Abstract
The main objective of this study was to evaluate the antimicrobial activity and anti-inflammatory activity of marine actinomycetes extract against ocular pathogen Pseudomonas aeruginosa. Actinomycetes isolated from Rameswaram coastal region, Tamilnadu, India were initially screened by primary screening and secondary screening against ocular pathogen P. aeruginosa. Followed by anti-conjunctivitis efficacy of actinomycetes ethyl acetate extract formulation versus ciprofloxacin ophthalmic solution was evaluated using rabbit as animal model. The bioactive compounds present in the best actinomycetes extract was identified by HPTLC and GC–MS analysis. Finally the screened best actinomycetes was identified by 16S rRNA sequencing method. In primary screening 28 actinomycetes that inhibited the growth of P. aeruginosa were taken for secondary screening. In secondary screening RAM24C2 extract had maximum activity against P. aeruginosa. In vivo study of conjunctivitis developed rabbits treated with RAM24C2 extract formulation showed the best clinical cure than ciprofloxacin ophthalmic solution. The RAM24C2 extract was chromatographically characterized and found to contain macrolides. In addition, the effective major pivotal molecule in the extract was detected as 1, 2 benzene dicarboxylic acid and Bis (2-ethylhexyl) phthalate by GC–MS analysis. The RAM24C2 strain was identified as Streptomyces sp. MAD01 and the sequence was submitted in NCBI with accession number JX050218. From our study it is found that the ethyl acetate extract obtained from marine actinomycetes is effective against ocular pathogen P. aeruginosa. Compared to ciprofloxacin ophthalmic solution our RAM24C2 extract formulation hastens the cure of conjunctivitis developed rabbits and need less dosage frequency.
1 Introduction
Conjunctivitis is a prevalent disease all over the world especially higher rate of infection was found in developing countries. Every people at least once in their lifetime gets affected by conjunctivitis. Conjunctivitis commonly described by red eye which shows symptoms like conjunctival congestion, eyelid edema, irritation in eye with discharge, sometimes in the morning difficult to open the eye due to sticking of the eyelid together by discharge. Conjunctiva is a mucous membrane which covers the bulbar conjunctiva and palpebral conjunctiva and safeguard the eye against invading microorganisms. Conjunctivitis caused by various external factors like bacteria, virus and fungi. Among these 80% of conjunctivitis occurred by bacteria [Citation1]. Among the different bacteria Pseudomonas aeruginosa is one of the common pathogen causing conjunctivitis with severe infection including capable of producing different toxins and proteases thereby damages ocular tissues [Citation2].
There are many topical antibiotics are available to cure for P. aeruginosa conjunctivitis. Fluoroquinolones like ciprofloxacin, ofloxacin, levofloxacin and aminoglycosides like tobramycin, gentamicin and some other antibiotics like polymyxin B and neomycin are used as ophthalmic eye drops in different concentration. These topical antibiotics possessed broad spectrum activity as antimicrobial agents. But the resistance problem of ophthalmic pathogens, side effects and expensive of antibiotics making the researchers to search for new antibiotics as ophthalmic solution. Especially P. aeruginosa highly resistant to most of the antibiotics [Citation3,Citation4].
In this regard here we have taken marine actinomycetes for our research purpose. Because marine actinomycetes reveals as source of natural products for human ailments. Marine actinomycetes exhibit different biological activity especially broad spectrum activity against gram positive and gram negative bacteria. Among marine actinomycetes, Streptomyces are the chief producer of novel antibiotics. Each day many new antibiotics are identified from actinomycetes. Among actinomycetes 80% of antibiotics were derived from Streptomyces family [Citation5,Citation6].
The present study was carried out to evaluate the biological activity (anti-conunctivitis) of actinomycetes isolated from marine coastal regions of Rameswaram, Tamilnadu, India. Their potential to control ocular pathogen P. aeruginosa both in vitro and in vivo animal model was analyzed. The efficacy of actinomycetes extract formulation, with available quinolone ophthalmic solution ciprofloxacin was also compared.
2 Materials and methods
2.1 Isolation and identification of Actinomycetes
Marine soil samples were collected from coastal regions of Rameswaram, Tamilnadu, India. Selective isolation of actinomycetes from soil samples was carried out by spread plate technique using Actinomycetes isolation agar medium. The isolation medium was supplemented with 0.2 mg/ml fluconazole [Citation7] and 20 mg/l nalidixic [Citation8] to inhibit fungal and bacterial colonization respectively. After incubation, actinomycetes colonies appeared on the agar medium were pure cultured by restreaking in the same agar medium and used for further assays.
