Abstract
Mobilization of immunoglobulins (Igs)-containing plasma cells (IgA, IgG and IgM) in the spleen, bursa of Fabricius and thymus was investigated in broiler chickens that were vaccinated with Newcastle disease virus (NDV) vaccine. In the thymus, the Igs-containing plasma cells were distributed in the cortex and medulla. Their frequency and distribution were higher at D14 and at D28. The number of IgG- and IgM-positive cells was greater than IgA-positive cells in thymus. In the bursa of Fabricius, Igs-containing plasma cells were distributed beneath the capsules; within and around the bursal follicles. Their frequency of occurrence significantly peaked at D14 and at D28 in comparison to day-old chickens, and IgG-positive cells were significantly greater than the IgA- and IgM-positive cells in the bursa of vaccinated chickens. In the spleen, Igs-containing plasma cells were distributed in the white pulp, around the trabeculae, and in the periarterial lymphatic sheath. In this secondary lymphatic tissue, IgG- and IgM-positive cell numbers significantly greater than IgA-positive cells. In conclusion, mobilization of more Igs-positive cells in lymphoid tissues of broiler chickens is due to the effect of NDV vaccine as well as the advancement of age.
1 Introduction
Poultry production, especially chickens and ducks has attained an important place in the agricultural economy of Bangladesh both through contribution to gross domestic product (GDP) and employment, especially in urban and pre-urban areas. Newcastle disease (ND) is the most common and harmful disease for commercial broilers. In addition, ND causes serious outbreaks in commercial layer and parent stocks every year and results in huge losses for the poultry industry. ND leads to necrosis, infiltration of heterophils and heterophilic changes in lymphoid organs [Citation1].
Most living beings manage not only to survive but indeed thrive in a potentially hostile milieu, with seemingly little effort. This freedom from disease is dependent on the existence of a complex and highly sophisticated defense system called lymphoid system [Citation2] which start to develop at embryonic day (ED) 10 in broilers [Citation3,Citation4].The lymphoid system of fowl is consisting of unique organs and divided into two morphologically and functionally distinct components [Citation5]. The lymphoid tissues have an independent phylogenetic origin, their function being to react to foreign antigens by producing antibodies, thereby providing “adaptive immunity”. An obvious characteristic of the lymphatic tissues of mammals and birds is that they are densely populated with lymphocytes. This is because they are involved in the lymphocytes production, immune responses or both of these occurring at the same time [Citation6]. When lymphocytes arrive in the lymphoid tissues or organs they become plasma cells and begin to synthesize immunoglobulins. The plasma cells-containing different classes of immunoglobulins (Igs) are distributed throughout the lymphoid tissues, [Citation7] including Harderian gland of broiler chickens [Citation8].
Chickens have been used as experimental animals for studies of the immune system, because chicken T and B cells mature in the thymus and bursa of Fabricius, respectively, and because these organs are easily manipulated. The development, differentiation, distribution and function of immune cells in these tissues, including the spleen of chicken, have been investigated using a wide variety of methodologies: surgical thymectomy, bursectomy, X-ray irradiation, and the colloidal carbon uptake method, as well as the administration of hormones, drugs, vitamins and minerals [Citation9–Citation14]. In the present study, we used NDV vaccine via both ocular and oral routes to study the frequency and distribution of Igs-containing plasma cells in the thymus, bursa of Fabricius and spleen of broiler chickens.
2 Materials and methods
The experiment was carried out in the Department of Anatomy and Histology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh. Part of the research was also conducted in the Laboratory of Anatomy and Histology, Faculty of Veterinary Medicine, University Malaysia Kelantan, Malaysia. The following materials and methodologies were followed in the present experiment:
2.1 Chickens
A total of 32 day-old “Cob-500” broiler chickens of both sexes were purchased from Kazi Farm Ltd. Mymensingh, Bangladesh. The chickens had no visible developmental disorders or detectable diseases that might influence the distribution of lymphocytes and plasma cells.
2.2 Management
The chickens were reared in a litter system in a poultry shed at the Department of Poultry Science, Faculty of Animal Husbandry, Bangladesh Agricultural University, Mymensingh. All the chickens were provided with broiler feed and mineral water ad libitum. Poultry shed bio-security and brooding management were both strictly maintained, as were optimum temperature, lighting and ventilation in the brooder.
