Abstract
The effect of supplementing purebred and crossbred Merino lambs with Arthrospira platensis (Spirulina) on plasma metabolite concentrations under pasture-based management system and the influences of sire breed and sex were investigated. A completely randomized experimental design balanced by 4 sire breeds (Merino, White Suffolk, Dorset and Black Suffolk), 3 Spirulina supplementation levels (0, 100 and 200 ml representing the control, low and high, respectively) and 2 sexes (ewe and wether lambs) was utilised. All lambs had ad libitum access to the basal diet of ryegrass pastures and barley. Lambs in the treatment groups were individually drenched daily with Spirulina prior to being released with the control group of lambs for grazing over a 6-week period following a 3-week adjustment phase. At the start and completion of the feeding trial, blood samples were centrifuged and plasma metabolites measured. Data were analysed with Spirulina supplementation level, sire breed, sex and their second-order interactions fitted as fixed effects and metabolite concentrations as dependent variables. Gamma-glutamyl transferase (GGT) concentrations decreased (from 79.40 to 69.25 UI) and glucose increased (from 3.81 to 4.19 mmol/L) as the level of Spirulina supplementation increased from 0 ml in the control to 200 ml in the high treatment groups (P < 0.05). Lambs supplemented at low Spirulina levels had the highest creatinine concentrations (61.75 μmol/L). Interactions between sex and supplementation level significantly affected glucose, aspartate aminotransferase (AST) and Mg concentrations (P < 0.05), while sire breed and supplementation level interactions influenced albumin to globulin (A/G) ratio, creatinine and GGT concentrations. It was demonstrated that Spirulina supplementation does not negatively impact lamb health and productivity.
1 Introduction
Spirulina (Arthrospira platensis) is a filamentous cyanobacterium which has been the recent subject of several feeding trials with agriculturally significant animal species [Citation1]. However, to the best of our knowledge, published information regarding the metabolite response of crossbred and Merino purebred lambs to Spirulina supplementation remains relatively scarce, hence the need to fill this knowledge gap.
In Australia, crossbred Merino lambs are generally a product of dual-purpose sheep production systems. These systems typically mate meat-type rams with a core Merino flock to introduce both desirable meat and wool traits into the subsequent progeny [Citation2]. Resultant lambs are routinely supplemented with protein-rich feed types to optimise growth and productivity [Citation3]. Consequently, dual-purpose producers are best situated to exploit the current high lamb meat prices [Citation4] without abandoning their traditional wool interests. In dual-purpose systems, as in other sheep producing systems, lamb health is equally as important as productivity and profitability.
Knowledge of haematological metabolite concentrations is valuable in understanding individual lamb health and productivity status [Citation5,Citation6]. Hence, quantifying key haematological metabolite concentrations has been applied to measure the response of lambs to alternative diets and feed supplements [Citation7]. The hypothesis tested was that: Spirulina supplementation will not be detrimental to the health and productivity of lambs as indicated by plasma metabolite and electrolyte profiles, but significant interactions between supplementation level, sire breed and sex will be the key drivers of variation. Therefore, the primary objective of this study was to investigate the effects of Spirulina supplementation at differing levels to crossbred and purebred Merino lambs on haematological metabolites as indicators of health and productivity. The secondary aim was to evaluate the interactions of Spirulina supplementation levels with sire breed and sex.
2 Materials and methods
This study was conducted at the University Farm, Cambridge, Hobart, Tasmania, Australia, and was approved by the Tasmanian Animal Ethics Committee in accordance with the 1993 Tasmania Animal Welfare Act and the 2004 Australian code of Practice for the Care and Use of Animals for Scientific Purposes.
2.1 Animal management and experimental design
Using a 1:100 mating ratio, approximately 1600 Merino ewes were mated with 16 terminal sire rams of different breeds – Black Suffolk, Dorset, Merino, and White Suffolk. All progeny were identified with National Livestock Identification ear tags before being weaned onto ryegrass pasture at 16 weeks of age. At 6 months old, 48 lambs were randomly allotted into a completely randomized block experimental design balanced by sire breed, Spirulina supplementation levels and sex, respectively.
