Abstract
Olive (Olea europaea L.) leaves are rich in phenolic substances, which exert important antioxidant and antibacterial effects. In this study, the effect of olive leaves extract (OLE) on the microbial load of raw peeled undeveined (PUD) shrimp (Penaeus semisulcatus) was evaluated. Alcoholic OLE extracts were prepared at 0.5%, 1% and 2% (w/v) concentrations. Raw PUD shrimp samples were immersed in the treatment solutions for 3 h at 4 °C and samples were taken for determination of total viable count (TVC) and total coliforms count (TCC). OLE at concentration of 1% (w/v) significantly (p < 0.01) reduced the count of the aerobic and coliforms bacteria at least 1 log cycle CFU/g in reference to the non-treated control group. Such antimicrobial activity was concentration dependent and usage of 2% OLE had the most beneficial effect in controlling microbial load in PUD shrimp stored at 4 °C. This study demonstrates the potential use of OLE formulations to improve the microbial quality of PUD shrimp and OLE might be useful to the seafood industry as a natural preservative.
1 Introduction
Amongst foods, seafood is the most perishable of all. It is harvested and processed in a wide array of circumstances, often in remote, under-equipped and unsanitary conditions. Therefore shrimp, whether wild-caught or cultured, is subjected to a wide range of safety hazards [Citation1].
Green tiger shrimp (Penaeus semisulcatus) is an indigenous species in Mediterranean environment and become one of the most common farm-raised shrimps in Egypt. After harvest shrimp is generally subjected to one or more steps, such as removing the head, peeling, icing, rinsing, etc., and is likely to become contaminated with spoilage and/or pathogenic microbes, unless proper sanitation measures are put into practice [Citation1]. Peeling refers to removing shrimp shell from the meat, whereas deveining refers to removal of shrimp vein (intestine) that runs down the dorsal side near the surface which is usually filled with food and sand; its removal improves product quality [Citation1]. Shrimp is peeled and deveined by machines, although it is done manually in most of the developing countries [Citation1]. Shrimp are further processed into a frozen (raw) or cooked and/or breaded product before being frozen and stored [Citation1].
The major thrust of sanitation quality assurance in shrimp culture, harvesting, handling and processing is geared towards preventing microbial contamination and controlling the growth of microbes [Citation1]. The use of natural preservatives to increase the shelf-life of food is a promising technology since many plant-derived substances such as pine bark extract (Pycnogenol) and Garlic can extend the shelf-life of fresh and cooked meat products [Citation2,Citation3].
Olive (Olea europaea L.) fruit, oil and leaves have an ancient history of nutritional, medicinal and traditional usages. Olive products are an important part of the Mediterranean diet, the highest value of which may be due to olive polyphenols that modulate part of the oxidative balance in vivo [Citation4,Citation5]. Olive leaves polyphenols become a subject of intense investigation because of their several beneficial effects on health due to their anti-hypertensive, anti-diabetic, anti-carcinogenic, anti-atherosclerotic, anti-inflammatory, and antimicrobial activities [Citation6–Citation11].
Olive leaves extract (OLE) is now a popular nutraceutical taken either as liquid or capsules [Citation12].The most abundant phenolic component of OLE content is Oleuropein which gives the bitter taste to olive and olive oil and give an in vitro inhibitory effect of OLE against many foodborne pathogens such as Campylobacter jejuni, Helicobacter pylori and Staphylococcus aureus [Citation7]. Therefore, the current study was conducted to investigate the beneficial effect of OLE to improve the microbiological quality of raw peeled shrimp.
2 Materials and methods
2.1 Preparation of the olive leaves extract (OLE)
Fine-quality olive leaves were collected from the pruning of trees of ‘Picual’ olive (Olea europaea L.) cultivar during ripening of the fruit. The leaves were cleaned from extraneous matter and properly washed then dried in hot air-oven for 24 h at 40 °C. The dried leaves were ground in a blender to form powder. Thereafter, 100 g of the powder were macerated in 1000 ml absolute ethanol and allowed to extract for 48 h [Citation13]. The resultant (dark green-brown mixture) was filtered and the filtrate was concentrated in a rotary evaporator under reduced pressure. The extraction yield% of the alcoholic extract was 11%. The dried extract residue was reconstituted in sterile distilled water to give final concentration of 0.5%, 1% and 2%.
2.2 Study design
Ten samples (frozen package weight 500 g) of raw peeled undeveined (PUD) shrimp (Penaeus semisulcatus) were randomly collected from several seafood retailers. Each sample was allowed to thaw at 4 °C and was equally subdivided into four parts, the first part was immersed in distilled water in 1:1 ratio (shrimp:distilled water w/v) and used as a control, meanwhile the second, third and fourth parts were immersed in 0.5%, 1% and 2% OLE (w/v) concentrations at 4 °C for 3 h, respectively. After treatment, all samples were drained and prepared for bacteriological examination.