2.3 Vaccines
The live attenuated NDV vaccine used in this study was the product of Mohakhali Research Centre, Directorate of Livestock Services, Dhaka, Bangladesh. The recommended dose is one drop of vaccine per eye in between one to 3-days-old chicken, with a booster dose usually given between 12 and 21 days of age.
2.4 Experimental design
The chickens were grouped into control and treatment groups. Chickens in the control group (n = 16) were given a drop of distilled water ocularly at 06:00 on D1. From this group 8 chickens were killed at 18:00 on the same day (D1), and the remaining 8 chickens were sacrificed on D10 at 18:00 to ascertain the mobilization of immune cells. Chickens in the treatment group (n = 16) were given NDV vaccine ocularly on D3 at 06:00 with a booster dose of the same vaccine being administered orally on D13 at 06:00. The thymus, bursa of Fabricius and spleen were collected from these chickens on D14 and D28 after sacrificing the chickens (8 broilers per group) through cervical subluxation, and specimens were fixed in Bouin’s fluid or ice cold periodate lysine paraformaldehyde (PLP) for histological and immunohistochemical study.
2.5 Antibodies
The following antibodies were used for detecting Igs-positive cells:normal rabbit serum (Biosource international, Inc., Camarillo, CA, USA), goat anti-chicken IgA (Bethyl Laboratories, Montgomery, TX, USA), goat anti-chicken IgG (Bethyl Laboratories), goat anti-chicken IgM (Bethyl Laboratories) and HRP-conjugated rabbit anti-goat IgG (Bethyl Laboratories).
2.6 Immunohistochemistry
The indirect immunoperoxidase method was performed to study the distribution pattern and frequencies of Igs-positive cells in the bursa of Fabricius, thymus, and spleen, of control and vaccinated broiler chickens. The tissues from bursa of Fabricius, thymus and spleen were fixed in Bouin’s fluid or PLP, dehydrated in a series of graded alcohol, cleared in xylene, and embedded in paraffin. Paraffin sections of 6 μm thickness were immunostained by the indirect immunoperoxidase method as described previously [Citation14].
2.7 Histoplanimetry
The Igs-containing plasma cells were counted in 20 fields of the histological sections using a light microscope at a magnification of 400× where Igs-plasma cells were diffusely distributed and their relative frequency per focus was calculated according to the point count method [Citation16].
2.8 Statistical analysis
The numbers of Ig-containing plasma cells and their variation in numbers in the thymus, bursa of Fabricius and spleen were compared (randomized block design) among broilers of various stages, and data were evaluated by student’s t-test [Citation17].
3 Results and discussion
Frequency and distribution of Igs-containing plasma cells (IgA, IgG and IgM) in the lymphoid tissues of broiler chickens:
3.1 Thymus
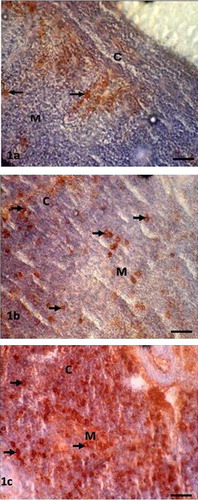
In the thymus, Igs-containing plasma cells were distributed in the cortex and medulla (–). The frequency of Igs-containing plasma cells was statistically greater (P < 0.05) in the thymus at D14 and D28 in comparison to the day-old chickens (–). When comparing frequency of occurrence of different Igs-containing plasma cells, it was found that IgG- and IgM-positive cells were greater (P < 0.05) at D14 and D28 ages. In the present study, the population of Igs-containing plasma cells and their infiltration to the lymphoid organs due to inoculations of NDV vaccine were clearly observed. These dynamic changes in the frequency and distribution of Igs-containing plasma cells were also observed in the Harderian gland, cecal tonsil and trachea of our previous studies [Citation14]. The thymus is responsible for the production of T-lymphocytes subsets in the chicken [Citation18] not Igs-containing plasma cells, however existence of statistically higher IgG- and IgM-positive cells in the thymus of the present study indicates that the B lymphocytes infiltrated in the thymus via blood circulation and differentiated into Igs-containing plasma cell for local defensive function as T-lymphocytes subpopulation do in the bursa of Fabricius [Citation19], which is the homing organ for B lymphocytes.