The Spirulina was commercially purchased (TAAU, Darwin, AUS) as a powder () which was then made into a water suspension using a Spirulina to water ratio of 1 g:10 mL. This was daily given to the lambs using a sheep drench to directly deliver each lamb’s assigned Spirulina level of supplementation – control (0 mL), low (100 mL), and high (200 mL). Supplementation continued over the 9-week feeding trial duration, consisting of a 3-week adjustment phase and 6-week experimental period [Citation8]. All experimental lambs were run together as a single mob with ad libitum access to drinking water and a basal diet of ryegrass pasture and cracked barley.
Table 1 Nutrient composition of Spirulina and basal diet of ryegrass pasture and barley grain.
2.2 Blood sampling and analysis
Blood samples were taken using jugular venipuncture [Citation9] at the completion of the feeding trial. These were stored in BA Vacutainer® tubes without anticoagulant (Becton, Dickson and Company, Belliver Industrial Estate, Plymouth, UK), immediately chilled in an ice-containing esky and later centrifuged at 3000 rpm for 20 min at 4 °C [Citation10]. Sub-samples of plasma and serum were taken and stored at −20 °C until analysis [Citation5].
All samples were analysed for haematological metabolite concentrations at the Animal Health Laboratory of the Tasmanian Department of Primary Industries, Parks, Water and Environment (DPIPWE, Launceston, AUS) using kits (Thermo Scientific) for all metabolites excluding GLDH which was supplied by Randox). Specifically, creatine kinase (CK), aspartate aminotransferase (AST), glutamate dehydrogenase (GLDH), gamma-glutamyl transferase (GGT), total bilirubin, creatinine, urea, protein, albumin, globulin, albumin to globulin ratio (A/G ratio), beta-hydroxybutyrate (BHB), glucose, calcium, magnesium, phosphate, sodium, potassium, sodium to potassium ratio (Na/K ratio), and chloride concentrations were analysed on a Konelab 20XTi Clinical Chemistry Analyser (Thermo Scientific).
Non-esterified fatty acids (NEFA) were measured enzymatically by Regional Laboratory Services (Benalla, Victoria, AUS) in a sample blanked endpoint reaction (Randox Laboratories, Crumlin, UK, product #FA 115) via Acyl CoA Synthetase/Acyl CoA Oxidase/Peroxidase as per Matsubara et al. [Citation11]. Cortisol was analysed at Gribbles Pathology (Clayton, Victoria, AUS) using an Immulite 2000 Systems analyser (Siemens; GERMANY) and a solid-phase, competitive chemiluminescent enzyme immunoassay.
2.3 Statistical analysis
All data were analysed using ‘Statistical Analysis System’ software [Citation12]. Summary statistics were initially computed and the unadjusted means, standard deviations, minimum and maximum values scrutinized for outliers and data entry errors. Data were then further analysed using Factorial ANOVA (PROC GLM) procedures with Spirulina supplementation level, sire breed, sex and their second-order interactions fitted as fixed effects and the following haematological metabolites as dependent variables: CK, AST, GLDH, GGT, bilirubin, creatinine, urea, protein, albumin, globulin, A/G ratio, BHB, glucose, calcium, magnesium, phosphate, sodium, potassium, Na/K ratio, chloride, NEFA, serum cortisol. Duncan’s multiple range and Bonferroni pairwise comparison tests were used for mean separation using a P < 0.05 level of significance.
3 Results
Nutrient compositions of the Spirulina supplement and the basal diet of ryegrass pasture and barley grain are shown in , wherein Spirulina’s protein-rich (61.0 g/100 g DM) content accounting for twice as much protein in the combined basal diet was apparent. In terms of metabolisable energy, Spirulina and the basal diets were iso-energetic ranging from 1701.1 to 1723.7 kJ/100 g DM. Dry matter and crude fibre contents were sufficient to meet lamb gut-fill requirements.
