Abstract
This study utilised epidemiological, haematological, pathological findings and serological detection of specific antibodies to evaluate and confirm a peste des petit ruminants (PPR) outbreak in a herd of West African dwarf (WAD) goats in Ibadan, Nigeria. The morbidity and mortality rates post exposure (PE) were 96% and 60% respectively. Laboratory analyses revealed significant differences (P < 0.05) in mean values of the haematological and serum biochemical indices between the PE and control groups. The PE group experienced a significant (P < 0.05) increase in white blood cell (WBC), lymphocyte and monocytes after 10 days PE; the drop in glucose and high levels of alkaline phosphatase (ALP) and aspartate amino transferase (AST) indicated liver damage, while increased serum creatinine, blood urea nitrogen (BUN) and uric acid arose from kidney impairment. The electrolyte imbalance (potassium, sodium and chloride ions) resulting from the symptomatic diarrhea affected the functionality of the Na+–K+ pump mechanisms, hence pathologic damage to the liver, kidneys, skin, gastrointestinal, respiratory and cardiovascular systems. The competitive enzyme linked immuno-sorbent assay (c-ELISA) detected varying antibody levels in the PPR infected WAD goats; the percent inhibition was highest (P < 0.001) in survivors (70.00 ± 1.73), then in contact group (60.00 ± 2.00), and least in infected (23.33 ± 1.53), which were sero-negative. This study confirmed a PPR outbreak in a WAD goat flock in Ibadan, Nigeria.
1 Introduction
Peste des petits ruminants (PPR), also referred to as ovine rinderpest, goat plague, plague of small ruminants, Kata or stomatitis-pneumoenteritis complex; is an acute, highly contagious and infectious viral disease of small ruminants, which is closely related antigenically to rinderpest in goats and sheep [Citation1]. The disease was first confirmed in Cote d’Ivoire, West Africa in 1942, and endemic to most of Saharan and sub-Saharan Africa, Turkey, the Middle East and the Indian subcontinents [Citation2–Citation4]. The PPR is a notifiable disease on account of the rapidity of its spread, high morbidity and mortality [Citation5,Citation6]. Transmission is mainly through aerosols between animals living in close contact, and confinement seems to favour outbreaks, but it may also happen by feeding of contaminated feed or water. Secretions and excretions of sick animals are the sources of infection, which can also occur during the incubation period [Citation7]. The PPR is characterized by a sudden onset of depression, fever, discharges from the eyes and nose, sores in the mouth, disturbed breathing, coughing and foul-smelling diarrhoea. The morbidity and mortality rates can be as high as 90–100% in virgin populations, dropping to about 20–40% in endemic areas [Citation2,Citation3]. The disease was first reported in Nigeria between 1950 and 1960, and thereafter outbreaks have been reported across the country on a yearly basis. The epidemiology of PPR in Nigeria has not been fully elucidated; and it is possible that it occurs in a more diverse range of hosts [Citation8,Citation9]. It has become a major threat to small ruminant existence and food security in Africa and neighbouring continents [Citation10]. Vaccination remains the most effective and viable tool for the prevention of this infection, but the vaccines are relatively scarce [Citation6,Citation7].
This study utilised the epidemiological, clinical, haematological, pathological findings and serological detection of specific antibodies to evaluate and confirm PPR outbreak in WAD goats.
2 Materials and methods
2.1 Study location and study animals
The PPR outbreak; occurred at the student research farm unit of the Federal College of Animal Health and Production Technology, Moor Plantation, Ibadan, South Western Nigeria (7° 24′ 3″ N, 3° 51′ 9″ E). The location is in a tropical rainforest-derived savannah transition zone with an average relative humidity of 75–90% and mean monthly temperature range of 26–28°C. There are two main seasons, with the rain season spanning from April to October and the dry season from November to March. The study animals were 25 apparently healthy female WAD goats between 6 and 8 months of age, purchased for research purposes, that were sourced from different locations and local livestock markets in Offa, Kwara State, Nigeria, about 170 km from Ibadan. The animals were kept in a well constructed ruminant holding units, intensively managed and placed on basal diets (grasses, legumes and concentrates). Animals were allowed to acclimatise prior to the date for the commencement of the initially planned research. During this period ectoparasitic treatment and deworming were carried out. Preliminary haematological analysis was carried out and the results served as control samples. After the acclimatization period, the PPR outbreak occurred. It started in April 2017 and ended in June 2017.
