Abstract
Research in the area of oxidative stress in pneumonic pathology still requires attention in small ruminants especially with the use of bronchoalveolar lavage (BAL) which may be a more sensitive indicator of respiratory diseases than blood. This investigation evaluates the role of oxidative stress in the pathogenesis of caprine pneumonia using BAL fluid (BALf) from healthy and pneumonic goats. A BALf from 192 goats (whose pneumonic histopathology had been characterized using standard techniques) was biochemically assayed for anti-oxidants and pro-oxidants. Malondialdehyde (MDA), hydrogen peroxide generation (H2O2), myeloperoxidase (MPO) and reduced glutathione (GSH) contents were measured to assess free radical activity in the BALf. Superoxide dismutase (SOD), Glutathione transferase (GST) and Glutathione peroxidase (GPx) activity were also determined colourimetrically. There were significant increases in the BALf supernatant of MDA, H2O2 and MPO with decreases in GSH level and SOD activity in the pneumonic goats (P < 0.05). There was also significant correlation of BALf oxidative assay to the type and severity of pneumonia. The levels of MDA, H2O2, and MPO increased significantly (P < 0.05) in bronchopneumonia and bronchointerstitial pneumonia than other pneumonic conditions and normal lungs. The management of caprine pneumonia should often incorporate antioxidant supplementation to correct the imbalance in pro-oxidant and anti-oxidant levels.
1 Introduction
Respiratory diseases in sheep and goat have been established from exposure to stressors which may include long period of starvation and transportation, housing and weather followed by invasion of bacterial and viral infectious agents [Citation1,Citation2]. Exposure to these stressful conditions leads to excessive production of free radicals and reactive species with potential membrane damaging effects [Citation3].
Oxidative stress involves oxidative modification by reactive oxygen species of biomolecules (proteins, nucleic acids, and lipids). It induces a variety of organ dysfunction as a result of imbalance between the pro-oxidant and anti-oxidant levels in cells and tissues [Citation4,Citation5].The pro-oxidant promotes oxidation while anti-oxidants checkmates the activities of these pro-oxidants. Oxidative stress may also result from defects in expression of the genes controlling anti-oxidant enzymes [Citation6].
The respiratory pathogens survive insults from reactive species generated by the host through detoxification mechanisms using enzymatic and non-enzymatic processes. The anti-oxidant enzymes are involved in enzymatic detoxification mechanisms and other adaptive mechanism controlled by gene expression [Citation5,Citation7].
Research in the area of oxidative stress in pneumonic pathology still requires attention especially in small ruminants. Hitherto, evaluations of oxidative stress in animals require estimation of certain blood biomarkers that reflect the oxidative profile of affected cases [Citation8,Citation9], as was done in Peste des petits ruminant (PPR) virus infected sheep [Citation10]. The assay of these markers in BAL may be a more sensitive indicator of respiratory diseases than blood. Hence, this study evaluates for the first time the oxidative stress parameters in bronchoalveolar lavage fluid of healthy and pneumonic goats.
2 Materials and methods
2.1 Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and also approved by University of Ibadan Animal Care Use and Research Ethics Committee (UI-ACUREC/17/0060).
2.2 Animals and bronchoalveolar lavage fluid
The source of animals and pattern of pneumonia have been previously described [Citation11]. The bronchoalveolar lavage sampling was as described by Khin and Zamri-Saad [Citation12]. Briefly, following slaughter, the trachea together with the lungs were resected and lavaged by introducing 40 mL of warm sterile phosphate buffered saline (PBS), pH 6.8 into the lungs. This was followed by gentle massage of the lungs before the fluid was re-collected into a measuring beaker. The colour and consistency of the BALf was noted before centrifuged at 3000 rfc for 15 min and the supernatant decanted. The BALf supernatant was stored at −20 °C. Out of 700 goats, 192 BALf were randomly selected for biochemical analysis of oxidative markers.
2.3 Oxidative biochemical assay of BALf
The total protein concentration was calculated using the biurets method [Citation13]. Fifty μL of BALf supernatant were added to 100 μL of biuret reagent in the 96-well microtitre plate. The plate was left for 30 min at room temperature before read on the spectrophotometer at a wave length of 490 nm using distilled water as blank. The readings were extrapolated on the total protein standard curve.
The superoxide dismutase (SOD) activity was calculated according to Misra and Fridovich [Citation14] and Omobowale et al. [Citation7]. Twenty μL of the BALf were added to 250 μL 0.05 M carbonate buffer (pH 10.2) followed by the addition of 300 μL of acidified reconstituted adrenaline. The change in absorbance was observed every 30 s for 180 s at 490 nm wave length.