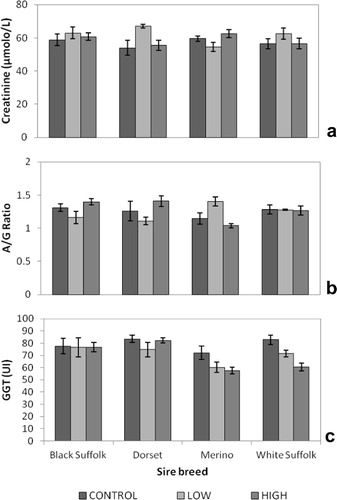
GGT concentrations were highest in unsupplemented (control) lambs compared to their supplemented counterparts in the low and high treatment groups (). Lambs supplemented at the low Spirulina level had the highest creatinine concentrations averaging 61.75 ± 1.72 μmol/L. Glucose concentrations were highest in lambs supplemented at the high Spirulina level ().
Table 2 Least square means and standard error (LSM ± SE) of haematological metabolites in Spirulina supplemented purebred and crossbred Merino lambs, as affected by Spirulina supplementation level.
Creatinine concentrations were highest in Dorset-sired lambs supplemented at low Spirulina level. Merino-sired lambs supplemented at the low Spirulina level had lowest creatinine concentrations (). A/G ratios were higher in Black Suffolk- and Dorset-sired lambs supplemented at the high Spirulina level compared to their counterparts supplemented at the low level. Purebred Merino lambs supplemented at the low Spirulina level had the highest A/G ratios (). GGT concentrations were highest in unsupplemented (control) purebred Merino lambs. In White Suffolk-sired lambs, GGT concentrations progressively decreased with increase in Spirulina supplementation levels ().
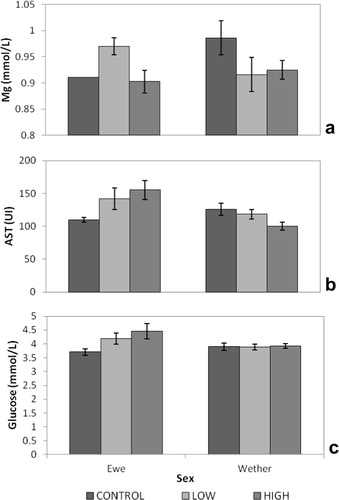
Glucose and AST concentrations were lowest in control ewe lambs (). Wether AST concentrations were lowest with high Spirulina supplementation levels (). Ewe lambs supplemented at low Spirulina levels and wether lambs in the high supplementation treatment group had the highest Mg concentrations ().
Urea, albumin and chloride concentrations exceeded their normal ranges in both supplemented and unsupplemented lambs. In contrast, creatinine and globulin concentrations were below their normal ranges in all supplemented and control lambs ().
Black Suffolk-sired lambs had the lowest creatinine and urea concentrations while Merino-sired lambs had the lowest albumin concentrations. Dorset-sired lambs had higher glucose concentrations than White Suffolk-sired lambs. Chloride concentrations were higher in Dorset- and White Suffolk-sired lambs than Merino (). GGT and glucose concentrations were higher in ewes than wethers, but Creatinine and protein concentrations were highest in wethers (). Dorset-sired ewes had higher glucose concentrations than their wether counterparts (). In Black Suffolk-sired lambs, ewes had higher GGT concentration than wethers ().
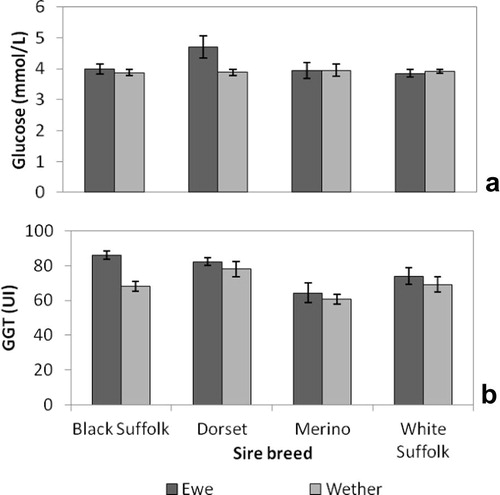
Table 3 Least square means and standard error (LSM ± SE) of haematological metabolites in Spirulina supplemented purebred and crossbred Merino lambs as affected by lamb sire breed and sex.