2.2 Haematology and biochemistry
Five mL of blood per goat were collected by jugular venipuncture using a sterile 5 mL needle and hypodermic syringe. Two mL of blood were put into a labelled tube containing EDTA anticoagulant, while 3 mL were put in tubes without anticoagulant. The first set of blood samples were collected prior to the outbreak of PPR, and subsequent blood collections were undertaken10 days and 15 days post exposure. Haematological and serum biochemical analyses were carried out at the laboratory of Department of Veterinary Physiology, University of Ibadan, Nigeria. Blood samples were analysed for hematological parameters; packed cell volume (PCV), haemoglobin concentration ([Hb]), red blood cell (RBC), white blood cell (WBC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), lymphocyte (LYM) and serum biochemical parameters and electrolytes (albumin, globulin, creatinine, ALT, AST, blood urea nitrogen, sodium, potassium and chloride). Haemoglobin concentration was determined spectrophotometrically, as previously described [Citation11], PCV and RBC counts were determined as described by Dacie and Lewis [Citation12]. Total WBC counts were determined using Neubauer haemocytometer. Blood constants (MCV and MCH) were determined using appropriate formulae as previously described [Citation13]. The second sets of samples without EDTA were centrifuged and serum decanted for serum biochemical analysis. Total serum protein was determined using appropriate kits with basic reported procedure [Citation14], albumin was measured using bromocresol green (BCG) binding technique as described [Citation15]. Electrolytes (sodium, potassium and chloride) were determined with an IDEXX Vetlyte Electrolyte and blood R gas analyzer.
2.3 Serology
Blood samples (2.5 mL /goat) were collected by venipuncture into sample bottles without anticoagulant. Sampling was performed 10 days post exposure for the PPR infected animals and 40 days post exposure for survivors and in contact WAD goats. The samples were transported cooled to a private laboratory in Ibadan metropolis for analysis. At the laboratory the sera were separated further by centrifugation.
The paired sera samples were analysed for the presence of PPRV specific antibodies using an anti- nucleocapsid monoclonal antibody (MAb) based competitive ELISA (c-ELISA) [Citation16]. ELISA plates (NUNCMaxisorp, Hamburg, Germany) were coated with the PPRV antigen (50 μL/well) incubated at 37°C for 1 h; thereafter the wells were washed three times with 0.002 mol/L phosphate-buffered saline (PBS) before 40 μL of blocking buffer (PBS with 0.2% PPR-negative goat serum and 0.1% Tween 20) was added to each well. Twenty μL of the test samples were added to duplicate sets of wells, followed by addition of 40 μL of MAb in each well with the exception of conjugate control wells, at a final dilution of 1:500. Anti-mouse–HRPO conjugate (Dako, Glostorp, Denmark) diluted 1:1000 in blocking buffer was added to each well (50 μL/well) after incubation. Finally, substrate solution orthophenylene diamine (OPD) was added to each well and the colour reaction allowed to develop for 10 min before the reaction was halted with 1 mol/L H2SO4. Optical density (OD) was measured at a wavelength of 492 nm.
2.4 Post-mortem examination
Post-mortem examination was carried out in 4 freshly dead goats with clinical symptoms of PPR.
2.5 Statistical analysis
All the data generated from this study (haematological and serum biochemical from blood and sera respectively, and percentage inhibition from c-ELISA) were subjected to analysis of variance (ANOVA) [Citation17]. If significant differences occurred, the means were separated by Duncan multiple range test (DMRT). The results were presented as mean with the standard error and P-value < 0.05 was considered significant.