2.2 Bacterial culture
The authenticated ophthalmic pathogen P. aeruginosa obtained from Aravind eye Hospital, Coimbatore, Tamilnadu, India. The pathogen was isolated form clinical specimen in Clinical Microbiology laboratory of the hospital.
2.3 Antibiotic susceptibility of the test organism
The antibiogram pattern of the ophthalmic pathogen P. aeruginosa was checked against the commercially available antibiotic discs (Himedia) using the standard disc diffusion test [Citation9]. The log phase culture broth was seeded in Muller Hinton agar plate by sterile swab. The antibiotics discs with the following concentrations were placed in equidistant with uniform contact between the antibiotic discs and the surface of the Muller Hinton agar plates, Amoxyclav (30 mcg), Ceftazidime (30 mcg), Chloramphenicol (30 mcg), Gentamicin (10 mcg), Nalidixic acid (30 mcg), Nitrofurantoin (30 mcg), Norfloxacin (10 mcg), Tetracycline (30 mcg), Kanamycin (30 mcg), Imipenem (15 mcg), Ampicillin (15 mcg) and Ciprofloxacin (15 mcg). The plates were then incubated at 37 °C in upright position for overnight. Inhibition zone diameters were measured and tabulated.
2.4 Screening of actinomycetes for antimicrobial activity
The primary screening of all 131 actinomycetes isolates were done by streaking perpendicular to the ophthalmic pathogen P. aeruginosa with four isolates streaked parallel per petridishes. The zones of inhibition were measured and tabulated [Citation10]. The actinomycetes isolates that which exhibited good antibiosis effect against P. aeruginosa were pure cultured respectively in culture flask. The culture was extracted by solvent extraction method [Citation11] using ethyl acetate as solvent. The actinomycetes culture extracts were secondary screened for their antagonistic activity against ophthalmic pathogen. The secondary screening was done by well diffusion method.
2.5 Determination of stability and shelf life of RAM24C2 ethyl acetate extract
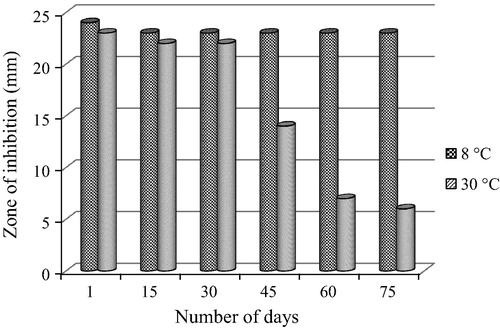
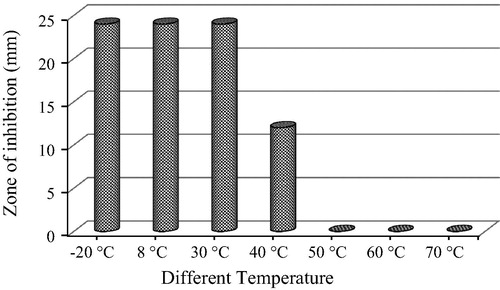
The best actinomycetes culture extract that had maximum antagonistic activity against ophthalmic pathogen was subjected for stability and shelf life test. The extract was aliquot into 7 screw cap vials with 5 ml each. These tubes were respectively incubated for an hour at different temperatures (−20 °C, 8 °C, 30 °C, 40 °C, 50 °C, 60 °C and 70 °C) maintained in deep freezer, fridge and in a water baths respectively. After incubation period the extracts were checked for their antagonistic activity by well diffusion method. Similarly, for the shelf life expectancy study 2 sets of 6 aliquots of 5 ml extract in vials were stored respectively in refrigerated condition (8 °C) and at ambient temperature (30 °C). Each aliquot was checked for its antagonistic activities at every 15 days’ time interval starting from 0th day to 75th day [Citation12].
2.6 Actinomycetes extract preparation
The marine actinomycetes isolates were inoculated in 50 ml of ISP2 broth and incubated at 30 °C, 150 rpm in orbital shaking incubator for two days. After incubation period 20 ml of the actinomycetes cultures were inoculated in 300 ml of ISP2 broth in shake flask and incubated at 30 °C in an orbitary shaker at 150 rpm for 7 days. The grown culture was centrifuged and the culture filtrate was subjected for solvent extraction for recovery of secondary metabolites. The solvent ethyl acetate was added to the filtrate in the ratio of 1:1 (v/v) and shaken vigorously for complete extraction. Two layers aqueous and the organic layer were separated, the solvent ethyl acetate layer was collected and aqueous layer was discarded. Extraction was continued up to three times with the same solvent and the solvent layer was accumulated and concentrated by rotary vacuum evaporator. The residue is weighed and resuspended in buffer, this was used as ophthalmic suspension [Citation6].