4 Discussion
Although significant differences attributable to level of Spirulina supplementation were only detected in GGT, creatinine and glucose profiles, there are vital animal welfare and well-being implications for the non-significant differences in cortisol, protein, albumin, globulin, urea and NEFA concentrations. It has been demonstrated that cortisol is an indicator of stress levels [Citation13]. The fact that lambs supplemented with Spirulina did not have elevated cortisol levels compared to the control lambs implies that oral drenching with Spirulina supplement did not trigger discomfort to the lambs nor did it compromise their welfare. Globulin, albumin, urea are all directly related to protein metabolism and their concentrations in both supplemented and control animals fell within the normal range (). Normality was also evident in the electrolyte concentrations of Ca, P, Mg, Na and K indicating that mineral metabolism was not negatively impacted by Spirulina supplementation. However, level of Spirulina supplementation had direct impact on GGT, creatinine and glucose concentrations () and interacted significantly with sire breed and sex to influence metabolites and electrolytes in lambs as depicted in.
GGT plays a critical role in cellular detoxification and is found in the kidneys, pancreas, intestine and liver [Citation14]. The liver is the main source of GGT [Citation5]. Hence, haematological GGT is a useful biomarker of optimal liver function [Citation15]. In sheep, haematological GGT concentrations will elevate from normal ranges during periods of hepatic tissue damage, injury and disease [Citation6]. Our results suggest that Spirulina supplementation amended elevated GGT concentrations towards normality. Hence, Spirulina supplementation can be associated with improved lamb liver health. Other researchers have reaffirmed this association by linking Spirulina consumption with improved liver health [Citation16,Citation17].
Assessment of haematological creatinine allows growth, muscularity and total body protein mass to be objectively determined [Citation7]. This stems from the positive linear relationship between creatinine concentrations and muscle tissue mass. Therefore, lambs with high haematological creatinine concentrations have greater muscularity than others with lower creatinine concentrations. Holman et al. [Citation18] found low Spirulina supplementation levels resulted in higher lamb liveweights than in both control and high Spirulina supplementation level groups. This finding aligns with similar outcomes from supplementing sheep with soybean meal [Citation19], canola meal and cracked lupins [Citation20]. This increase in liveweight, thus lamb muscularity, with low supplementation levels, is reflected in current study and associated with haematological creatinine concentration.
Ruminant glucose requirements are met using gluconeogensis pathways sourcing carbon from complex carbohydrates [Citation21], lipids [Citation22], and protein [Citation23]. The observed change in haematological glucose with increased dietary protein, through Spirulina supplementation is confirmatory of this pathway. Spirulina supplementation would have also affected the dietary energy intake of lambs, which is directly related to haematological glucose concentrations [Citation24]. Trenkle et al. [Citation25] found similar increases in haematological glucose concentrations of supplemented lambs on a basal diet of grains as opposed to Spirulina. However, glucose concentrations rapidly and frequently change dependent on individual lamb and other factors [Citation22].
Spirulina supplementation level and sex interaction were shown to affect glucose, aspartate amino transferase and magnesium concentrations. Haematological glucose concentrations have been shown to have a negative linear association with lamb liveweight [Citation24] and wethers have been reported to have higher liveweights [Citation18] and muscularity [Citation26] than ewes under identical diets. In this study, it can be suggested that dietary protein in these experimental lambs was likely partitioned more towards total body protein mass stores rather than gluconeogenesis in wethers compared to ewes. Furthermore, as AST catalyses gluconeogenesis [Citation14], its response to Spirulina supplementation level and sex interactions is identical to haematological glucose concentration.