3.2 Bursa of Fabricius
In the bursa of Fabricius, Igs-containing plasma cells were found principally beneath the capsule; in the cortex, and medulla of the bursal follicles; and around the bursal follicles ( and ). The frequency of Igs-containing plasma cells in this gland was greater at D14 and at D28 in comparison to day-old chickens (–). When comparing the data, it was found that, the frequency of IgG-positive cells was statistically higher (P < 0.01) at D28. In contrast, IgM-positive cells were insignificantly greater than IgA-and IgG-positive cells at D1 and at D14. The findings of our present study varied greatly [Citation20]. Honjo et al. [Citation15] reported that most of the bursal lymphoid cells of 4 week old inbred line P chickens were IgM positive. This variation at the same age is possibly due to the strain differences of chickens used in the present study. In the vaccinated chickens of the present study Igs-containing plasma cells were sharply increased from the D1 chickens and peaked from D14 to D28 and this might be due to immunomodulation of the vaccine.
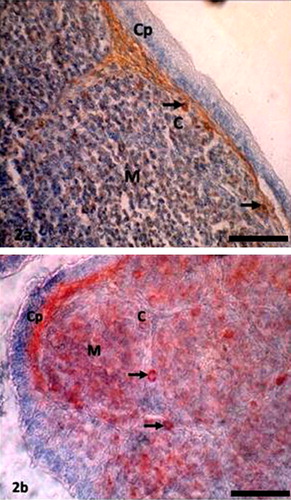
3.3 Spleen
In the spleen, Igs-containing plasma cells were numerous in the white pulp (–), around the trabeculae, around the central artery and in the periarterial lymphatic sheath. These distribution patterns of Igs-containing plasma cells in the vaccinated chickens were similar to our previous study [Citation13], where we used vitamins and minerals to observe the distribution pattern of Igs-containing plasma cells. A similar distribution pattern of Igs-containing plasma cells was also observed in the spleen of the control group in the present study. The frequency and distribution of IgG-positive cells were statistically greater (P < 0.01) at D14 (); and IgA- and IgM-positive cells were higher at D28 ( and ). When comparing the data of Igs-containing plasma cells in this gland, it was observed that, both IgG- and IgM-positive cells were statistically higher (P < 0.01–0.05) than the IgA-positive cells throughout the period of postnatal development. This finding was in agreement with our previous statement that the NDV vaccine initiates mobilization of similar types of immune cells in the Harderian gland of broiler chickens [Citation14].
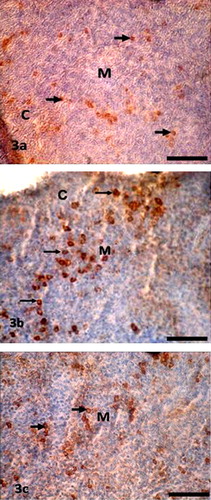
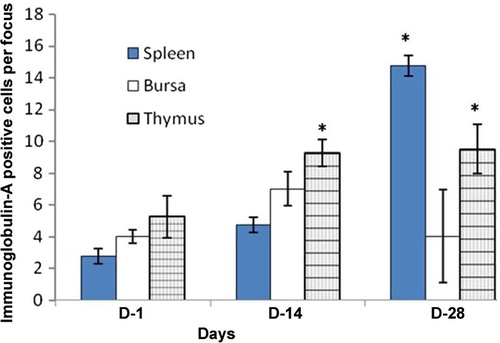
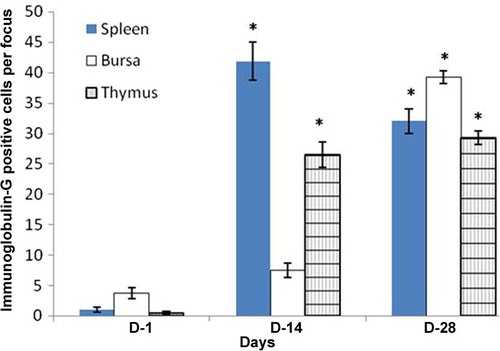
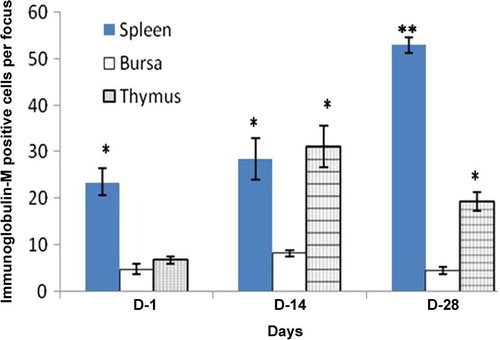
Chickens were inoculated with NDV vaccine at D3, and the booster dose was given at D13 in the present study. We compared the data of Igs-containing plasma cells between vaccinated chickens and control up to D10, and it was found that the number of Igs-containing plasma cells were significantly greater in vaccinated chickens than in control. In the present study, it was observed that the frequency and distribution of IgA-, IgG- and IgM-positive cells were more in D14 and D28 groups of vaccinated chickens than the chickens of D1. This finding was similar to the report of Salam et al. [Citation20] and Solcan et al. [Citation21]. Among the Igs-containing plasma cells, the number of IgG- and IgM-positive cells was significantly greater at D14 and D28 groups of chickens. This finding is in partial agreement with the results of Bienenstock et al. [Citation22] and Ohshima et al. [Citation23], who reported that both IgA- and IgG-positive cells formed the bulk of the lymphoid cell population in the early stages of life and that IgA-positive cells were predominant in the later stages of life.