2.7 In vivo animal model
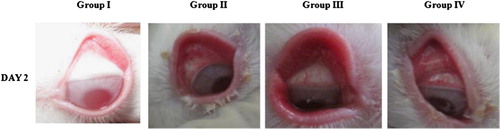
To analyse the anti-inflammatory efficiency of the actinomycete extract the anticonjunctivitis study was done in the eye balls of young New Zealand albino male rabbits, weighing between 2 and 2.5 kg. The conjunctivitis animal model studies experimental protocol was subjected to Institutional animal ethical committee and obtained certificate No (IAEC/KU/BT/13/10). The designed study has 4 groups with 2 animals in each group and used both eyes of each animal. From the acclimatized ten rabbits housed for study two were caged uninfected and named group I (uninfected). Both eyeballs of other eight animals were individually infected with the log phase culture of clinical isolate P. aeruginosa log phase culture. Holding the animal at ease the lower eyelid were gently made as cavity into which 50 μl of 8 h old log phase culture of P. aeruginosa containing approximately 100 CFU/ml was instilled and holded for few seconds and ensured the culture spreaded on the entire eyeball and not spilled out.
After 24 h the 8 infected rabbits eyeball were examined, six animals who’s both eyeballs have inflammated (with reddening) were chosen and randomized into 3 groups [Citation13].
Group II: treated with normal sterile saline (Negative control/untreated group).
Group III: treated with commercial eye drop containing (0.3% W/V) ciprofloxacin (Positive control).
Group IV: treated with actinomycete extract resuspended in saline (Test group).
Treatment was initiated from 2nd day that is after this infection development (Day 2).
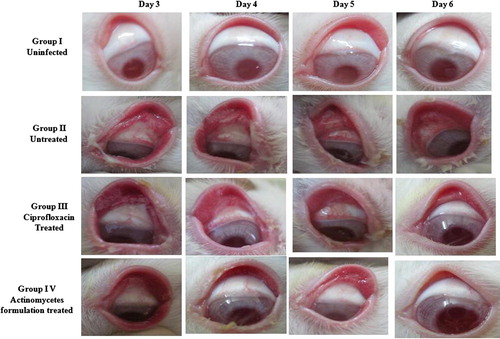
From day 2 to day 6 all these 3 groups received treatment respectively with 50 μl/eyeball. Before administration of drug the rabbit’s eyeballs were observed daily for ocular inflammation. Scoring was done by observing the curing and symptoms like mucopurulent discharge, chemosis, conjunctival congestion and palpebral fissure ().
Table 1 Scoring of ocular inflammation based on conjunctivitis symptoms.
2.8 Statistical analysis
The ophthalmic scores were expressed as Mean ± SD. The data was evaluated by one way ANOVA followed by Dunnett’s T-Test for comparison of multiple variations using SPSS/16 software. The values ∗P < 0.05 and ∗∗P < 0.01 were considered as significant.
2.9 GC–MS and HPTLC analysis
The RAM24C2 ethyl acetate extract was checked for bioactive compounds by GC–MS analysis and HPTLC profile analysis for macrolides, quinolones and terpenoids. The Gas Chromatography and Mass Spectrometry was analysed by GC–MS electron impact ionization (EI) method on GC-8000 gas chromatograph (FISONS Instruments) coupled to a MD-800 Mass Spectrometer (FISONS Instruments). Compound identification was done by comparing the NIST (National Institute of Standards and Technology-Chemistry web book by WILEY) library data of the peaks with those reported in literature. The HPTLC analysis was performed using Hamilton syringe and CAMAG LINOMAT 5 instrument, CAMAG REPROSTAR 3 photo-documentation chamber and CAMAG TLC SCANNER 3.
2.10 Strain identification at molecular level
16S rRNA analysis was performed for RAM24C2 isolate. The actinomycete genomic DNA was isolated using commercial kit then run in Applied biosystem veriti 96well thermo cycler PCR machine. Finally, the sequencing done in ABI 3730XL Genetic Analyser with sequence scanner V1.0 applied biosystem (By Eurofins Genomics India PVT. Ltd., India). The sequence of isolate was compared by alignment against 16S rRNA sequences, available in the GenBank Database using the BLAST program. Alignment and similarity comparison were initially conducted by the Clustal W method [Citation14] and the phylogenetic tree was constructed using Clustal W with the neighbour joining method. A boost strap analysis of 1000 replicates was carried out using MEGA 5.05 software.