3 Results
3.1 History, epidemiological and field observations
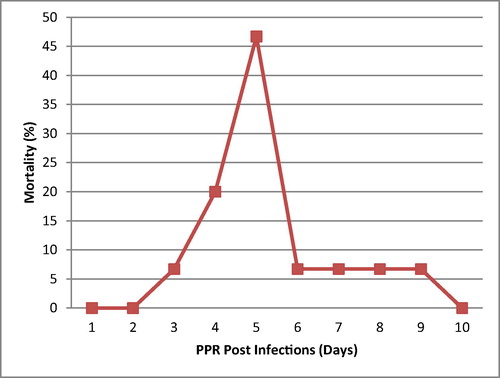
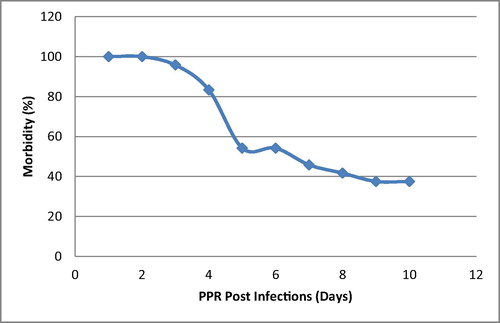
There was no record or any history of vaccination against PPR for any of the goats. An epidemiological survey revealed that there have been previous PPR outbreaks in the same locality as the study location. Also the morbidity rate was 96%. However, 15 out of 25 goats died during the outbreak with a mortality rate of 60% ( and ).
3.2 Clinical findings
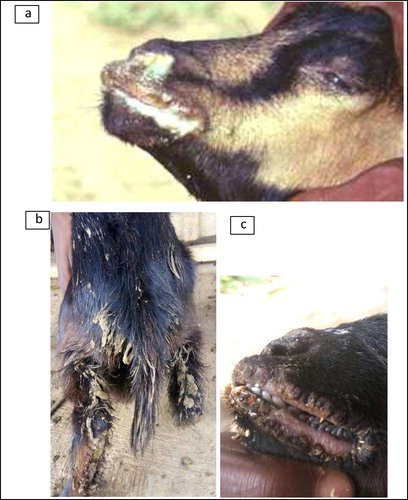
During the clinical examination, the affected animals were coughing, dyspneic and pyrexic, (temperature between 39°C and 42°C). Other signs and symptoms included conjunctivitis, nasal discharges, depression, anorexia and swollen lips. The affected animals showed signs of severe dehydration and the hindquarters were pasted with diarrhoea. The mouth lesions were found in all the affected animals with eroded areas on outer and inner side of the lips, lower gums, and necrosis of the dorsal surface of the tongue. There was a whitish membranous covering all over the buccal cavity, and stomatitis was observed (–).
3.3 Haematological and serum biochemical findings
There were significant differences (P < 0.05) in the haematological indices between pre-infection and post-exposure to PPR among the WAD goats (). Goats tested before infection with PPR exhibit relatively high values of PCV, [HB], RBC, MCV, MCH, neutrophil and eosinophil compared to goats that were infected and tested after 10 and 15 days. Goats that were infected and tested after 15 days had highest values of WBC, lymphocytes and monocytes (P < 0.05). There were significant differences (P < 0.05) in the serological indices between pre-infection and post exposed WAD goats to PPR (). Pre-infected goats with PPR exhibit relatively high values of total protein (TP), albumin, globulin, glucose, potassium, sodium and chloride compared to goats infected with PPR and tested after 10 and 15 days. Goats infected with PPR and tested after 15 days had highest values of creatinine, blood urea nitrogen, uric acid, potassium, ALP and AST.
Table 1 Haematological indices of WAD Goats naturally infected by PPR.
Table 2 Serum biochemical indices of WAD Goats naturally infected by PPR.
3.4 Post-mortem findings
There were different degrees of erosion and necrosis, and nodular lesions on the lips depending on the stage of infection. At necropsy, internal examination revealed varying degrees of erosions and necrosis, and a whitish membranous covering lining the buccal cavity and oesophagus. The lungs appeared heavy and dark (pneumonic lesions) with enlargement of mediastinal and bronchial lymph nodes. There were areas of congestion and haemorrhagic lesions on the mucosal surfaces of the rumen, abomasum and large intestine. Moreover, there were necrotic areas in the small intestine. Localised petechial haemorrhages were observed on the liver lobes and kidneys of dead animal. A marked inflammation of the mesenteric lymph nodes was also noticed.
3.5 Detection of PPR antibodies
Specific antibodies for small ruminant morbilli virus were detected in all the eight paired sera samples collected from WAD goats in the flocks that experienced the outbreak, following the usage of PPR c-ELISA kit, sero-positivity and sero-negativity were determined through the values of percent inhibition (PI). The antibody level differed significantly among the PI sera values for the three categories of infected WAD goats (infected, survivor and in contact /un-infected). Any sera with PI ≥ 40% is considered positive for the presence of PPRV antibodies, while the reverse is the case for PI < 40%. This result however, shows that the survivors had the highest antibody titre due to a PI (70 ± 1.73), followed by the in contact goats (60 ± 2.00) and the infected (23.33 ± 1.53) which is sero-negative, at P < 0.0001 ().