The reduced glutathione (GSH) was determined as described by Jollow et al. [Citation15]. Addition of 250 μL of 4% sulfosalicylic acid to 250 μL of BALf in test tube was carried out, the tube was centrifuged at 4000 rfc for 5 min. Twenty μL of the supernatant were aliquoted into wells of the 96-well microtitre plates, 180 μL of Ellman's reagent (containing 0.04g of DTNB in 100 mL of 0.1 M phosphate buffer, pH 7.4) was added to the well. The absorbance of the reaction was read on the spectrophotometer at a wavelength of 405 nm against distilled water as blank.
The glutathione peroxidase (GPX) was estimated as described by Beutler et al. [Citation16]. The tube contained 250 μL 0.1 M phosphate buffer (pH, 7.4), 50 μL of Sodium azide, 100 μL of GSH solution, 100 μL of H2O2, 250 μL of BALf and 300 μL of distilled water. The tube was incubated at 37 °C for 5 min in water bath. Now 250 μL of TCA were added to the tube before centrifugation at 3000 rpm for 5 min. An amount of 50 μL of the supernatant was aliquoted into well of the 96-well microtitre plate, 100 μL of K2PHO4 and 50 μL of DTNB were added. The reaction absorbance was evaluated at a wavelength of 405 nm with distilled water as blank.
The glutathione transferase (GST) activity was estimated via the conjugation of 1-chloro, 2,4-dinitrobenzene (CDNB) with reduced glutathione [Citation17]. A unit of enzyme will conjugate 10.0 nmol of CDNB with reduced glutathione per minute at 25 °C. The changes in absorbance were monitored at a wavelength of 405 nm. The rate of the linear reaction was set at Δ405 nm. The absorbance Δ405 nm for the blank reaction was subtracted from the absorbance Δ405 nm for each BALf sample reaction. The molar extinction coefficient of CDNB was 0.0096 μM−1/cm. Thus, GST activity = [(Adjusted Δ405 nm)/0. 0096 μM−1/cm] × (1.0 mL /0.1 mL) × sample dilution = U/mL.
The malondialdehyde (MDA) level was as described by Varshney and Kale [Citation18]. Each wells of the 96-well microtitre plate contained 400 μL of Tris-KCl, 125 μL of 30% TCA, 100 μL of BALf and 125 μL of 0.75% TBA prepared in 0.2 M HCl. The plate was incubated at 80 °C for 45 min in water bath. It was cooled on ice and centrifuged at 3000 rfc for 15 min. The reaction absorbance was read against distilled water as blank at wavelength of 490 nm. The level of lipid peroxidation (units/milligram protein) was valued on a molar extinction coefficient of 1.56 × 105/M/cm.
The hydrogen peroxide generation was evaluated according to Woff's [Citation19]. Each wells of the 96-well microtitre plate contained 100 μL of 0.1 M phosphate buffer (pH 7.4), 50 μL of Ammonium ferrous sulphate, 20 μL of sorbitol, 10 μL of Xanthine Oxidase (XO), 25 μL of sulphuric acid and 50 μL of BALf. The plate was vortexed slightly change in colour (pink colour). The plate was then incubated at room temperature for 30 min. The reaction absorbance was read at 490 nm wavelength using distilled water as blank. The H2O2 standard curve was used for extrapolation of H2O2 level generated.
The myeloperoxidase (MPO) activity was evaluated as described by Xia and Zweier [Citation20]. The well of the microtitre plate contained 200 μL O-dianisidine mixture and 10 μL of BALf. The reaction absorbance was monitored at 0, 30 and 60 s at 450 nm wavelengths.
2.4 Statistics
All statistical analyses were performed using Computer Software (SPSS version 16.0, Chicago, USA). Data were presented as Mean ± SEM, and compared using Pearson correlation and ANOVA at 5% significance.
3 Results
3.1 Pattern of lung lesion
Of the 192 BALf; 35 were of normal lungs, 29 of congestion and oedema, and 72 of bronchopneumonia, 29 of broncho-interstitial pneumonia, 22 of interstitial pneumonia and 3 of granulomatous pneumonia.
3.2 Pro-oxidant and anti-oxidant parameters
The total protein (TP) concentration in the BALf of goats with histologically normal lung was 9.56 ± 0.7 mg/ml. It was significantly lower than that in congestion and oedema (11.6 ± 1.1 mg/ml), bronchopneumonia (16.7 ± 1.7 mg/mL), broncho-interstitial pneumonia (13.5 ± 1.7 mg/ml), interstitial pneumonia (15.4 ± 3.5 mg/mL) and granulomatous pneumonia (15.2 ± 4.8 mg/ml).