3 Results
3.1 In vitro antimicrobial activity
Out of 131 actinomycetes isolated from Rameswaram coastal region, Tamilnadu, India, 28 actinomycetes (21%) were able to control the growth of gram negative ocular pathogen P. aeruginosa in primary screening while the remaining 79% actinomycetes did not exhibit antimicrobial activity against P. aeruginosa. Based on the primary screening results the 28 active actinomycetes ethyl acetate extracts were prepared and subjected to secondary screening to evaluate its antimicrobial activity. Out of 28 actinomycetes four actinomycetes ethyl acetate extracts displayed maximum antimicrobial activity. Among the four actinomycetes extracts, the highest antimicrobial activity against P. aeruginosa was observed in RAM24C2 extract (24 mm) followed by RAM25B4 (16 mm), RAM23C1 (14 mm) and RAM25C4 (14 mm). The isolate RAM24C2 which highly active against ocular pathogen in vitro was taken for next study.
3.2 Antibiotic susceptibility testing of P. aeruginosa
The ophthalmic clinical isolate P. aeruginosa was evaluated for its antibiotic susceptibility pattern against twelve antibiotics. P. aeruginosa was highly sensitive to imipenem with a zone of inhibition 26 mm followed by ciprofloxacin (23 mm). But it was resistant to many antibiotics tested like nitrofurantoin, norfloxacin, amoxyclav, ampicillin, tetracycline and kanamycin. But some antibiotics like, ceftazidime, chloramphenicol, gentamycin and nalidixic acid showed intermediate response to the ocular pathogen P. aeruginosa. Our actinomycetes extract RAM24C2 exhibited better activity than the commercial antibiotics nitrofurantoin, amoxyclav, ampicillin, tetracycline, kanamycin, Ceftazidime, gentamycin and nalidixic acid. RAM24C2 extract displayed greater activity than ciprofloxacin and activity equivalent with imipenem antibiotic ().
Table 2 Antibiotic susceptibility testing of ocular pathogen Pseudomonas aeruginosa.
3.3 Determination of thermal inactivation point of RAM24C2 ethyl acetate extract
The RAM24C2 ethyl acetate extract retained its antimicrobial activity against P. aeruginosa up to 40 °C. The optimum temperature for storage was observed between −20 °C and 30 °C. But at 40 °C reduction in the antimicrobial activity was observed up to 50%. The extract had no activity above 40 °C ().
3.4 Shelf life of RAM24C2 ethyl acetate extract
The RAM24C2 ethyl acetate extract withhold its antimicrobial activity for the whole testing period (75 days at 8 °C). So we can infer that the extract was stable at 8 °C for the entire testing period. But the extract stored at 30 °C withhold its activity for 30 days. The extract stored at 30 °C after 30 days exhibited loss of antimicrobial activity against P. aeruginosa ().
3.5 Anti-inflammatory activity of RAM24C2 ethyl acetate extract formulation
On the second day after infection with P. aeruginosa the rabbit’s eyeballs developed beefy red conjunctiva, mucopurulent discharge, palpebral fissure and chemosis. There was no much difference observed among the group II, III and IV on the second day after infection (). The infection was severe in rabbits especially in the conjunctival congestion but the symptom like mucopurulent discharge formation was less. The treatment was started from the second day onwards. The RAM24C2 extract formulation was effective in controlling P. aeruginosa induced conjunctivitis while compared to ciprofloxacin ophthalmic solution.
Based on the clinical score results () we infer that after first day of treatment (day 3) the mucopurulent discharge was reduced between treated groups. But there is no reduction in other symptoms like chemosis, palpebral fissure and conjunctival congestion in treated groups. After, two days of treatment (day 4) significant reduction in inflammation was observed in treatment groups. In ciprofloxacin treated group the mucopurulent discharge formation was less; the beefy red conjunctiva changed to bright red conjunctiva, showed obvious conjunctival edema with eversion of eyelid and palpebral fissure was in moderate level. Whereas in RAM24C2 formulation treated group the mucopurulent discharge formation was absent, showed minimal congestion, palpebral fissure was moderate and minimal edema of the conjunctiva was observed. While comparing to ciprofloxacin treated group, the RAM24C2 formulation treated group showed effective clinical cure after 2 days of treatment itself (4th day). After three days of treatment (day 5), the ciprofloxacin treated group showed absence of mucopurulent discharge, minimal congestion, mild palpebral fissure and minimal edema of the conjunctiva whereas RAM24C2 formulation treated group minimal edema of the conjunctiva and mild palpebral fissure observed. After four days of treatment (day 6) the complete clinical cure was observed in the RAM24C2 formulation treated group whereas in ciprofloxacin treated group mild palpebral fissure observed ().