Table 3 Percentage Inhibition of sera for different categories of WAD goats exposed to PPR infection.
4 Discussion
This recent outbreak of PPR in a small herd of goats in Ibadan has confirmed the presence of PPR in Nigeria and sub-Saharan Africa. It remains a major cause for concern and a problematic issue that needs urgent attention because of its aftermath effects on food security, transboundary transmission and unquantifiable economic losses, especially to small scale farmers [Citation5]. All the epidemiological findings associated with this study and history of previous PPR outbreaks are known to have predisposed the WAD goats to the PPR outbreak; this is in consonance with the submissions of FAO and Baazizi et al. [Citation2,Citation6]. The mortality recorded for this PPR outbreak peaked (67% of total mortality recorded) between 6 and 8 days PE, which supports the results of Baron et al. [Citation21], that the severity of clinical signs generally peaks between 6 and 8 days post infection and can continue for up to 14 days leading to death or recovery from infection. This study shows that PPR is associated with very high morbidity (96%) and mortality (60%), this may be due to the acute nature, pathogenesis, mode of spread of the infection and the ability of the causative agent (morbilli virus) to cause immuno-suppression in infected WAD goats, through the reduction of CD4+T cells; which is in line with previous studies [Citation22–Citation23]. This immuno-suppression is further corroborated by a significant drop in levels of total protein and globulin which is a precursor for immunoglobulins (antibodies) of the infected WAD goats [Citation24]. This study shows that the WAD goats in the post exposure groups have higher values for WBC, when compared with the pre-infection group; this is contrary to the leukopenia synonymous with PPR outbreaks; which has been previously reported [Citation25,Citation26], that within the 4–10 days PE to PPR, there is destruction of the WBC and subsequent immuno-suppression [Citation27]. The high values for WBC could be attributed to the regeneration of damaged cells after the acute phase of the infection; and subsequently there were varying levels of antibodies, which is in line with a previous study [Citation26], which concluded that the level of antibodies increased significantly after days 28–35 post infection with virulent PPR virus. On the other hand, the low levels of RBCs, [Hb], PCV, MCV and MCH compared with the control and normal physiological range of values [Citation18,Citation19], could be attributed to the erosion, necrosis and congestion associated with PPR, and also to the different phases of the disease, presence of secondary infections, nutritional status, and/or level of dehydration [Citation28,Citation29]. There was a sharp drop in the blood glucose level, termed hypoglycaemia; this condition occurs whenever there is a difficulty with the conversion of glycogen to glucose (glycogenolysis). The liver is normally a principal store house for glycogen, but due to the functional impairment as a result of PPR infection, hence the short fall [Citation2,Citation8]. Liver damage, as a result of PPR infection is further confirmed by an excessively high level of ALP and AST when compared with the control and normal physiological values [Citation18,Citation20], these are serum enzymes ordinarily found in the liver, but when there is damage to the liver they are found at high levels in the serum. The low levels of potassium ions in the infected WAD goats resulted from the dehydration arising from the symptomatic diarrhoea due to PPR infection, this will lead to metabolic acidosis as a result of the drop in pH level in the gastrointestinal tract (reason for increase in serum chloride level). This pH change will in turn stimulate a compensatory mechanism leading to respiratory alkalosis, and hence the rapid respiration for the expiration of carbon dioxide. This condition will result in an increase in blood sodium that will bind to bicarbonates to serve as buffer during PPR infection to aid in the reduction of the pH [Citation30]. This drop in potassium will automatically affect the Na+–K+pump mechanism which will evidently disrupt the functionality of most of the systems (especially nervous, cardiovascular and urinary systems) [Citation25]. The high values for uric acid, blood urea nitrogen and creatinine in the serum are indicative of a functional damage to the kidney, under normal condition these substances are not suppose to be present in the blood at high levels, because the function of the kidney is to excrete them through the urine. But when there is functional defects or damage to the kidney, such as in PPR infection (possibly due to disruption of the Na+-K+ pump), these substances are found at high levels in the serum; this is in consonance with the findings of Sharma et al. [Citation31]. It was however observed that, as the infection progressed from the 10th to 15th day post infection, the haematological and serum biochemical parameters became more significant, due to progression of infection from acute stage to death or recovery [Citation21]. All the aforementioned and previously discussed factors are presumptive and tentative diagnosis for PPR infection. The detection of morbilli virus specific antibodies in the sera of PPR infected WAD goats is indicative of recent PPR outbreak in Ibadan; this is also in line with previous diagnostic procedures carried out by FAO and Saliki [Citation2,Citation8], to confirm outbreaks of PPR in different locations. It was further confirmed through the percentage inhibition, that out of the three categories of WAD goats exposed to PPRV, the survivors of this infection have the highest antibody titres, which is in tandem with the findings of Santhosh et al. [Citation32]. Also, the in-contact WAD goats with no clinical signs of PPR presented a high level of antibody, probably they were nursing a sub-clinical PPR infection; which is similar to the findings of Sen et al. [Citation33] that exposed or in-contact cattle seroconverted and maintained PPR virus specific antibodies after day 21 post infection.
