?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Green tea (Camellia sinensis) is one of the most popular and widely consumed beverages in the world. In the current study, aqueous extract of green tea (C. sinensis) was evaluated for mast cell stabilizing and anti-anaphylactic activities. Green tea extract (11, 13, 15 mg/ml) significantly (P < 0.05) inhibited compound 48/80-induced rat mesentric mast cell degranulation in a dose dependent manner. Anti-anaphylactic activity of green tea extract was performed in female mice. At a dose of 400, 500, 600 mg/kg BW, green tea extract showed significant reduction in the mortality of mice subjected to anaphylactic shock by compound C48/80. Ketotifen was used for comparison. In addition, IR and UV–Visible spectroscopy analysis of green tea extract revealed the presence of functional groups of bioactive compounds. These results suggest that green tea could be useful in the treatment of asthma and allergic rhinitis.
1 Introduction
Mast cells derive from the bone marrow and mature under the influence of local tissue microenvironmental conditions [Citation1]. Mast cells are important mediators of inflammatory responses, such as allergy and anaphylaxis. The activated mast cells secrete numerous vasoactive and proinflammatory mediators. These include pre-formed molecules such as histamine, serotonin, TNF, kinins and proteases stored in secretory granules. The scientific evidence indicates that mast cells are critical for the pathogenesis of inflammatory diseases, such as arthritis, atopic dermatitis, psoriasis, and multiple sclerosis [Citation1].
Plants are well known for their health benefiting activities since antiquity. According to World health organization (WHO) about 80% of the World’s population mainly depends on traditional medicine. Medicinal plants constitute the major component of the traditional medicine due to economical viability, accessibility and ancestral experience. Therefore, the search for safe and more effective agents from plant origin has continued to be an important area of active research [Citation2]. Green tea is one of the most popular beverages in the world and is produced from the leaves of the plant Camellia sinensis. Recently, green tea has attracted great attention of scientists and common public because of its health promoting effects. Several biological activities have been reported for the green tea such as antioxidant [Citation3], antifibrotic [Citation3], immunomodulatory [Citation4], antimicrobial activity [Citation5] and anticancer properties [Citation6]. The major bioactive compounds present in the green tea are polyphenols. These are mainly comprised of catechins and catechin derivatives, including (−)-epigallocatechin-3-gallate (EGCG), (−)-epicatechin (EC), (−)-epigallocatechin (EGC), (−)-epicatechin gallate (ECG) and (−)-gallocatechin gallate (GCG) [Citation7]. Though several biological activities have been reported for green tea, the studies pertaining to mast cell stabilization and anti-anaphylactic are scanty; hence we have taken up this investigation to study the mast cell stabilization and anti-anaphylactic activity of green tea extract.
2 Materials and methods
2.1 Plant material
Green tea powder was purchased from a local market and it was identified and authenticated by Botanist, Prof. T. Sreedhar murthy, Department of Botany, Govt. College for men, kadapa.
2.2 Preparation of green tea extract
Hot water extract of green tea was prepared by soaking tea powder (10 g) in 100 ml of boiling distilled water for 5 min. The extract was filtered and filtrate was dried at room temperature (27 °C). The dried extract was weighed and used for further experimental work.
2.3 Chemicals
C48/80 (condensation product of N-methyl-P-methoxy Phenethylamine with formaldehyde) was purchased from Sigma–Aldrich Co. (St. Louis, MO, USA). All other chemicals used in this work were of analytical grade.
2.4 Animals
Male albino rats (150–250 g) and female mice (20–25 g) were procured from Sri Venkateswara Enterprises, Bangalore, India. Animals were acclimatized for 10 days to our animal house and housed three animals per cage. Animals were provided with standard rodent pellet diet and maintained in a temperature of 22 ± 2 °C and humidity-controlled environment on a 12-h dark/light cycle. Animals were handled according to the rules and regulations of Institutional Animal Ethical Committee (IAEC), Sri Krishnadevaraya University, Anantapur, India.
2.5 Ultraviolet visible absorption (UV)
The aqueous extract of C. sinensis was analyzed in UV–Visible range between 190 and 400 nm using UV–Visible spectrophotometer (UV-18-1885-01-0043).
2.6 Infra-red spectroscopy (IR)
The IR spectrum of aqueous extract of C. sinensis was performed using IR Bruker Alpha-T model over the frequency range from 4000 to 400 cm−1 [Citation8].