Magnesium concentrations have been shown to increase proportionally with growth [Citation27]. Hence, this finding can be attributable to the liveweight responses to Spirulina supplementation level and sex interactions observed previously (Holman et al. [Citation18]). Moreover, Spirulina has a high concentration of calcium [Citation1] and dietary calcium levels have been found to negate haematological magnesium concentrations [Citation10]. However, this relationship was only observed in wethers in this study.
This study observed Spirulina supplementation level and sire breed interactions affect gamma-glutamyl transferase, creatinine and albumin/globulin ratio. It has been reported that GGT concentrations naturally vary between lambs of differing sire breeds [Citation28]. This stems from different levels of disease resistance, liver functionality and response to dietary protein sources found between lambs of differing genotypes [Citation29]. This is confirmed in our current study given the genetically divergent sire breeds. Lamb sire breed has also been shown to affect lamb liveweight [Citation30], and predisposition for muscle growth rather than fat deposition [Citation31]. These can be attributed to influences on feed use efficiency varying between sire breeds [Citation32]. This study found haematological creatinine concentrations reflecting these differences in liveweight and muscularity that we previously found between sire breeds supplemented with Spirulina [Citation18].
Albumin and globulin are the main protein components of plasma which are typically monitored as A/G ratio [Citation5]. Haematological albumin concentrations are indicative of long term dietary protein intake [Citation33]. Consequently, lambs supplemented with high Spirulina levels have greater long term dietary protein intakes which would influence albumin levels and, in return, alter the A/G ratios, as shown. Merino-sired lambs’ divergence from other sire breed A/G ratio trends may have arisen from previously discussed differences in feed use efficiency.
Independent to Spirulina supplementation, sex and sire breed affected metabolite concentrations. Wethers generally have greater liveweights and muscularity than ewes [Citation32], hence the observed differences in creatinine, protein and glucose concentrations between wethers and ewes in our current study. Sex interacts with sire breed to affect liveweight and muscularity mainly by influencing feed use efficiency [Citation34] and growth potential [Citation35].
Creatinine concentration was outside the normal range and since it is associated with muscularity, this finding was expected as all experimental lambs were actively growing weaners whose liveweights and muscularity had not yet reached the levels expected in mature wethers and ewes.
Urea, the principal by-product of protein metabolism synthesised in the liver, is transported for excretion by the kidneys or for recycling in the gut [Citation5]. Hence, urea concentrations relate to dietary nitrogen supply [Citation36] and dietary protein intake. Therefore, increased provision of protein through Spirulina supplementation would have induced the observed findings. However, urea concentrations only represent short-term changes in dietary protein intake [Citation33].
Albumin concentrations are a function of dietary protein [Citation37]. Furthermore, globulin concentrations are typically calculated by subtracting albumin concentrations from total protein concentrations [Citation5]. This study found total protein concentrations to be within the normal range, therefore observed globulin concentrations would have resulted from elevated albumin concentrations.
Haematological chloride concentrations are useful indicators of animal hydration [Citation5]. Also, these concentrations are only indicative of the animal’s status at the time of sampling [Citation33]. Therefore, we can assume that the experimental lambs were probably slightly dehydrated during blood sampling.
In conclusion, our findings demonstrate that Spirulina supplementation can lower haematological GGT concentrations by 10 UI on the average, compared to unsupplemented lambs, but this difference varies depending on interactions between lamb sire breed and sex. Creatinine levels were indicative of muscularity and lambs supplemented at low Spirulina levels had the highest muscularity. High Spirulina supplementation levels resulted in the highest glucose concentrations indicative of available energy for driving protein metabolism and other metabolic pathways including gluconeogenesis. Furthermore, Spirulina supplementation, sex and sire breed interacted to affect glucose, AST, magnesium, A/G ratios, creatinine and GGT concentrations. These findings highlight the beneficial impact of Spirulina supplementation on lamb health and productivity, hence the acceptance of the tested hypothesis that Spirulina supplementation of crossbred and purebred Merino lambs will not be detrimental to health and productivity as indicated by haematological metabolite and electrolyte profiles. The fact that significant interactions between level of supplementation, sire breed and sex were the key drivers of observed variation in metabolite profiles, gives dual-purpose sheep farmers the flexible options of choosing appropriate sire breeds to match the level of Spirulina supplementation for optimal lamb productivity and health.