5 Conclusions
PPR is a contagious infection that affects number of organs and systems in small ruminants. The outcomes of this study will serve as a guide and template to subsequent research and diagnostic procedures in this field; and will contribute to the repository of knowledge. Regional vaccination of sheep and goats before the age of 6 months is hereby recommended in endemic areas.
Competing interests
The authors declare no competing interests.
Acknowledgements
The authors of this study sincerely wish to appreciate the management and staff of Federal College of Animal Health and Production Technology, Moor Plantation, Ibadan; especially workers in the student research farm and ruminant unit; and Prof. F.O. Fasina (Country Team leader ECTAD, FAO-United Nations, Kenya).
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- P.PastoretRinderpest: a general introductionRinderpest and Peste Des Petits Ruminants Virus: Plague of Large and Small Ruminants; Biology of Animal Infections2006Academic PressLondon, UK112
- FAO (Food and Agriculture Organisation). Recognizing Peste des Petits Ruminants Field Manual; 1999. http://www.fao.org/docrep/003/x1703e/x1703e00.HTM.
- A.C.BanyardS.ParidaC.BattenC.OuraO.KwiatekG.LibeauGlobal distribution of peste des petits ruminants virus and prospects for improved diagnosis and controlJ Gen Virol91201028852897
- M.AbubakarS.AshiqA.Bin ZahoorM.J.ArshedA.C.BanyardDiagnosis and control strategies for peste des petits ruminants virus: global and Pakistan perspectivesPak Vet J312011267274
- FAO (Food and Agriculture Organisation). Supporting livelihoods and supporting livelihoods and Peste des petits ruminants (PPR) and small ruminant diseases control 2013. http://www.fao.org/docrep/017/aq236e/aq236e.pdf.
- R.BaaziziM.MahapatraB.D.ClarkeK.Ait- OudhiaD.KhelefS.ParidaPeste des petits ruminants (PPR): a neglected tropical disease in Maghreb region of North Africa and its threat to EuropePLoS ONE122017e0175461
- OIE (World organization for animal health). Animal Health in the World – Overview; 2017. http://www.oie.int/animal-health-in-the-world/.
- Saliki JT. An overview of Peste des Petit Ruminants. Merck Veterinary Manual. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA; 2015. http://www.merckvetmanual.com/generalized-conditions/peste-des-petits-ruminants/overview-of-peste-des-petits-ruminants.
- A.OzkulY.AkcaF.AlkanT.BarrettT.KaraogluS.B.DagalpPrevalence, distribution, and host range of Peste des petits ruminants virus, TurkeyEmerg Infect Dis82002709712
- FAO (Food and Agriculture Organisation). Peste des petits ruminants; 2017. http://www.fao.org/ppr/en/.