2.7 Experimental dose
The dose (400, 500 and 600 mg/kg BW) selected for this study was based on the toxicity study of C. sinensis reported by Hsu et al. [Citation9].
2.8 In vitro mesentric mast cell degranulation
The rats were sacrificed by Co2 inhalation. The abdomen was cut open to expose the intestine and the pieces of mesentery were collected in petri dish containing Ringer Locke solution (NaCl 9.0, KCl 0.42, CaCl2 0.24, NaHCo3 0.5 and glucose 1 gm/L in double distilled water; pH 7.4) at 37 °C and then subjected to the following treatment schedules.
Petri dish no. 1 – Ringer Locke solution (vehicle control)
Petri dish no. 2 – Ringer Locke solution (positive control)
Petri dish no. 3 – Ketotifen (10 μg/ml)
Petri dish no. 4 – Aqueous extract of green tea (11 mg/ml)
Petri dish no. 5 – Aqueous extract of green tea (13 mg/ml)
Petri dish no. 6 – Aqueous extract of green tea (15 mg/ml)
Each Petri dish was incubated for 15 min at 37 °C later compound 48/80 (0.1 ml, 10 μg/ml) was added to each petri dish except for vehicle control and again incubated for 10 min at 37 °C [Citation10]. After incubation, all pieces were immersed in 4.0% formaldehyde solution containing 0.1% toluidine blue and kept a side for 15 min. After staining the pieces were transferred and kept in acetone for 10 min and then kept in xylene for 2 min and mounted on slides. All the pieces were examined under a digital light microscope (M/s Motic, Korea) at 450× magnification. Minimum 100 cells were counted and percentage of intact and disrupted mast cells was determined. Each cell was considered either disrupted or not disrupted, and percentage protection from degranulation of mast cells by the drug was calculated using the following formula [Citation11].
2.9 Anti-anaphylactic activity
Compound 48/80 induced systemic reaction was carried out according to the previous method of Chitme et al. [Citation12]. The female mice were divided into six groups each containing 10 animals. Green tea extract was administered daily for 7 days by oral gavage using three different doses (400, 500 and 600 mg/kg). The standard drug ketotifen (50 mg/kg) was administered intraperitoneally. The following schedule of treatment was administered.
Group I: distilled water (control)
Group II: C48/80 (sensitized)
Group III: standard drug (ketotifen)
Group IV: green tea extract (400 mg/kg)
Group V: green tea extract (500 mg/kg)
Group VI: green tea extract (600 mg/kg)
One hour after the last dose of drug administration (on 7th day), all the animals except group I were given an injection of 8 mg/kg i.p. of C48/80 (toxicant). Mortality was monitored for 1 h after induction of anaphylactic reaction. Mortality was calculated using the following formula.
3 Statistical analysis
All data were reported as Mean ± SD. The statistical analysis was done using one-way analysis of variance (ANOVA) followed by Duncan’s Multiple Range (DMR) test. The level of significance was evaluated at P < 0.05.
4 Results
4.1 Ultraviolet visible absorption (UV)
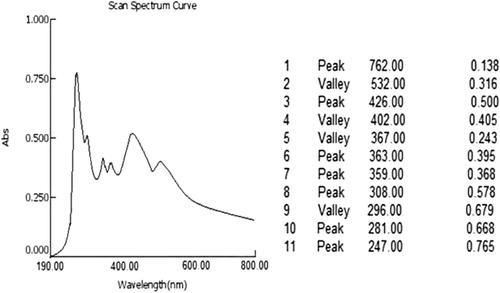
UV–Visible spectrum of aqueous extract of C. sinensis is depicted in . The UV spectrum of aqueous extract of C. sinensis showed absorption maxima at 762, 426, 363, 359, 308, 281 and 247 nm.
4.2 Infra-red spectroscopy (IR)
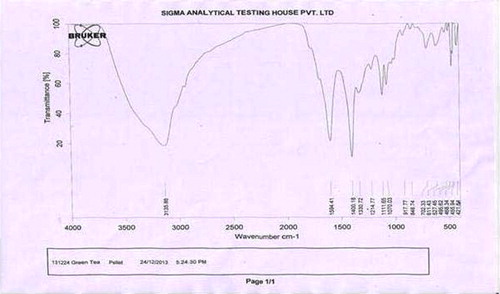
Infra-red spectrum of aqueous extract of C. sinensis is shown in . The mid infrared, approximately 4000–400 cm−1 was used to study the fundamental vibrations and related rotational vibrational spectrum. Interpretation of spectrum of C. sinensis is presented in .