2.3 Bacteriological evaluation
Preparation of samples, decimal dilutions, culturing and enumeration techniques of bacteria were performed according to the methods described by the American Public Health Association (APHA). Briefly, 25 g from each sample was aseptically transferred into a sterile polyethylene stomacher bag and blended with 225 ml of sterile normal saline in a stomacher homogenizer (Stomacher 400, Seaward medicals, UK.) at 230 rpm for 60 s. Serial dilutions were made using sterile 0.1% peptone water. Determination of the total viable count (TVC) was performed using plate count agar (Difco, Detroit, Ml, USA) plates, which were inoculated with serial dilutions and incubated at 37 °C for 48 h. Countable plates are those containing from 25 to 250 colonies [Citation14]. Determination of coliforms count was performed using Violet Red Bile Lactose agar (VRBA-Oxoid, CM0107). VRBA plates were incubated at 37 °C for 24 h. Purple-red colonies, 0.5 mm in diameter or larger, surrounded by a zone of precipitated bile acids were counted [Citation15].
2.4 Statistical analysis
Data analysis was performed by using SPSS statistical software program (SPSS for Windows version 16, Spss Inc., USA). Data were expressed as mean ± standard deviation (SD). One-way analysis of variance (ANOVA) with Duncan post-hoc multiple comparisons test. Result was considered significant at p < 0.01.
3 Results and discussion
Nutraceuticals can be used alone or in combination with other novel preservation technologies to facilitate the replacement of traditional approaches of food preservation [Citation16,Citation17]. However, use of natural plant extracts or essential oils on food may not always reproduce the results obtained from preliminary in vitro studies of the same compounds as foods are complex, multicomponent systems consisting of different and connected microenvironments [Citation16]. For example, rosemary extracts revealed activity against lactic acid bacteria and Listeria in agar diffusion test but when used in meatballs, they only slightly reduced the lactic acid bacterial counts [Citation18].
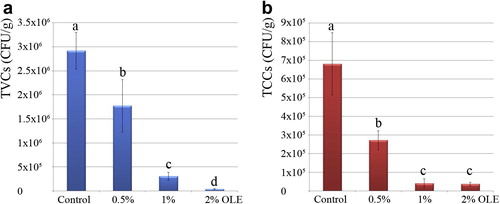
The current study was designed to investigate the application of OLE to reduce the bacterial load in raw PUD shrimp. PUD shrimp samples were treated with 0.5%, 1% and 2% OLE (w/v) in 1:1 ratio (shrimp:distilled water with OLE) for 3 h at 4 °C. After the treatments, samples were drained and bacteriologically evaluated for determination of TVCs and TCCs ().
Table 1 Limits of estimated minimum and maximum total viable counts (TVCs) and total coliforms counts (TCCs) in raw peeled undeveined (PUD) shrimp samples treated with OLE at concentrations of 0.5%, 1% and 2% (w/v) for 3 h. (CFU/g).
The average value of initial microbial load of shrimp samples (control group) was 2.9 × 106 ± 3.8 × 105 CFU/g. The TVCs in OLE-treated samples were significantly (p < 0.01) decreased to (1.7 × 106 ± 5.4 × 105, 3 × 105 ± 8 × 104 and 4.1 × 104 ± 1.2 × 104) by increasing the OLE concentration to (0.5%, 1% and 2%, respectively) ().
OLE with a concentration of 0.6% (w/v) killed Escherichia coli, Pseudomonas aeruginosa, S. aureus and Klebsiella pneumonia in 3 h exposure [Citation11]. Whereas Bacillus subtilis was inhibited only when the concentration was increased to 20% (w/v) possibly due to spore forming ability of this species [Citation11].
Coliforms count (Escherichia, Enterobacter, Citrobacter and Klebsiella) is used as an indicator of shrimp post-harvest contamination, particularly that of fecal origin [Citation1]. The average value of initial TCCs in shrimp samples (control group) was 6.8 × 105 ± 1.6 × 105 CFU/g. The TCCs in OLE-treated samples were also significantly (p < 0.01) decreased to 2.7 × 105 ± 5 × 104 and 4.1 × 104 ± 2.4 × 104 by increasing the OLE concentration to 0.5% and 1%, respectively (). However, increasing the concentration of OLE to 2% resulted in a similar decrement of TTCs (3.7 × 104 ± 9.7 × 103) obtained by 1% OLE treatment ().
Raw black tiger shrimp (Penaeus indicus) products produced by a processor operating under Hazard Analysis Critical Control Point (HACCP) programme, exerted good process control in order to maintain superior bacteriological quality of their products as few samples exceeded the aerobic plate count of 105 CFU/g, whereas Coliforms were detected in all the products, though at a low level [Citation19]. Coliforms were prevalent in headless shell-on shrimps followed by raw, peeled, and deveined tail-off, raw, peeled tail-on, and cooked, peeled tail-on shrimps [Citation19]
Seven phenolic compounds are identified in OLE including caffeic acid, verbascoside, oleuropein, luteolin 7-O-glucoside, rutin, apigenin 7-O-glucoside and luteolin 4′-O-glucoside [Citation20]. The unique olive plant polyphenol is oleuropein, which is most abundant in the leaves (up to 264 mg/g of dry leaf, when expressed as tyrosol equivalents) [Citation21]. The use of whole extracts of olive leaves may be more beneficial than isolated constituents since a bioactive component can change its properties in the presence of other compounds present in the extract [Citation20]. Ingested OLE effectively delivers oleuropein and hydroxytrosol metabolites to plasma in humans, as conjugated (sulfated and glucuronidated) metabolites of hydroxytyrosol were the primary oleuropein metabolites recovered in plasma and urine following OLE consumption [Citation12].