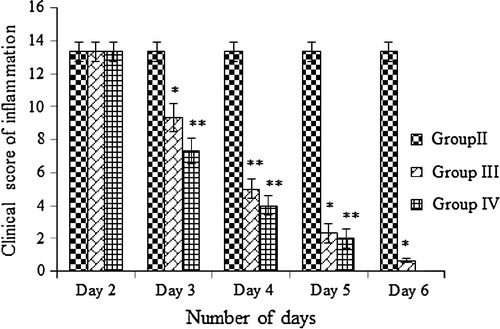
From our study results for comparing the efficacy of the curing of bacterial conjunctivitis using RAM24C2 extract formulation in comparison with standard drug ciprofloxacin; it is observed that 50 μl actinomycetes formulation (RAM24C2) administered twice daily for four days after infection maybe efficacious in the complete treatment of P. aeruginosa induced conjunctivitis. While the same dosage of ciprofloxacin treated group, the inflammation subsidized only after five days of treatment. In the actinomycetes formulation treated group the mucopurulent discharge disappeared even in the 1st day of treatment itself.
3.6 HPTLC and GC–MS analysis of RAM24C2 extract
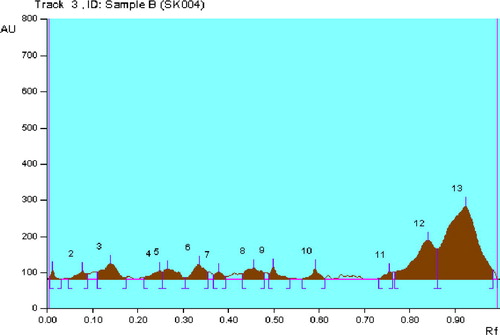
The ethyl acetate extract of RAM24C2 strain checked for macrolides (erythromycin), terpenoids (solanesol) and quinolone (nalidixic acid) profile by HPTLC. Brown and brownish violet colour zones at UV 366 nm was present in the standard track and the RAM24C2 ethyl acetate extract track, it was observed from the chromatogram after derivatization which confirmed the presence of macrolide in the standard track and also in the ethyl acetate extract track ( and , ). But terpenoids and quinolones were absent in the extract.
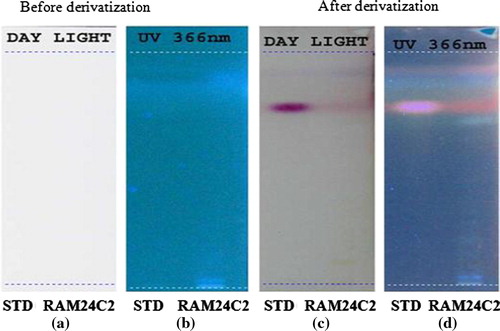
Table 3 Peak table for macrolide profile of RAM24C2 isolate.
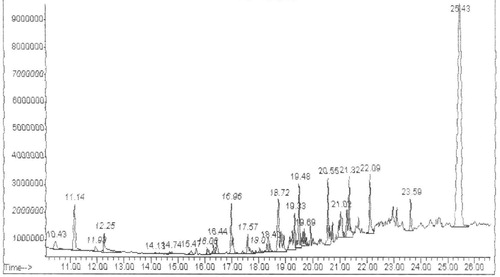
In GC–MS analysis 14 compounds were observed (). The compound name with molecular weight, molecular formula, retension time and area percentage were tabulated (). The major constituents are 1, 2 benzene dicarboxylic acid and Bis (2-ethylhexyl) phthalate (44.67%). The structure of major compounds were shown in . There are many pivotal molecules were present in the RAM24C2 extract. Fatty acid compounds, aromatic compounds which plays a major role as antimicrobial agents were present in the extract.
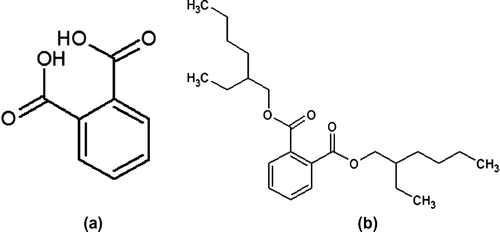
Table 4 Bioactive compounds of RAM24C2 isolate identified by GC–MS analysis.
3.7 Molecular identification of RAM24C2 actinomycete
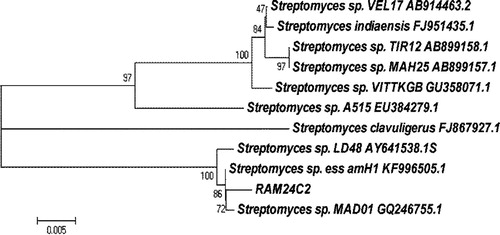
The BLAST analysis of the 16S rRNA gene sequence of the RAM24C2 strain compared with the sequences contained in the data bank (GenBank). The Phylogenetic tree was constructed with boot strap values using Mega 5.05 software. Neighbour joining tree based on 16S rRNA gene sequences showing relationship between the RAM24C2 sequences with other Streptomyces species. However the RAM24C2 strain revealed 100% similarity with Streptomyces sp. MAD01 strain (). The sequence of the Streptomyces sp. MAD01 strain RAM24C2 was submitted in NCBI with accession number JX050218.