IgG and IgM cells protect the body from infection are found in the lymphoid organs, whereas IgA cells are found in the secretory organs [Citation24]. The frequency of these cells and the antibody titre in the serum increased with the NDV vaccine alone [Citation25] or perform synergistic effect with immune adjuvant [Citation26]. In the present study, the number of IgG- and IgM-positive cells was statistically higher than IgA-positive cells in the thymus and bursa of Fabricius. In contrast to the Harderian gland of the duck [Citation27] and chicken [Citation8]; and to the mucosa of the gastrointestinal tract of chickens [Citation7], IgA-positive cells were significantly greater. This observation from avian species was similar to the mammalian context, particularly the rat [Citation24]. This finding suggests that the frequency and distribution of Igs-containing plasma cells are in similar in avian and mammals despite belonging to different phyla.
4 Conclusion
In the present study, immunoglobulins-positive cells were detected in the lymphoid organs using immunohistochemistry. The results demonstrate that IgG- and IgM-containing plasma cells are abundantly found in the thymus, bursa of Fabricius and spleen of broilers chickens. Cells numbers were statistically greater at D14 and at D28 due to an immune response resulting from the NDV vaccine as well as the advancement of age.
Acknowledgements
The University Grants Commission, Bangladesh, supported this project financially. Special Thanks to Greggory Wroblewski (Lecturer, English Communication, Yamaguchi University Graduate School of Medicine, Japan) for a critical reading of this manuscript.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- E.L.JungherrPathology of NDV for chickenR.P.HansonNewcastle disease virus. An evolving pathogens1964University of Wisconsin252272
- R.S.CortanV.KumarS.L.RobbinRobbin’s pathogenic basis of disease4th edition1998W.B. SaundersPhiladelphia [p. 163–164]
- M.N.IslamM.Z.I.KhanM.R.JahanR.FujinagaA.YanaiK.KokubuPrenatal histomorphological development of the lymphoid organs of native chickens of BangladeshPak Vet J31201116
- M.N.IslamM.Z.I.KhanM.R.JahanR.FujinagaK.ShinodaOntogenic development of immunoglobulins (Igs)-positive lymphocytes in the lymphoid organs of native chickens of BangladeshInt J Vet Sci Med1201396101
- R.D.CooperA.PetersonR.A.GoodIndication of the thymic and bursal lymphoid system of the chickenNature2051965143146
- D.H.CormackHAM’s histology9th ed.1987J.B. Lippincott CompanyPhiladelphia [p. 234–263]
- M.N.IslamM.Z.I.KhanM.R.JahanM.R.KarimY.KonComparative studies of mucosa and immunoglobulin (Ig)-containing plasma cells in the gastrointestinal tract of broiler and native chickens of BangladeshJ Poult Sci452008125131
- M.Z.I.KhanM.R.JahanM.N.IslamZ.HaqueM.R.IslamY.KonImmunoglobulin (Ig)-containing plasma cells in the Harderian gland in broiler and native chickens of BangladeshTissue Cell392007141149
- B.W.PapermasterR.A.GoodRelative contribution of the thymus and the bursa of Fabricius to the maturation of the lympho-reticular system and immunological potential in chickenNature21962838840
