxygenase and the prevention of cytokines induced hyperalgesia as well as meloxicam decrease of tramadol doses which is called “an opioid-sparing effect” [Citation28].
In the acute preemptive groups; hyperalgesia to thermal stimulations were achieved at maximum severity 2 h after surgery [Citation29]. So the selected time point of 2 h post-operatively was adequate for the assessment of analgesic drug properties on neuropathic pain.
In this study, the preemptive saline-treated rats which have received medetomidine and ketamine as anesthetic combination showed attenuation of the thermal hyperalgesia within 120–150 min. Medetomidine induced analgesic effect by decreasing the release of norepinephrine [Citation29] while ketamine induced analgesia by norketamine (a major metabolite of ketamine) through binding to the noncompetitive site at the N-methyl d-aspartate (NMDA)-receptor complex and blocking NMDA-mediated central sensitization [Citation30].
Meloxicam is ineffective in enhancing thermal nociceptive thresholds while it is effective in preventing the development of the thermal hyperalgesia as NSAIDs which is able to reduce the thermal hyperalgesia related to facilitation of spinal neuron activity [Citation26]. Moreover, tramadol at dose of 15 mg/kg significantly attenuated the thermal hyperalgesia resulted from their peripheral and central effects [Citation27].
In preemptive tramadol and meloxicam group; the withdrawal latency time had a maximum significant reduction compared to control. This might be attributed to the synergism between tramadol and meloxicam resulting in increased drugs activity.
On the other hand, the chronic sciatic nerve ligation in rats lead to exaggerated persistent pain-related behavior, demonstrated by increased responsiveness to a noxious heat stimulus “hyperalgesia”, that was increased 4 days post-operative and achieved the maximum severity 8 days after surgery. These result lead to an assumption that, IL-6 is one of most common cytokines that induces the hyperalgesia [Citation31,Citation32]. Therefore, analgesics were injected after confirming the development of thermal hyperalgesia from 5 till 11 days post-operatively to mimic a clinical scenario in which analgesic administration commences only after the manifestation of the symptoms and signs of painful neuropathic disorders [Citation33]. Also, hyperalgesia appeared 7 days post-operative, which might be due to different of number of chromic cat gut ligation around sciatic nerve [Citation20].
Tail withdrawal latency time was affected after sciatic nerve ligation in rats, which could be due to development of secondary hyperalgesia to non-injured areas of the body distal to the injury due to central sensitization (secondary hyperalgesia). This is because it is not directly associated with the primary injury site [Citation33].
Thermal hyperalgesia in an animal model after chronic post-operative neuropathy had a significant decrease after chronic systemic administration of tramadol/meloxicam combination at injection days. A micro-opioid agonist therapy suppresses thermal hyperalgesia and modulates the nociceptive input C-fibers responsible for thermal hyperalgesia [Citation34], while at 12 and 14 days post-surgery thermal hyperalgesia relieves, which may lead to drug withdrawal syndrome of tramadol [Citation35].
In the current study, the detection of PGE-2 and IL-6 as a pro-inflammatory cytokines which are an early sensitive biomarker for tissue damage, healing process modulation. Both pro-inflammatory cytokines had a role in recognition of central nervous system inflammation and immune responses, which are often associated with persistent pain states [Citation36–Citation38]. Consequently; the selection of 2 h for detection of IL-6 and PGE-2 in preemptive treatment was based on the fact that saline treatment rats feel thermal stimulations at its maximal severity during that time.
The preemptive tramadol/meloxicam combination treatment resulted in attenuation of serum IL-6, which could be explained by the fact that tramadol stimulate the serotonergic descending inhibitory system resulting in decreased stimulation of lymphocyte proliferation and decrease of IL-6 production [Citation14]. In addition, meloxicam had decreased the inflammatory cells production such as immune cells, fibroblasts, endothelial cells, and neurons which are responsible for IL-6 secretion after surgical incision [Citation14].
Unlike serum IL-6, serum PGE-2 was significantly reduced by the preemptive or post-operative using of tramadol or meloxicam alone. The action of NSAIDs meloxicam is based on the inhibition of cyclooxygenase which converts arachadonic acid into prostaglandins including PGE-2 [Citation27], prostacyclin, thromboxane and tramadol, which had inhibited noradrenalin reuptake [Citation27]. Thus, when rats treated with preemptive or post-operative tramadol/meloxicam combination, it had a maximum significant reduction of PGE-2 than other treatment [Citation28,Citation39] due to the synergetic interactions between tramadol and meloxicam.