4 Discussion
This study mainly focused on the anti-inflammatory activity of actinomycete extract against P. aeruginosa induced conjunctivitis in rabbit model. P. aeruginosa cause severe eye infection which pose a threat in many countries due to its multidrug resistant ability. Previously aminoglycosides are used for the treatment of conjunctivitis but the resistance problem necessities the search for different antibiotics. In this regard recently many fluoroquinolones are used for bacterial conjunctivitis [Citation15].
In our study we have compared the antimicrobial activity of different actinomycetes extracts with commercially available ciprofloxacin antibiotic disc in vitro studies. Followed by that for the in vivo we have used 0.3% ciprofloxacin (fluoroquinolones) ophthalmic solution as standard to compare its efficacy with our actinomycete RAM24C2 extract formulation.
The ethyl acetate extracts of actinomycetes showed inhibitory activity against P. aeruginosa. The results exhibit that the actinomycetes producing antimicrobial compounds as secondary metabolites in the extracellular secretion which controls the pathogen growth [Citation16]. Especially RAM24C2 extract had greater biological activity (antimicrobial) than other actinomycetes extracts.
We examined antibiotics susceptibility of P. aeruginosa towards twelve antibiotics. P. aeruginosa was controlled well by imipenem. Similar result was observed with our RAM24C2 actinomycete ethyl acetate extract. Our RAM24C2 extract exhibit significant antimicrobial activity against ocular pathogen P. aeruginosa in in vitro studies demonstrates that the RAM24C2 extract have pivotal bioactive molecules which can control the multidrug resistant Pseudomonas even in lower concentration. In a previous study with regard antibiotic susceptibility testing of P. aeruginosa showed 100% resistance to norfloxacin, ampicillin but exhibited high-sensitivity towards ciprofloxacin [Citation17]. Our study also supports the above result, ciprofloxacin controlled the growth of P. aeruginosa while norfloxacin, ampicillin had no inhibitory activity. While compared to ciprofloxacin, RAM24C2 extract exhibited greater efficacy in controlling the pathogen and this extract taken for further study for their bioactive compound identification and shelf life, stability analysis.
In thermal inactivation point studies, RAM24C2 ethyl acetate extract retained its antimicrobial activity up to 40 °C and the extract was stable until 75 days when stored at 8 °C. The active compounds present in the extract may be heat labile which lost its antimicrobial activity above 40 °C. Sweetline et al. [Citation18] has reported earlier that crude culture filtrate of actinomycete was able to withstand up to 80 °C. Depending on the species of actinomycetes and kind of secondary metabolite in the extract the thermal inactivation point may differ. For most of the crude extracts that which had antimicrobial activity the optimum temperature was found below 300C in earlier reports [Citation19]. In our study also the optimum temperature for storage was found between −20 °C and 30 °C for RAM24C2 extract. So for the biological control of ocular pathogen P. aeruginosa by RAM24C2 extract should be stored below 30 °C. In another study it is reported that culture filtrate of Streptomyces VPTS3-1 had maximum antimicrobial activity when filtrate stored at 30 °C [Citation20].
In our previous studies we observed severe mucopurulent discharge and bright red conjunctiva in Staphylococcus aureus infected rabbits. But in the case of P. aeruginosa induced conjunctivitis the infection was severe with beefy red conjunctiva but moderate mucopurulent discharge was observed. In anti-inflammatory studies the RAM24C2 extract showed faster cure than ciprofloxacin ophthalmic solution. For the complete eradication of P. aeruginosa induced conjunctivitis five days treatment with ciprofloxacin necessary whereas four days treatment is so efficient for RAM24C2 extract formulation. In earlier findings Leibowitz [Citation21] reported that topical application of ciprofloxacin eradicated all isolated ocular bacterial pathogens. Our study correlates with a study conducted by Alex Azuka Ilechie [Citation13] demonstrated that the topical application of honey twice daily for four days eradicate the bacterial conjunctivitis caused by P. aeruginosa.
The outcome of in vivo study demonstrated that the RAM24C2 extract formulation had greater efficacy for controlling P. aeruginosa induced conjunctivitis which can be used to treat ophthalmic infection caused by P. aeruginosa.
The macrolides are important antibacterial antibiotics. The presence of this compound may responsible for the antimicrobial activity of the RAM24C2 extract. The mode of action of the ciprofloxacin is DNA synthesis inhibition, ciprofloxacin acts on the DNA gyrase of P. aeruginosa there by inhibits DNA replication of bacteria and finally leads to death. Our RAM24C2 extract formulation contain different bioactive compounds like macrolides which may interact with toxins produced by pathogen and thereby subsidize the inflammation. Macrolides also act as bacteriostatic agent. The other bioactive compounds present in the extract may also control pathogen [Citation22–Citation25].