- M.A.FranceschiniS.FantiniA.CerussiB.BarbieriB.ChanceE.GrattonQuantitative spectroscopic determination of haemoglobin concentration and saturation in a turbid medium: analysis of the effect of water absorptionJ Biomed Opt21997147153
- J.N.DacieS.M.LewisPractical haematology7th ed.1991ELBS with Churchill and LivingstoneEngland3785
- O.W.SchalmN.C.JainE.J.CarrolVeterinary haematology4th ed.1986Lea and FebigerPhiladelphia
- R.A.KohnM.S.AllenEnrichment of proteolysis activity relative to nitrogen in preparation from the rumen for in vitro studiesAnim Feed Sci Technol52195514
- Hameed T.AbdulMeasurement of serum Albumin by three different methodsKerbala J Pharm Sci42012111118
- R.P.SinghB.P.SreenivasaP.DharL.C.ShahS.K.BandyopadhyayDevelopment of monoclonal antibody based competitive ELISA for detection and titration of antibodies to peste des petits ruminants (PPR) virusVet Microbiol982004315 10.1016/j.vetmic.2003.07.007
- SAS (Analytical software). User's Guide (release 8.03) SAS Institute, Cany North Carolina USA; 2004. 7.
- Daramola JO, Adeloye AA, Fatoba TA, Soladoye AO. Haematological and biochemical parameters of West African Dwarf goats. Livestock Res Rural Dev 2005; 17, Art. #95.
- M.N.OparaN.UdeviI.C.OkoliHaematological parameters and blood chemistry of apparently healthy West African Dwarf (Wad) Goats in Owerri, South Eastern NigeriaNew York Sci J320106872
- M.A.WaziriA.Y.RibaduN.SivachelvamChanges in the serum proteins, hematological and some serum biochemical profiles in the gestation period in the Sahel goatsVet Arhiv802010215224
- J.BaronA.Bin-TarifR.HerbertL.FrostG.TaylorM.D.BaronEarly changes in cytokine expression in peste des petits ruminants’ diseaseVet Res45201422
- J.BaronE.FishbourneE.Couacy-HymanM.AbubakarB.A.JonesL.FrostDevelopment and testing of a field diagnostic assay for peste des petits ruminants virusTransbound Emerg Dis612014390396
- R.HerbertJ.BaronC.BattenM.BaronG.TaylorRecombinant adenovirus expressing the haemagglutinin of peste des petits ruminants virus (PPRV) protects goats against challenge with pathogenic virus; a DIVA vaccine for PPRVet Res45201424
- H.W.SchroederL.CavaciniStructure and Function of ImmunoglobulinsJ Allergy Clin Immunol1252010S41S52
- S.DasR.NathV.BalamuruganR.ChoudhuryM.DeviHaemato-biochemical analysis of goat naturally infected with Peste des petit RuminantsInt J Res Emerg Sci Tech220151924
- M.El HarrakN.TouilC.LoutfiM.HammouchiS.ParidaG.SebbarReliable and reproducible experimental challenge model for Peste des petits ruminants virusJ Clin Microbiol50201237383740
- K.K.RajakB.P.SreenivasaM.HosamaniR.P.SinghS.K.SinghR.K.SinghExperimental studies on immunosuppressive effects of peste des petits ruminants (PPR) virus in goatsComp Immunol Microbiol Infect Dis282005287296
- Aytekın İ, Mamak N, Ulucan A Kalinbacak, Aslan. Clinical, Haematological, Biochemical and Pathological Findings in Lambs with Peste des Petits Ruminants. Kafkas Universitesi Veteriner Fakultesi Dergisin 2011;17:349.
- C.S.SharmaH.K.MehtaM.M.PrakashP.C.ShuklaStudies on clinico-haemato-biochemical changes in peste des petits ruminants in goatsVet Pract132012322325
- Lewis JL. Metabolic Acidosis. Merck Veterinary Manual 2016. Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ; USA. http://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/acid-base-regulation-and-disorders/metabolic-acidosis.
- S.K.SharmaJoshi M.TarunpreetClinico-haemato-biochemical characterization of peste des petits ruminants in sirohi goats and its managementInt J Sci Environ Tech5201622002204
- A.K.SanthoshA.R.GomesR.HegdeD.RathnammaB.M.VeeregowdaS.M.ByregowdaComparative immunogenicity of two peste des petitis ruminants (PPR) vaccines in South Indian sheep and goats under field conditionsIndian J Virol242013373379
- A.SenP.SaravananV.BalamuruganV.BhanuprakashG.VenkatesanJ.SarkarDetection of subclinical peste des petits ruminants virus infection in experimental cattleVirus Dis252014408411