The measure of Glutathione peroxidase (GPx) activity was 24.1 ± 1.5 units mg−1 protein in the BALf of normal goats. There were slight reduction in the GPx activity from congested and oedematous (21.4 ± 1.7 unit mg−1 protein), bronchopneumonia (18.9 ± 1.4 unit mg−1 protein), broncho-interstitial pneumonia (20.1 ± 1.8 unit mg−1 protein), interstitial pneumonia (22.3 ± 2.7 unit mg−1 protein) and granulomatous pneumonia (20.4 ± 4.9 unit mg−1 protein). The difference was significant in BALf from goats with bronchopneumonia (P < 0.05).
The level of reduced glutathione (GSH) was 9.9 ± 0.3 µg mL−1 form BALf of normal lungs. There were slight decrease to 9.4 ± 0.2 µg mL−1 in congested and oedematous lungs, 9.7 ± 0.10 µg mL−1 in bronchopneumonia, 9.5 ± 0.1 µg mL−1 in broncho-interstitial pneumonia, and similar level or slight increase in interstitial pneumonia (9.9 ± 0.3 µg mL−1) and granulomatous pneumonia (10.2 ± 0.4 µg mL−1).
The activity of Glutathione transferase (GST) was 8.0 ± 1.1 unit mg−1 protein in the BALf from normal lungs. It decreased in congested and oedematous lungs (6.5 ± 1.0 unit mg−1 protein), bronchopneumonia (7.0 ± 0.8 units mg−1 protein), and in broncho-intersttial pneumonia (6.3 ± 0.9 unit mg−1 protein) Nearly similar activity of GST was recorded in interstitial pneumonia (8.6 ± 1.1 units mg−1 protein) and granulomatous pneumonia (8.1 ± 3.3 unit mg−1 protein).
Similar pattern was also observed in Superoxide dismutase (SOD) activity; 4.1 ± 0.3 unit mg−1 protein in normal, 3.6 ± 0.3 units mg−1 protein in congestion, decreasing further to 3.2 ± 0.2 unit mg−1 protein in bronchopneumonia, 3.4 ± 0.3 unit mg−1 protein in broncho-interstitial pneumonia, 3.7 ± 0.4 unit mg−1 protein in interstitial pneumonia and 3.4 ± 0.8 unit mg−1 protein in granulomatous pneumonia.
Hydrogen peroxide generation was least in the BALf of normal lungs (3.6 ± 0.5 µmol mg−1 protein). It increased to 5.0 ± 0.4 µmol mg−1 protein in congestion and oedema, 4.9 ± 0.3 µmol mg−1 protein in bronchopneumonia, 5.0 ± 0.6 µmol mg−1 protein in broncho-interstitial pneumonia, 5.2 ± 0.4 µmol mg−1 protein in interstitial pneumonia and 5.6 ± 0.7 µmol mg−1 protein in granulomatous pneumonia.
Malondialdehyde (MDA) level was low in normal (2.3 ± 0.1 µmol/mL), oedematous lungs (2.1 ± 0.1 µmol/mL), bronchopneumonia (2.3 ± 0.1 µmol/mL). It was remarkably high in broncho-interstitial pneumonia (4.43 ± 0.1 µmol/mL), 2.2 ± 0.1 µmol/mL in interstitial pneumonia, and 3.0 ± 0.1 in granulomatous pneumonia.
Myeloperoxidase (MPO) activity was least in BALf from histologically normal lungs (2.0 ± 0.2 µmol/min) but significantly higher in congestion and oedema (16.0 ± 0.1 µmol/min), bronchopneumonia (20.0 ± 0.1 µmol/min), broncho-interstitial pneumonia (30.0 ± 0.2 µmol/min), interstitial pneumonia (10.0 ± 0.1 µmol/min) and granulomatous pneumonia (11.0 ± 0.1 µmol/min).
The pattern of the anti-oxidants in BALf from examined goats is shown in while that of the pro-oxidants is shown in and . There was also significant positive correlation of BALf pro-oxidant assay to the type and severity of pneumonia (+0.65, P < 0.05).