In GC–MS analysis 14 mixture of compounds were identified. Most of the identified compounds in GC–MS analysis of RAM24C2 extract reported to have antimicrobial properties. The major constituent present was 1,2 benzene dicarboxylic acid, dissoctylester also called phthalic acid an aromatic dicarboxylic acid compound reported to have many properties like antimicrobial, antifouling. The same compound also present in the extract of Nocardiopsis dassonvillei MAD08 which reported to have broad spectrum antimicrobial activity [Citation26,Citation27]. Tertradecaonic acid also called myristic acid a fatty acid compound said to have antimicrobial activity in a study conducted by Agoramoorthy et al. [Citation28]. Pryan derivatives are reported to have antimicrobial and anti-inflammatory activity [Citation29]. Hence the above mentioned compounds either individually or in synergism with other compounds may be responsible of antimicrobial and anti-inflammatory activity of RAM24C2 extract.
The 16S rRNA sequencing method, reveals that the RAM24C2 isolate as Streptomyces sp. MAD01. Till now there is no report on the anti-conjunctivitis activity of this Streptomyces species. Hence we provide the first hand information on report the biological activity (anti-inflammatory and antimicrobial) and bioactive compounds of Streptomyces sp. MAD01. This will enable researchers to carry out further studies on the structural elucidation of the bioactive compounds, clinical trials and different biological activity of the extract of this potent strain.
5 Conclusion
By selective isolation actinomycetes were isolated and pure cultured from Rameswaram coastal soil samples. The actinomycetes were screened for their antibiogram pattern against ophthalmic pathogen P. aeruginosa obtained from clinical isolate in microbiological laboratory of Aravind eye hospital. The best actinomycetes exerting antimicrobial inhibitory activity against the ophthalmic pathogen P. aeruginosa was mass cultivated in broth and extracted the secondary metabolites in ethyl acetate by phase separation process. The extracts were checked for the inhibition activity against ophthalmic pathogen in in vitro. The Streptomyces sp. MADO1 strain RAM242 was identified as an eminently suitable strain for isolating antibiotics against multidrug resistant ophthalmic pathogen P. aeruginosa. The ophthalmic pathogen was cultured and used to create ophthalmic conjunctivitis in rabbit model. The conjunctivitis was treated with antibiotic ciprofloxacin ophthalmic solution in comparison with actinomycete ophthalmic suspension. The actinomycete ophthalmic suspension was found to be effective in accelerated curing of conjunctivitis. The presence of macrolides identified through HPTLC analysis inferred that the RAM24C2 strain is rich source for antimicrobial profile.
Acknowledgements
The authors thank the Chancellor Dr. Paul Dhinakaran; Karunya University for his constant motivation and providing the infrastructure facilities for the research. The author Femina Wahaab sincerely acknowledge the Moulana Azad National Fellowship grant issued by UGC India, for the scholar during this research work under the Minority Students Category (Grant No: F1-17.1/2012-13/MANF-2012-13-MUS-TAM-16965.)
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- F.E.AbdullahM.I.KhanS.WaheedCurrent pattern of antibiotic resistance of clinical isolates among conjunctival swabsPak J Med Sci29120138184
- Bruce E.SilversteinTimothy W.MorrisLynne S.GearingerHeleen H.DeCoryTimothy L.ComstockBesifloxacin ophthalmic suspension 0.6% in the treatment of bacterial conjunctivitis patients with Pseudomonas aeruginosa infectionsClinical Ophthalmol6201219871996
- Christopher J.QuinnDennis E.MathewsRichard F.NoyesGary E.OliveJ.James ThimonsOptometric clinical practice guideline care of the patient with conjunctivitisAm Optometric Assoc1995 243 N. Lindbergh Blvd; St. Louis
- M.J.MannisBacterial conjunctivitisW.TasmanE.A.JaegerDuane’s clinical ophthalmology1990JB LippincottPhiladelphia 4, p.5.3–5.7
- N.ThajuddinA.PanneerselvamR.SaravanamuthuOptimization of antimicrobial production by a marine actinomycete streptomyces afghaniensis VPTS3-1 isolated from Palk Strait, East Coast of IndiaIndian J Microbiol5222012230239