There was no apparent detectable effect for the systemic administration of COX inhibitors in animal models for neuropathic pain. However, a pronounced effect has been noticed with their local administration into the injured nerve itself or into the hyperalgesic hind paw [Citation8]. The present study proved that the prolonged administration of meloxicam at 6, 8, 10 days post neuropathic pain, have significantly increased the withdrawal latency time. Consequently, pre- or post-operative injection of systemic NSAIDs markedly reduced PGE-2 levels, which resulted in a moderate attenuation of thermal hyperalgesia [Citation17].
In conclusion, the preemptive combination between tramadol and meloxicam could be a good choice to overcome the neuropathic pain by decreasing serum IL-6 level and preventing PGE-2 release as well as by attenuation of hyperalgesia following sciatic nerve injury.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- N.I.CherneyH.T.ThalerH.Friedlander-KlarJ.LapinK.M.FoleyR.HoudeR.K.PortenoyOpioid responsiveness of cancer pain syndrome caused by neuropathic or nociceptive mechanism: a combined analysis of controlled, single-dose studiesNeurology441994857861
- M.ZimmermannPathobiology of painEur J Pharmacol42920012327
- L.R.WatkinsS.F.MaierL.E.GoehlerImmune activation: the role of proinflammatory cytokines in inflammation, illness responses and pathological pain statesPain631995289302
- S.M.SweitzerR.W.ColburnM.D.RutkowskiJ.A.DeleoAcute peripheral inflammation induces moderate glial activation and spinal IL-1β expression that correlates with pain behavior in the ratBrain Res8291999209221
- S.LacroixL.ChangS.Rose-JohnM.H.TuzynskiDelivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growthJ Comp Neurol4542002213228
- R.F.DejonghK.C.VissersT.F.MeertL.H.D.BooijC.S.DeDeyneR.J.HeylenThe role of interleukin-6 in nociception and painAnesth Analg96200310961103
- H.G.SchaibleR.F.SchmidtExcitation and sensitization of fine articular afferents from cat’s knee joint by prostaglandin E2J Physiol403198891104
- T.L.YakshD.M.DirigC.M.ConwayC.SvenssonD.Z.LuoP.C.IsaksonThe acute antihyperalgesic action of nonsteriodal, anti-inflammatory drugs and release of spinal prostaglandin E2 is mediated by the inhibition of constitutive spinal cyclooxygenase-2 [COX-2], but not COX-1J Neurosci21200158475853
- T.MinamiR.UdaS.HoriguchiS.ItoM.HyodoO.HayaishiAllodynia evoked by intrathecal administration of prostaglandin E2 to conscious micePain571994217223
- S.AhmadiS.LipprossW.L.NeuhuberH.U.ZeilhoferPGE2 selectively blocks inhibitory glycinergic neurotransmission onto rat superficial dorsal horn neuronsNat Neurosci520023440
- G.J.BennettY.K.A.XiePeripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in menPain33198887107
- Z.SeltzerR.DubnerY.A.ShirNovel behavioral model of neuropathic pain disorders in rats by partial sciatic nerve injuryPain431990205218
- S.H.KimJ.M.ChungAn experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the ratPain501992355363
- Y.LiuS.ZhuK.WangZ.FengQ.ChenEffect of tramadol on immune responses and nociceptive thresholds in a rat model of incisional painJ Zhejiang Univ Sci9112008895902
- P.SacerdoteM.BianchiL.GaspaniB.ManfrediA.MaucioneG.TernoM.AmmatunaA.E.PaneraiThe effects of tramadol and morphine on immune responses and pain after surgery in cancer patientsAnesth Analg906200014111414
- G.EngelhardtD.HommaK.SchlegelR.UtzmannC.SchnitzlerAnti-inflammatory, analgesic, antipyretic and related properties of meloxicam, a new non-steroidal anti-inflammatory agent with favorable gastrointestinal toleranceInflamm Res441995423433