Table 1 Interpretation of IR spectrum of aqueous extract of C. sinensis.
4.3 Mast cell degranulation
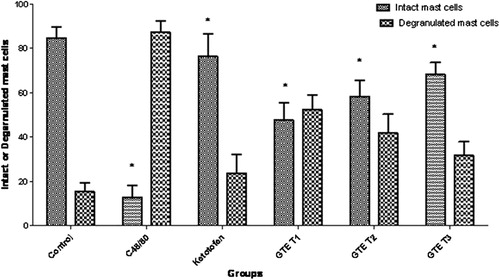
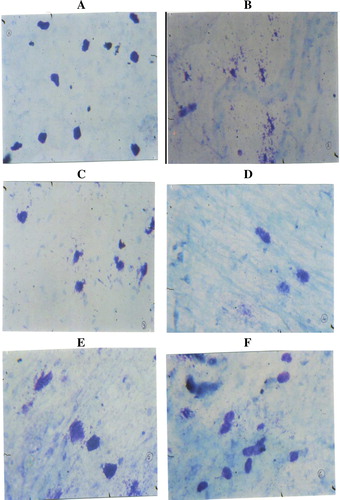
Compound 48/80 (10 μg/ml), a known mast cell degranulating agent, produced significant rat mesenteric mast cell degranulation (87.1%), as evidenced by histopathology observation (). Green tea extract (11, 13 and 15 mg/ml) produced significant reduction in the compound 48/80 induced mast cell degranulation in a dose dependent manner (–). The percentage inhibition of mast cell degranulation was found to be 47.6%, 58.2% and 68.2%, respectively for 11, 13 and 15 mg/ml of green tea extract (). Kitotifen, a known mast cell stabilizing agent, also brought significant (P < 0.05) reduction in degranulating mast cells ().
4.4 Anti-anaphylactic activity
Compound 48/80 induced 100% mortality in mice. Kitotifen, a standard drug, showed 70% protection from mortality. Treatment with green tea extract (400, 500, and 600 mg/kg) showed dose dependent protection from mortality (). The mortality was 70%, 50% and 40%, respectively for 400, 500 and 600 mg/kg green tea extract treated groups. Green tea extract 600 mg/kg showed similar reduction in mortality when compared to Kitotifen standard drug.
Table 2 Effect of aqueous extracts of C. sinensis on anaphylactic shock induced by C48/80 in mice.
5 Discussion
The qualitative UV–Vis profile of aqueous extract of C. sinensis was taken at wavelength of 190–400 nm. The profile showed the peaks at 762, 426, 363, 359, 308,281 and 247 nm with absorption of 0.138, 0.500, 0.395, 0.368, 0.578, 0.668 and 0.765, respectively. Infrared spectroscopy is one of the influential analytical techniques which recommend the possibility of chemical identification. It is an excellent method for the qualitative analysis because excluding optical isomers, the spectrum of compound is unique. It is most useful for the identification of purity and gross structural details. In the current study, IR spectrum was used to identify the functional groups of the active components based on the peak value in the region of infrared radiation. The IR analysis confirmed the presence of phenolic alcohols, carboxylic acids, ethers, esters and aromatics compounds which showed major peaks at 3135.86, 1594.41 and 1214, respectively (; ). The presence of phenolic and aromatic compounds in aqueous extract of C. sinensis shows the pharmacological properties.
Allergic appearance includes allergic rhinitis, anaphylaxis, purities and asthma diseases allied with inflammatory conditions. Mast cells are known to be the primary responders in allergic reactions, most of which are triggered by cross-linking of high affinity IgE receptors [Citation10]. Anaphylaxis is a severe and systemic allergic reaction caused by systemic release of histamine and inflammatory mediators [Citation13]. The frequent cause of anaphylaxis was IgE mediated hypersensitivity reaction. Anaphylaxis treatment involves the use of immunotherapeutic agent which decreases production of IgE [Citation12]. Mast cell degranulation can be induced by synthetic compound 48/80 and it has been used as a direct and convenient model to study the mechanism of anaphylaxis. Several reports have demonstrated that stimulation of mast cell by compound 48/80 initiates activation of signal transduction pathway through G-protein, which leads to histamine release [Citation10]. In the present study, green tea extract produced dose dependent reduction in the mortality of mice subjected to anaphylactic shock. In the present investigation, green tea extract was evaluated for its anti anaphylactic and mast cell stabilizing activities. Green tea extract represent marked protection of rat mesenteric mast cells from disruption caused by compound 48/80. The anti-anaphylactic and mast cell stabilizing effect of green tea extract might be attributed to the suppression of IgE antibody production, which is responsible for degranulation mast cells [Citation14]. Ketotifen is an antihistaminic (H1) with some crymoglycate like action; stimulation of immunogenic and inflammatory cells and mediator release is inhibited. It is not bronchodilator but produces sedation [Citation15].