- V.VimalBenita MercyRajanK.KannabiranAntimicrobial activity of marine actinomycete, Nocardiopsis sp. VITSVK 5 (FJ973467)Asian J Med Sci1220095763
- G.AdinaryanM.R.VenkateshanV.V.BpirajuP.SujathaJ.PremkumarP.EllaiahA.ZeeckCytotoxic compounds from the marine ActinobacteriumBioorganicheskaia Khimiia322006328334
- S.ParthasarathiC.J.KimP.K.KimS.SathyaM.ManikandanT.ManikandanTaxonomic characterization and UV/VIS analysis of antagonistic marine actinomycete isolated from south west coast of South KoreaInt J Med Res12201099105
- R.W.BauerM.D.K.KirbyJ.C.SherrisM.TurckAntibiotic susceptibility testing by single standard disc diffusion methodAm J Clin Pathol451966493496
- M.OskayAntifungal and antibacterial compounds from Streptomyces strainsAfr J Biotechnol813200930073017
- S.RavikumarM.GnanadesiganA.SaravananN.MonishaV.BrindhaS.MuthumariAntagonistic properties of seagrass associated Streptomyces sp. RAUACT-1: a source for anthraquinone rich compoundAsian Pac J Trop Med2012887890
- W.M.MuiruE.W.MutituD.M.MukunyaCharacterization of antibiotic metabolites from actinomycete isolatesAfr Crop Sci. Conf Proc8200721032107
- Alex AzukaIlechieP.K.KwapongE.Mate-koleS.KyeiC.Darko-takyiThe efficacy of stingless bee honey for the treatment of bacteria-induced conjunctivitis in guinea pigsPharmacology20126368
- J.D.ThompsonD.G.HigginsT.J.GibsonCLUSTALW: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choiceNucleic Acids Res22199446734680
- SumanYadavNayyarParvezA comparative evaluation of 0.3% eye drops and in situ forming gel of Pefloxacin mesylate in experimentally induced Pseudomonas conjunctivitisCont J Pharmacol Toxicol Res2200815
- RofiqSunaryantoBambangMarwotoMarine Actinomycetes screening of Banten West Coast and their antibiotics purificationBiodiversitas1142010176181
- Tamil SelviSivanmaliappanMuruganSevananAntimicrobial Susceptibility Patterns of Pseudomonas aeruginosa from diabetes patients with foot ulcersInt J Microbiol201114
- C.SweetlineR.UshaM.PalaniswamyStudies on anticandidal activity of the actinomycetes isolated from Coimbatore region of Tamil NaduInt J Pharm Bio Sci5320144653
- GebreselemaGebreyohannesFelekeMogesSamuelSahileNagappanRajaIsolation and characterization of potential antibiotic producing actinomycetes from water and sediments of Lake Tana, EthiopiaAsian Pac J Trop Biomed362013426435
- R.VijayakumarK.PanneerselvamC.MuthukumarN.ThajuddinA.PanneerselvamR.SaravanamuthuOptimization of antimicrobial production by a marine actinomycete Streptomyces afghaniensis VPTS3-1 isolated from Palk Strait, East Coast of IndiaIndian J Microbiol5222012230239
- H.M.LeibowitzAntibacterial effectiveness of ciprofloxacin 0.3% ophthalmic solution in the treatment of bacterial conjunctivitisAm J Ophthalmol112199129S33S
- LaurentVerdierJosyaneGharbi-BenarousGildasBerthoPascaleMauvaisJean.-PierreGiraultAntibiotic resistance peptides: interaction of peptides conferring macrolide and ketolide resistance with Staphylococcus aureus Ribosomes. Conformation of bound peptides as determined by transferred NOE experimentsBiochemistry4113200242184229
- Shanooba M.PalamthodiVishnu J.GaikwadNitin V.GhasghaseSandip S.PatilAntibacterial targets in Pseudomonas aeruginosaInt J Pharm Appl232011159164
- T.MazzeiE.MiniA.NovelliP.PeritiChemistry and mode of action of macrolidesJ Antimicrob Chemother31199319
- Frederic.CollinShantanu.KarkareAnthony.MaxwellExploiting bacterial DNA gyrase as a drug target: Current state and perspectivesAppl Microbiol Biotechnol922011479497
- B.EzhilanR.NeelamegamGC–MS determination of bioactive compounds of Polygonum glabrum (Wild)J Phytol3920112325
- JosephSelvinS.ShanmughapriyaR.GandhimathiG.Seghal KiranT.Rajeetha RavijiK.NatarajaseenivasanOptimization and production of novel antimicrobial agents 5 from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08Appl Microbiol Biotechnol8332009435445
- G.AgoramoorthyM.ChandrasekaranV.VenkatesaluM.J.HsuAntibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India”Braz J Microbiol32007739742
- VadivelGopalakrishnanGC-MS analysis of some bioactive constituents of Mussaenda frondosa linnInt J Pharma Biosci212011313320