Abstract
The pharmacokinetic profile of cefoperazone was studied in goats following intravenous and intramuscular administration of 20 mg/kg body weight. Cefoperazone concentrations in serum were determined by microbiological assay technique using Escherichia coli (ATCC 10536) as test organism. Following i.v. administration, the cefoperazone serum concentration–time curve was best fitted in a two compartment open model. Cefoperazone has moderate distribution in the body of goats with Vdss of 0.44 ± 0.03 L/kg. The elimination half-life (T0.5(β)), area under curve (AUC) and total body clearance (Cltot) were 1.97 ± 0.14 h, 149.63 ± 8.61 μg ml−1 h−1, and 2.17 ml/min/kg, respectively. Following i.m. administration, the drug was very rapidly absorbed, with an absorption half-life (T0.5(ab)) of 0.12 ± 0.01 h. The maximum serum concentration (Cmax) of 30.42 ± 3.53 μg ml−1 was attained at (Tmax) 0.58 ± 0.02 h, with an elimination half-life (T0.5(el)) of 2.53 ± 0.11 h. The systemic bioavailability of cefoperazone in the goats after i.m. administration was 83.62% and in vitro protein binding was 20.34%. The serum concentrations of cefoperazone along 12 h post i.m. injection in this study were exceeding the MIC of different susceptible micro-organisms responsible for serious disease problems. Consequently, a suitable intramuscular dosage regimen for cefoperazone was 20 mg/kg repeated at 12 h intervals in goats. The drug was detected in urine up to 12 and 18 h following i.v. and i.m. administration, respectively.
Keywords:
1 Introduction
Cefoperazone is a semi-synthetic third generation, piperazine β-lactam antibiotics that possesses broad spectrum activity against aerobic and anaerobic gram-positive and gram-negative bacteria [Citation1]. Cefoperazone is used in the treatment of bone and joint infections of horses [Citation2], calf diseases such as diarrhea and pneumonia [Citation3] and has good penetration into the pancreas indicating its usefulness for the prophylaxis and therapy of secondary pancreatic infections [Citation4]. Only a few cephalosporins have a high biliary excretion, cefoperazone being one of them. Cefoperazone exhibits a longer half-life of elimination than older members of the group [Citation5] and good penetration into organic bone [Citation6]. The pharmacokinetics of cefoperazone had been investigated in a number of animal species including unweaned calves [Citation7], horse [Citation8], dog [Citation9], buffalo calves [Citation10,Citation11], cross bred calves [Citation12,Citation13] and sheep [Citation14]. The aim of the study was to determine the pharmacokinetic parameters of cefoperazone following a single intravenous and intramuscular administration at the dose of 20 mg/kg b.wt. in goats.
2 Materials and methods
2.1 Drugs and chemicals
Cefoperazone sodium powder (CEFOBID®, produced by Smithkline Beecham Egypt LLc for Pfizer Egypt) was diluted with sterile water just prior to administration. Mueller–Hinton agar was purchased from Mast Group Ltd., Merseyside, UK.
2.2 Animals
Ten clinically normal goats were used in this investigation. The body weight and age ranged from 23 to 31 kg and from 2 to 3 years old, respectively. Animals were housed in hygienic stable, fed on barseem, drawa and concentrate. Water was provided ad libitum. None of the animals were treated with antibiotics for one month prior to the trial.
2.2.1 Experimental design
The study was performed in two phases, following a crossover design (2 × 2) with a 15-day washout period between the two phases. Five animals were given a single i.v. injection into the left jugular vein at a dose of 20 mg/kg body weight (b.wt.) cefoperazone, and the other five were injected i.m. into the gluteal muscle with the drug at the same dose. Five milliliter venous whole blood samples were taken. The sampling times were 0.08, 0.166, 0.25, 0.5, 1, 2, 4, 6, 8, 12, 18 and 24 h after treatment. Blood samples were left to clot; the clear sera were separated by centrifugation at 3000 r.p.m for 15 min and stored at −20 °С until assayed. After washout period of 15 days, the animals that had been injected i.v. with the drug were injected i.m. and vice versa. Blood was collected and processed as above. Goats were catheterized with an indwelling balloon catheter (Foley Urinary Catheter, No. 12, Timedco, Atlanta, GA, USA). 5 ml urine samples were collected at 0.5, 1, 2, 4, 6, 8, 12, 18, 24, 36 and 48 h after administration of the drug. The samples stored at −20 °С until assayed.