- G.EngelhardtPharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improve safety profile through prefrentail inhibition of COX-2Br J Rheumatol3511996412
- J.D.ClarkX.ShiX.LiY.QiaoD.LiangM.S.AngstD.C.YeomansMorphine reduces local cytokine expression and neutrophil infiltration after incisionMol Pain31200728
- M.BianchiA.E.PaneraiAntihyperalgeic effects of tramadol in the ratBrain Res7971998163166
- Y.C.TsaiP.J.ChangI.M.JouDirect tramadol application on sciatic nerve inhibits spinal somatosensory evoked potentials in ratsAnesth Analg92200115471551
- M.ZimmermannEthical guidelines for investigations of experimental pain in conscious animalsPain1983161983109110
- E.D.MilliganV.ZapataM.ChacurD.SchoenigerJ.BiedenkappK.A.O’ConnorEvidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in ratsEur J Neurosci20200422942302
- W.MaR.QuirionUp-regulation of interleukin-6 induced by prostaglandin E2 from invading macrophages following nerve injury: an in vivo and in vitro studyJ Neurochem932005664673
- T.S.JensenH.GottrupS.H.SindrupF.W.BachThe clinical picture of neuropathic painEur J Pharmacol4291-32001111
- D.E.MoulinA.J.ClarkA.PanjuG.B.RollmanA.VellyPharmacological management of chronic neuropathic pain: consensus statement and guidelines from the Canadian Pain SocietyPain Res Manage1220071321
- H.VanegasH.G.SchaibleProstaglandins and cyclooxygenases correction of cyclooxygenases in the spinal cordProg Neurobiol642001327363
- B.DriessenW.ReimannInteraction of the central analgesic, tramadol, with the uptake and release of 5-hydroxytryptamine in the rat brain in vitroBr J Pharmacol1051992147151
- M.A.Isiordia-EspinozaF.Teran-RosalesG.Reyes-GarcıaV.Granados-SotoSynergism between tramadol and meloxicam in the formalin test involves both opioidergic and serotonergic pathwaysDrug Dev Res7320114350
- H.S.JangH.S.ChoiS.H.LeeEvaluation of the anaesthetic effects of medetomidine and ketamine in rats and their reversal with atipamezoleVet Anaesth Analg362009319327
- J.R.HoltmanP.A.CrooksJ.K.Johnson-HardyM.HojomatM.KlevenE.P.WalaEffects of norketamine enantiomers in rodent models of persistent painPharmacol Biochem Behav902008676685
- J.A.DeleoR.W.ColburnA.NicholsInterleukin-6 mediated hyperalgesia/allodynia and increased spinal IL-6 expression in a rat mononeuropathy modelJ Interferon Cytokine Res161996695700
- M.S.SandraW.L.JeungL.O.AnneNeedlestick distal nerve injury in rats models symptoms of complex regional pain syndromeInt Anesth Res Soc105200718201829
- Y.C.TsaiY.H.SungP.J.ChangF.C.KangK.S.ChuTramadol relieves thermal hyperalgesia in rats with chronic constriction injury of the sciatic nerveFundam Clin Pharmacol142000335340
- K.E.JosephD.J.LevineMu and delta opioid receptors on nociceptors attenuate mechanical hyperalgesia in ratNeuroscience17112010344350
- C.E.SenayH.E.AdamsA.GellerJ.A.InciardiA.MunozS.H.SchnollE.G.WoodyJ.T.CiceroPhysical dependence on Ultram† [tramadol hydrochloride]: both opioid-like and atypical withdrawal symptoms occurJ Drug Alcohol Depend692003233241
- J.A.DeleoR.P.YezierskiThe role of neuroimmune activation in persistent painJ Pain90200116
- I.NagahiroA.AndouM.AoeY.SanoN.ShimizuPulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedureAnn Thorac Surg J7222001362365
- S.WangG.LimQ.ZengB.SungJ.MaoExpression of central glucocorticoid receptors after peripheral nerve injury contributes to neuropathic pain behaviors in ratsJ Neurosci24200485958605
- M.BianchiC.MartucciP.FerrarioS.FranchiP.SacerdoteIncreased tumor necrosis factor and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: the effects of analgesic drugsInt Anesth Res Soc10442007949954