- M.SugimuraY.HashimotoQuantitative histological studies on the spleen of ducks after Neonatal thymectomy and bursectomyJ Anat1311980441452
- M.Z.I.KhanY.HashimotoY.IwamiT.IwanagaHormonal regulation of T-cell subsets in the oviduct: an immunohistochemical study using sex-hormone-treated chickensJ Vet Med Sci58199611611167
- M.R.KarimM.Z.I.KhanZ.HaqueMorphometrical analysis of major lymphoid organs of chemotherapy treated chickensBangladesh J Vet Med32005106109
- M.Z.I.KhanS.H.AkterM.N.IslamM.R.KarimM.R.IslamY.KonThe effect of Selenium and vitamin E on the lymphocytes and immunoglobulin-containing plasma cells in the lymphoid organ and mucosa-associated lymphatic tissues of broiler chickensAnat Histol Embryol3720085259
- K.HonjoT.HagiwaraK.ItohE.TakahashiY.HirotaImmunohistochemical investigations of lymphocytes in the lymphoid organs of cyclophosphamide treated chickensJ Vet Med Sci551993895897
- M.NasrinM.Z.I.KhanM.N.H.SiddiqiM.A.MasumMobilization of immunoglobulin (Ig)-containing plasma cells in Harderian gland, cecal tonsil and trachea of broilers vaccinated with Newcastle disease vaccineTissue Cell452013191197
- E.R.WeibelStereological principles for morphology in electron microscopic cytologyInt Rev Cytol261969235302
- J.H.ZarBiostatistical analysis 31996Prentice-HallUpper Saddle River, New Jersey [p 123–129]
- M.Z.I.KhanY.HashimotoM.AsaduzzamanDevelopment of T-cells subpopulations in postnatal chicken lymphoid organsVeterinarski Arh681998183189
- M.Z.I.KhanY.HashimotoAn immunohistochemical analysis of T-cell subsets in the chicken bursa of Fabricius during postnatal stages of developmentJ Vet Med Sci58199612311234
- R.SalamA.AslamS.A.KhanK.SaeedG.SaleemEffect of different routes of vaccination against Newcastle disease on lymphoid organs of broilersPak Vet J2320037883
- Solcan C, Paul I, Cotea C. The dynamic of the lymphoid tissue associated to the Harderian gland in chickens after the conjunctival vaccination against New Castle disease.Lucrai-Stiinifice-Medicina-Veterinara,-Universitatea-de-Stiinte-Agricole-si-Medicina-Veterinara-“Ion-Ionescu-de-la-Brad”-Iasi 2000; 43: 433–436.
- J.BienenstockJ.GauldieD.Y.E.PereySynthesis of IgG, IgA and IgM by chicken tissuesJ Immunol111197311121118
- K.OhshimaK.HiramatsuImmunohistochemical localization of three different immunoglobulin classes in the Harderian gland of young chickenCell Tissue Res342002129133
- M.R.McDermottJ.BienenstockEvidence for a common mucosal immunologic system 1. Migration of B Immunoblasts into intestinal, respiratory and genital tissuesJ Immunol122197918921898
- Z.S.ChimenoE.GomezE.CarrilloA.BerinsteinLocally produced mucosal IgG in chickens immunized with conventional vaccines for Newcastle disease virusBraz J Med Biol Res412008318323
- X.ZhangX.ZhangQ.YangEffect of compound mucosal immune adjuvant on mucosal and systemic immune responses in chicken orally vaccinated with attenuated Newcastle-disease vaccineVaccine25200732543262
- C.A.OliveiraL.F.TellesA.G.OlivieraE.KalapothakisH.G.DornelasG.A.B.MahechaExpression of different classes of immunoglobulin in intraepithelial plasma cells of the Harderian gland of domestic ducks, Anas PlatyrhynchosVet Immunol Immunopathol1132006257266