2.2.2 Drug bioassay
Concentrations of cefoperazone in samples were determined by the microbiological assay method described by [Citation15] using Escherichia coli (ATCC 10536) as test organism [Citation13]. This method estimated the level of drug having antibacterial activity, without differentiating between the parent drug and its active metabolites. The application of microbiological assay for measuring cefoperazone concentration is suitable [Citation13]. Six wells were made at equal distances in standard petri-dishes containing 25 ml seeded agar. The wells were filled with 100 μl of either the test samples or the cefoperazone standard concentrations. The plates were kept at room temperature for 2 h before being incubated at 37 °C for 18 h. Zones of inhibition were measured using micrometers and the cefoperazone concentrations in the test samples were calculated from the standard curve. Cefoperazone standard solution of concentrations of 0.5–100 μg/ml were prepared in antibiotic-free goats serum and phosphate buffer saline. Standard curves of cefoperazone were prepared in antibacterial-free goat serum by the appropriate serial dilution. The standard curve in goat serum was linear over the range from 0.5 to 100 μg/ml and the value of correlation coefficient (r) was 0.991. The limit of quantification was 0.5 μg/ml. Protein binding of cefoperazone was estimated according to [Citation16].
2.3 Pharmacokinetic analysis
A pharmacokinetic computer program (R-strip, Micro-math, Scientific software, USA) was used to analyse the concentration–time curves for each individual animal after the administration of cefoperazone by different routes. Following i.v. and i.m. administrations, the appropriate pharmacokinetic model was determined by visual examination of individual concentration–time curves and by application of Akaike’s information criterion (AIC) [Citation17]. The pharmacokinetic parameters were reported as mean ± SE. All statistical analysis was carried out according to [Citation18].
3 Results
Mean serum concentrations of cefoperazone in goats following i.v. and i.m. administrations of 20 mg/kg are summarized in (). These data are best fitted to a two-compartment open model and the drug was detected in serum up to 8 and 12 h following i.v. and i.m. administration, respectively. The pharmacokinetic parameters of cefoperazone in goats following i.v. and i.m. administration of 20 mg/kg are summarized in ( and ). Following i.v. administration, cefoperazone has moderate distribution in the body of goats with Vdss of 0.44 ± 0.03 L/kg. Cefoperazone was rapidly eliminated (T0.5(β): 1.97 ± 0.14 h) from the body. Following i.m. administration, the drug was very rapidly absorbed with a short absorption half life T0.5(ab) of 0.12 ± 0.01 h. The mean peak serum concentration (Cmax) was 30.42 ± 3.53 μg ml−1 achieved at (Tmax) 0.58 ± 0.02 h. The systemic bioavailability of cefoperazone in the goats after i.m. administration was 83.62%. In vitro protein binding was 20.34%. Mean urine concentrations of cefoperazone in goats following i.v. and i.m. administration of 20 mg/kg are summarized in ().
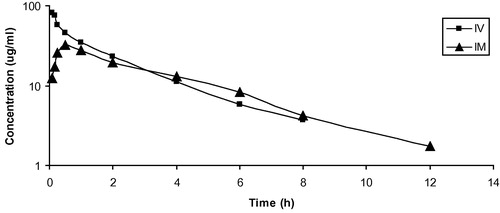
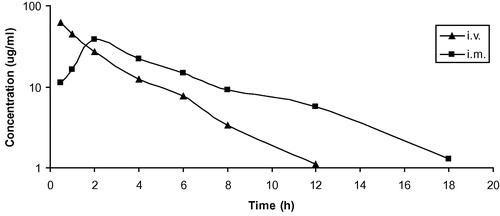
Table 1 Mean (±SE) kinetic parameters of cefoperazone (20 mg/kg) following a single intravenous injection in goats (n = 10).
Table 2 Mean (±SE) kinetic parameters of cefoperazone (20 mg/kg) following a single intramuscular injection in goats (n = 10).