Acknowledgments
This research was funded by grants and postgraduate scholarships from the Australian Wool Education Trust (AWET) and The University of Tasmania (UTAS). We appreciate this financial assistance by AWET and UTAS. We are grateful to Arash Kashani, Chris Gunn, Will Bignell and Barrie Wells for their assistance during the sheep breeding and feeding trials.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- B.W.B.HolmanA.E.O.Malau-AduliSpirulina as a livestock supplement and animal feedJ Anim Physiol Anim Nutr9742013615623
- N.M.FogartyE.SafariA.R.GilmourV.M.InghamK.D.AtkinsS.I.MortimerWool and meat genetics – the joint possibilitiesInt J Sheep Wool Sci5420062227
- E.KopkeJ.YoungR.KingwellThe relative profitability and environmental impacts of different sheep systems in a Mediterranean environmentAgr Syst961–320088594
- ABARE, Agricultural commodities: June quarter 2012. Australian Bureau of Agricultural and Resource Economics and Sciences, Canberra; 2012.
- K.E.RussellA.J.RousselEvaluation of the ruminant serum chemistry profileVet Clin North Am Food Anim Pract2332007403426
- J.P.BraunC.TrumelP.BezilleClinical biochemistry in sheep: a selected reviewSmall Rumin Res921–320101018
- R.S.HegartyJ.R.McFarlaneR.BanksS.HardenAssociation of plasma metabolites and hormones with the growth and composition of lambs as affected by nutrition and sire geneticsAust J Agric Res5762006683690
- A.TrenkleRelation of hormonal variations to nutritional studies and metabolism of ruminantsJ Dairy Sci6131978281293
- C.H.LongD.E.UllreyE.R.MillerB.H.VincentC.L.ZutautSheep haematology from birth to maturity III. Serum calcium, phosphorus, magnesium, sodium and potassiumJ Anim Sci241965145150
- E.N.PonnampalamA.R.EganA.J.SinclairB.J.LeuryFeed intake, growth, plasma glucose and urea nitrogen concentration, and carcass traits of lambs fed isoenergetic amounts of canola meal, soybean meal, and fish meal with forage based dietSmall Rumin Res5832005245252
- C.MatsubaraY.NishikawaY.YoshidaK.TakamuraA spectrophotometric method for the determination of free fatty-acid in serum using acyl-coenzyme a synthetase and acyl-coenzyme a oxidaseAnalyt Biochem13011983128133
- SAS Institute, Statistical analysis system. Version 9.2., Cary: USA; 2009.