Mast cell stabilizing and anti allergic activities of medicinal plants were due to the presence of saponin, glycosides and flavonoids [Citation16]. The phytochemical screening of aqueous extract of green tea showed the presence of saponins, glycosides, flavonoids, tanins, and phenolic compounds. Saponins are reported to possess mast cell stabilizing, anti allergic and anti histaminic activities. Several flavonoids have been shown to inhibit basophil histamine release and neutrophil betaglucuronidase release, and thereby possess in vivo anti allergic activity [Citation16]. Thus, the presence of flavonoids and saponins in the green tea might be responsible for the mast cell stabilizing and anti anaphylactic activity.
In conclusion, the results obtained in the present study suggest that green tea aqueous extract possess significant antianaphylactic activity and this might be due to stabilization of mast cell membrane. Based on this, green tea is effective in treatment of asthma as it shows anti allergic and mast cell stabilization potential.
Acknowledgements
The authors are thankful to Sri Krishna Devaraya University for providing facilities and financial support.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- T.C.TheoharidesK.AlysandratosA.AngelidouD.DelivanisN.SismanopoulosB.ZhangMast cells and inflammationBiochim Biophys Acta182220122133
- G.BalajiM.ChalamaiahB.RameshY.Amarnath ReddyAntidiarrhoeal activity of ethanol and aqueous extracts of Carum copticum seeds in experimental ratsAsian Pac J Trop Biomed22201211511155
- C.F.TsaiY.W.HsuH.C.TinC.F.HuangC.C.YenThe in vivo antioxidant and antifibrotic properties of green tea (Camellia sinensis, Theaceae)Food Chem1363–4201313371344
- M.HamerThe beneficial effects of tea on immune function and inflammation: a review of evidence from in vitro, animal, and human researchNutr Res272007373379
- A.B.SharangiMedicinal and therapeutic potentialities of tea (Camellia sinensis L.)Food Res Int422009529535
- A.M.BodeZ.DongEpigallocatechin 3-gallate and green tea catechins: united they work, divided they failCancer Prev Res22009514517
- H.WangG.J.ProvanK.HelliwellHPLC determination of catechins in tea leaves and tea extracts using relative response factorsFood Chem81200307312
- C.SumitraB.YogeshN.KrunalSpectral analysis of methanol extract of Cissus quadrangularis L. stem and its fractionsJ Pharmacogn Phytochem242013132140
- Y.HsuC.TsaiW.ChenC.HuangC.YenA subacute toxicity evaluation of green tea (Camellia sinensis) extract in miceFood Chem Toxicol49201126242630
- P.V.GohilA.A.MehtaEvaluation of mast cell stabilizing and anti-anaphylactic activity of polyherbal formulationAdv Biomed Res562011304308
- Ch.Venkateswara RaoV.MadhavanK.SairamK.VikasAntidiarrhoeal activity of the Cinnamomum tamala in experimental ratsJ Nat Med622008396402
- H.R.ChitmeM.MallikarjunV.M.ChandrashekharP.M.PrasantAntiallergic activity of Aristolochia bracteolata Lank in animal modelIndian J Exp Biol4820104652
- S.S.AnilR.B.PrakashC.J.ManjunathAnti anaphylactic and mast cell stabilization activity of Cynodond actylonInt J Pharm Pharm Sci2220106973
- G.PalitS.P.SinghN.SinghR.P.KohliK.P.BhargavaAn experimental evaluation of anti-asthmatic plant drugs from ancient ayurvedic medicineAspects Aller Appl Immunol1619833641
- Tripathi KD. Essentials of Medical Pharmacology. 5th ed. p. 206.
- D.J.TaurR.Y.PatilMast cell stabilizing and antiallergic activity of Abrus precatorius in the management of asthmaAsian Pac J Trop Med20114649