4 Discussion
Following i.v. administration of cefoperazone in goats at a dose of 20 mg/kg, no adverse effects or toxic manifestation was observed. The results revealed that serum cefoperazone concentration versus time decreased in a bi-exponential manner, demonstrating the presence of distribution and elimination phases and justifying the use of two-compartment open model. This finding is in agreement with cefoperazone in horse [Citation8], in dog [Citation9] and in cross bred calves [Citation12]. The drug was rapidly distributed with a short T0.5(α): 0.15 h. This value was close to those reported in calves T0.5(α): 0.15 h [Citation19] and dog T0.5(α): 0.20 h [Citation9], shorter than those reported in sheep T0.5(α): 0.53 h [Citation14].
The elimination half-life and MRT were 1.97 and 2.15 h, respectively, the results were near to those reported in cross bred calves 2.05 and 2.28 h, respectively [Citation12] and in dog 1.40 and 1.55 h, respectively [Citation9], shorter than those reported in sheep 3.80 and 3.29 h, respectively [Citation14]. The value of Vdss (0.44 L/kg) indicated moderate extravascular distribution of the drug. This value was close to those reported in sheep Vdss: 0.51 L/kg [Citation14]. The total body clearance of cefoperazone following i.v. administration was 2.17 ml/min/kg. The value was close to those reported in dog 1.96 ml/min/kg. [Citation9], while it is slower than 5.16 ml/min/kg reported in sheep [Citation14], 8.16 ml/min/kg reported in unweaned calves [Citation7] and 11.50 ml/min/kg reported in cross bred calves [Citation12].
Following i.m. administration of cefoperazone in goats at a dose of 20 mg/kg, the drug was very rapidly absorbed with a short absorption half life T0.5(ab) of 0.12 h. This value was shorter than those reported in dog 0.48 h [Citation9]. The maximum serum concentration (Cmax) of 30.42 μg ml−1 was attained at (Tmax) 0.58 h post administration. However, lower value of Cmax like 25.67 μg ml−1 at 0.5 h in sheep [Citation14], 24.5 μg ml−1 at 1.5 h in dog [Citation9] and 9.76 μg ml−1 at 0.75 h in cross bred calves [Citation13] had been reported.
The elimination half-life of cefoperazone following i.m. administration was 2.53 h. This value was close to those reported in unweaned calves 2.28 h [Citation7], dog 2.24 h [Citation9] and cross bred calves 2.31 h [Citation13]. While it is shorter than value reported in sheep 3.32 h [Citation14]. The mean residence time (MRT) was 3.34 h. This value was similar to those reported in cross bred calves 3.62 h [Citation13]. However it is shorter than value reported in sheep 4.27 h [Citation14] and dog 4.05 h [Citation9], but longer than value recorded in unweaned calves 2.34 h [Citation7].
The value of systemic bioavailability (83.62%) indicated good absorption of cefoperazone from i.m. injection site. This value was higher than those reported in unweaned calves 76.3% [Citation7] and sheep 71.83% [Citation14]. While this value was close to those reported for cefepime in goats 86.45% [Citation20] and ewes 86.8% [Citation21]. High bioavailability of cefoperazone and maintenance of therapeutic concentration up to 12 h after i.m. administration suggests that the drug is suitable for i.m. administration for the treatment of systemic bacterial infections in goats. In vitro protein binding was 20.34%, compared with 24.9% reported in cross bred calves [Citation13].
The serum level of ⩾0.2 μg ml−1 for third generation cephalosporin is considered adequate against most sensitive bacteria, including Enterobacteriaceae Spp. [Citation22]. However, a serum concentration of 0.25–2.0 μg ml−1 has been reported as MIC90 of cephalosporin against animal pathogens [Citation23]. In the present study an average value of MIC (1.0 μg ml−1) has been taken into consideration. The drug was detected above MIC in serum up to 8 and 12 h following i.v. and i.m. administration, respectively.
Cefoperazone was detected in urine at high concentrations following i.v and i.m. administration for 12 and 18 h, respectively. The detected concentrations of the drug in urine exceed the MIC90 for all bacterial pathogens responsible for urinary tract infections in goats. The result suggests the possibility of using cefoperazone in treatment of such disease conditions in goats.