- R.BradshawS.HallD.BroomBehavioural and cortisol response of pigs and sheep during transportVet Rec138101996233234
- S.A.CenterInterpretation of liver enzymesVet Clin North Am Small Anim Pract3722007297333
- T.J.ByrneC.I.LudemannP.R.AmerM.J.YoungBroadening breeding objectives for maternal and terminal sheepLivest Sci1441–220122036
- L.M.CollaA.L.Muccillo-BaischJ.A.V.CostaSpirulina platensis effects on the levels of total cholesterol, HDL and triacylglycerols in rabbits fed with a hypercholesterolemic dietBraz Arch Biol Technol512008405411
- M.F.IsmailD.A.AliA.FernandoM.E.AbdrabohR.L.GaurW.M.IbrahimChemoprevention of rat liver toxicity and carcinogenesis by SpirulinaInt J Biol Sci542009377387
- B.W.B.HolmanA.KashaniA.E.O.Malau-AduliGrowth and body conformation responses of genetically divergent Australian sheep to Spirulina (Arthrospira platensis) supplementationAm J Exp Agric222012160173
- P.G.HatfieldJ.A.HopkinsW.S.RamseyA.GilmoreEffects of level of protein and type of molasses on digestion kinetics and blood metabolites in sheepSmall Rumin Res2821998161170
- A.E.O.Malau-AduliR.E.WalkerC.W.BignellVariation in sire genetics is an irrelevant determinant of digestibility in supplemented crossbred sheepY.ChilliardInternational symposium on ruminant physiology2009Wageningen Academic PublishersClermont-Ferrand
- P.B.CronjeSupplementary feeding in ruminants – a physiological approachS Afr J Anim Sci2031990110117
- N.FilipovicZ.StojevicT.MasekZ.MikulecN.PrvanovicRelationship between fructosamine with serum protein, albumin and glucose concentrations in dairy ewesSmall Rumin Res96120114648
- E.F.AnnisonR.R.WhiteGlucose utilisation in sheepBiochem J8011961162169
- T.J.KemptonR.A.LengGlucose metabolism in growing lambsTrop Anim Prod841983244253
- A.TrenkleEffects of short-chain fatty acids, feeding, fasting and type of diet on plasma insulin levels in sheepJ Nutr10011197013231331
- G.H.GeesinkH.ZerbyMeat productionD.J.CottleInternational sheep and wool handbook2010Nottingham University PressNottingham395406
- C.H.LongD.E.UllreyE.R.MillerB.H.VincentC.L.ZutautSheep haematology from birth to maturity 3. Serum calcium, phosphorus, magnesium, sodium and potassiumJ Anim Sci2411965145152
- Phua SH, Johnstone P, Henry H, Findlay A, Morris CA. Sheep selected for resistance to facial eczema disease also show higher tolerance to acetaminophen challenge. In: Matching genetics and environment: a new look at an old topic. Proceedings of the 18th conference of the association for the advancement of animal breeding and genetics, Barossa Valley, p. 636–39.
- P.G.HatfieldJ.A.HopkinsW.S.RamseyA.GilmoreEffects of level of protein and type of molasses on digesta kinetics and blood metabolites in sheepSmall Rumin Res2821998161170
- E.N.PonnampalamD.L.HopkinsK.L.ButlerF.R.DunsheaR.D.WarnerGenotype and age effects on sheep meat production 2. Carcass quality traitsAust J Exp Agric4710200711471154
- P.G.AllinghamG.E.GardnerM.TaylorR.S.HegartyG.S.HarperEffects of sire genotype and plane of nutrition on fascicular structure of M-longissimus thoracis et lumborum and its effect on eating qualityAust J Agric Res5762006641650
- G.H.ScalesA.R.BrayD.B.BairdD.O’ConnellT.L.KnightEffect of sire breed on growth, carcass, and wool characteristics of lambs born to Merino ewes in New ZealandN Zeal J Agr Res431200093100
- N.D.SargisonP.R.ScottThe implementation and value of diagnostic procedures in sheep health managementSmall Rumin Res921–3201029
- A.E.O.Malau-AduliC.F.RansonC.W.BignellWool quality and growth traits of Tasmania pasture-fed crossbred lambs and relationships with plasma metabolitesJ Anim Sci872009499
- A.B.S.SobrinhoI.T.KadminR.W.PurchasEffect of genotype and age on carcass and meat quality of ram lambsJ Agr Mar Sci8220037378
- G.B.HuntingtonS.L.ArchibequePractical aspects of urea and ammonia metabolism in ruminantsJ Anim Sci772000111
- B.K.I.MeijersB.BammensK.VerbekeP.EvenepoelA review of albumin binding in CKDAm J Kidney Dis5152008839850
- D.UndersanderJ.E.MooreRelative forage qualityFocus Forag42200212
- J.NobletJ.M.PerezPrediction of digestibility of nutrients and energy values of pig diets from chemical analysisJ Anim Sci7112199333893398