Despite OLE has no broad-spectrum antibacterial activity, it has appreciable activity on H. pylori and C. jejuni [Citation7]. In addition, it exerts antimicrobial activities against several microorganisms including; E. coli, S. aureus, K. pneumoniae, Bacillus cereus, Salmonella Typhi and Vibrio parahaemolyticus [Citation11].
The current study investigated the antibacterial activity of crude OLE depending on the decreased bacterial counts in samples of PUD shrimp kept in escalating concentrations of the plant leaves extract. Essentially, application of OLE at 2% (w/v) concentration had a beneficial effect in controlling the microbial load of raw PUD shrimp. This data indicated a possible utilization of nutraceutical OLE to improve the food safety during shrimp processing.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- L.KanduriR.A.EckhardtFood safety in shrimp processing: a handbook for shrimp processors, importers, exporters and retailers2008Wiley
- J.AhnI.U.GrunA.MustaphaEffects of plant extracts on microbial growth, color change, and lipid oxidation in cooked beefFood Microbiol242007714
- K.I.SallamM.IshioroshiK.SamejimaAntioxidant and antimicrobial effects of garlic in chicken sausageLebenson Wiss Technol372004849855
- A.TaamalliD.Arraez-RomanM.ZarroukJ.ValverdeA.Segura-CarreteroA.Fernandez-GutierrezThe occurrence and bioactivity of polyphenols in Tunisian olive products and by-products: a reviewJ Food Sci772012R83R92
- M.G.SoniG.A.BurdockM.S.ChristianC.M.BitlerR.CreaSafety assessment of aqueous olive pulp extract as an antioxidant or antimicrobial agent in foodsFood Chem Toxicol.442006903915
- L.WangC.GengL.JiangD.GongD.LiuH.YoshimuraThe anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosisEur J Nutr472008235243
- A.N.SudjanaC.D’OrazioV.RyanN.RasoolJ.NgN.IslamAntimicrobial activity of commercial Olea europaea (olive) leaf extractInt J Antimicrob Agents332009461463
- Khayyal MT, el-Ghazaly MA, Abdallah DM, Nassar NN, Okpanyi SN, Kreuter MH. Blood pressure lowering effect of an olive leaf extract (Olea europaea) in L-NAME induced hypertension in rats. Arzneimittelforschung. 2002;52:797–802.
- H.F.Al-AzzawieM.S.AlhamdaniHypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbitsLife Sci78200613711377
- O.H.LeeB.Y.LeeAntioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extractBioresour Technol101201037513754
- D.MarkinL.DuekI.BerdicevskyIn vitro antimicrobial activity of olive leavesMycoses462003132136
- M.de BockE.B.ThorstensenJ.G.DerraikH.V.HendersonP.L.HofmanW.S.CutfieldHuman absorption and metabolism of oleuropein and hydroxytyrosol ingested as olive (Olea europaea L.) leaf extractMol Nutr Food Res57201320792085
- A.M.DubA.M.DuganiAntithrombotic effect of repeated doses of the ethanolic extract of local olive (Olea europaea L.) leaves in rabbitsLibyan J Med8201320947
- Morton RD. Aerobic Plate Count. In: Downes FP, Ito K, editors. Compendium of Methods for The Microbiological Examination of Foods. 4th ed: American Public Health Association; 2001. p. 63–7.
- Kornacki JL, Johnson JL. Enterobacteriaceae, coliforms, and escherichia coli as quality and safety indicators. In: Downes FP, Ito K, editors. Compendium of methods for the microbiological examination of foods. 4th ed: American Public Health Association; 2001. p. 69–82.
- B.K.TiwariV.P.ValdramidisC.P.O’DonnellK.MuthukumarappanP.BourkeP.J.CullenApplication of natural antimicrobials for food preservationJ Agric Food Chem57200959876000
- J.E.HayesV.StepanyanP.AllenM.N.O’GradyJ.P.KerryEffect of lutein, sesamol, ellagic acid and olive leaf extract on the quality and shelf-life stability of packaged raw minced beef pattiesMeat Sci842010613620
- J.Fernández-LópezN.ZhiL.Aleson-CarbonellJ.A.Pérez-AlvarezV.KuriAntioxidant and antibacterial activities of natural extracts: application in beef meatballsMeat Sci692005371380
- A.A.Mohamed HathaT.K.MaqboolS.Suresh KumarMicrobial quality of shrimp products of export trade produced from aquacultured shrimpInt J Food Microbiol822003213221
- A.P.PereiraI.C.FerreiraF.MarcelinoP.ValentaoP.B.AndradeR.SeabraPhenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leavesMolecules12200711531162
- Preedy VR, Watson RR. Olives and Olive Oil in Health and Disease Prevention: Elsevier Science; 2010.