5 Conclusion
A cefoperazone dose of 20 mg/kg is sufficient to maintain serum concentration of the drug above the MIC90 when it is administered at 8 and 12 h interval following intravenous and intramuscular administration, respectively. Consequently, cefoperazone can be used safely and effectively for treat the infections in goats.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- T.B.BarragryBeta-lactam antibioticsVeterinary drug therapy1994Lea & FebigerPhiladelphia, PA [p. 221–40]
- A.L.SoraciN.MestorinoJ.ErrecaldeConcentration of cefoperazone in synovial fluid and in bone after intramuscular administrationRevist Med Vet Buen Aire7911998148150
- S.SobackA.BorR.PazG.ZivClinical pharmacology of mecillinam in calvesJ Vet Pharmacol Ther911986385393
- L.JiangQ.PengY.YaoPenetration of ciprofloxacin and cefoperazone into human pancreasHua Xi Yi Ke Da Xue Xue Bao2821997365368
- T.BerganPharmacokinetic properties of the cephalosporinsDrugs342198789104
- Wittman D. “Bone concentrations of six new betalactam antibiotics”, 6th International cefoperazone symposium. Program and abstracts. April–May Tokyo; 1982. p. 9.
- S.SobackG.ZivPharmacokinetics of single doses of cefoperazone given by the intravenous and intramuscular routes to unweaned calvesRes Vet Sci4711989158163
- A.L.SoraciN.MestorinoJ.ErrecaldePharmacokinetics of cefoperazone in horsesJ Vet Pharmacol Ther19119963943
- C.MontesissaR.VillaP.AnfossicR.ZanonicS.CarlibPharmacodynamics and pharmacokinetics of cefoperazone and cefamandol in dogs following single dose i.v. and i.m. administrationVet J16612003170176
- S.GoyalR.K.ChaudharyA.K.SrivastavaPharmacokinetics of cefoperazone in buffalo calves following its intravenous administrationJ Vet Pharmacol Toxicol312003104108
- S.GoyalR.K.ChaudharyA.K.SrivastavaPharmacokinetics following intramuscular administration and dosage regimen for cefoperazone in buffalo calvesIndian J Anim Sci75120053132
- J.K.GuptaR.K.ChaudharyV.K.DumkaCefoperazone pharmacokinetics following single intravenous administration in cross bred calveIsr J Vet Med62120078790
- J.K.GuptaR.K.ChaudharyV.K.DumkaPharmacokinetics after single intramuscular administration and in vitro plasma protein binding of cefoperazone in cross bred calvesVet Arhiv7822008441448
- H.H.SoniR.J.PatelK.A.SadariyaS.S.DevadaS.K.BhavsarA.M.ThakerSingle dose pharmacokinetics of cefoperazone following intravenous and intramuscular administration in sheepIsr J Vet Med6742012220224
- B.ArretD.P.JohnsonA.KirshboumOutline of details for microbiological assay of antibiotics, second revisionPharmacol Sci6011197116891694
- A.W.CraigB.SuhProtein binding and the antibacterial effects: methods for determination of protein bindingV.LorianAntibiotics in laboratory medicine1980Williams and WilkinsBaltimore, MD265297
- K.YamaokaT.NakagawaT.UnoStatistical moment in pharmacokineticsJ Pharmacokinet Biopharm661978547558
- Snedecor GW, Cochran T. “Statistical methods”. 6th ed. Ames, lowa U.S.A.; 1976. p.502–3.
- S.CarliC.MontesissaO.SonzogniM.MadonnaPharmacokinetics of sodium cefoperazone in calvesPharmacol Res Commun1861986481490
- S.PrawezR.RainaD.DimitrovaN.PankajA.AhangerP.VermaThe pharmacokinetics of cefepime in goats following single-dose i.v. and i.m. administrationTurk J Vet Anim Sci3412010427431
- M.IsmailPharmacokinetics of cefepime administered by i.v and i.m. routes to ewesJ Vet Pharmacol Ther2812005499503
- S.BarriereJ.FlahertyThird generation cephalosporins: a critical evaluationClin Pharmacol311984351373
- A.W.CraigPharmacokinetic/pharmacodynamic parameters, rationale for antibacterial dosing of mice and menClin Infect Dis2611998112