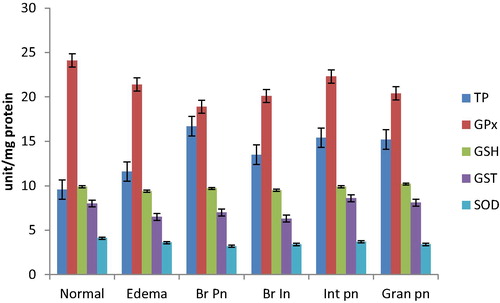
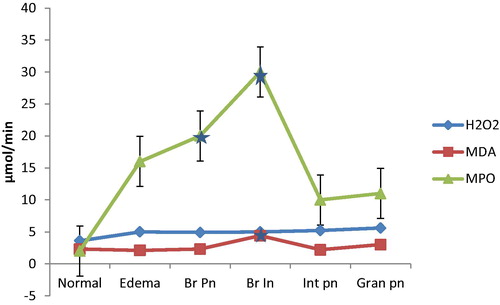
Table 1 The total protein, anti-oxidants and pro-oxidants values in bronchoalveolar lavage fluid from normal and pneumonic goats.
4 Discussion
This study recorded for the first time the levels of pro-oxidants and anti-oxidants in bronchoalveolar lavage fluid (BALf) in goats. Hitherto, the available few studies on oxidative stress in goats and ruminants quantified the markers in plasma. However, this study quantified oxidative stress biomarkers including GST, SOD, GPx, MDA, H2O2 and MPO in BALf of normal and pneumonic goats with different histologic classifications [Citation2].
The MDA and SOD values obtained in BALf of goats in this study are not too different from that of blood of sheep and goats reported by various studies. In goats, the MDA and SOD values of 250 nmol/mL and 80 µM were reported in goats in humid tropical environment [Citation21], while Kaya et al. [Citation22] reported 4.68 ± 0.26 and 6.09 ± 0.69 (nmol/mL) for MDA, 4.35 ± 0.13 and 3.67 ± 0.12 (μmol/L) for Glutathione in non-grazing and grazing cattle in similar environment. Kataria and Kataria [Citation10] reported 167.98 ± 10.00 kU L−1 and 294.22 ± 9.91 kU L−1 for SOD; 5.87 ± 0.10 μmol L−1 and 3.10 ± 0.06 μmol L−1 for Glutathione from healthy and pneumonic sheep affected PPR respectively in India.
The difference in oxidative parameters from BALf observed in pneumonic lungs was significant, this could be associated with the severity of inflammation and cellular damage commonly observed in bronchopneumonia and broncho-interstitial pneumonia with destruction of epithelial cells and fibrinous reaction from vascular damage. This clearly showed that the measure of MDA, H2O2 and MPO correlated with the inflammatory reactions and mechanisms of cellular response.
The role of anti-oxidant should therefore be stressed especially in ruminants, where vitamin supplementation may not be part of treatment because it is assumed that forage taken could adequately supply enough anti-oxidants. It should be noted also that pneumonia induces loss of condition and anorexia in these animals thereby further depleting anti-oxidant levels which are known first line of defense against the dangerous effects of free radical generation in living tissues in disease state.
The protein concentration of BALf in the pneumonic goats was significantly different to that observed in non-pneumonic goats. This is unique and quite contrasting to the observations of Lewis et al. [Citation23] in pneumonic, asthmatic and normal human patients.
The significant decrease in most of the enzymes (GPx, GST and SOD) indicates an overwhelming exhaustion of these enzymes. Consequently there would be damage to macromolecules, cells, tissues and organ dysfunction with health implications on the affected animal. Significant reductions were observed in broncho-interstitial pneumonia and bronchopneumonia.
Myeloperoxidase activity is unregulated in inflammation as well as in oxidative stress because of the increased phagocytic activity and release of reactive oxygen species (ROS) from neutrophils [Citation24]. The high MPO values recorded in bronchopneumonia and broncho-interstitial pneumonia further corroborate cellular damage and local neutrophilic inflammation. This is similar to findings of Schmekel et al. [Citation25], who linked origin of myeloperoxidase in bronchoalveolar lavage to local neutrophilic reaction. The increases in H2O2 generation and MDA levels may have underscored the cellular injury, membrane lipid peroxidation and acute inflammatory reactions induced by pathogens (bacterial and/or viral) in the lower respiratory tract.
More so, the role of ROS in the pathogenesis of viral infections has been emphasized [Citation26,Citation27]. The cellular injury, damage of tissues and inflammation induced by respiratory pathogens make them potent pro-oxidant agents [Citation10]. They are in turn eliminated by specific cell defense mechanisms which may also generate reactive oxygen species [Citation28]. This may explain the high levels of MDA in goats with broncho-interstitial pneumonia. Nisbet et al. [Citation29] suggested a shift towards pro-oxidant production in the pathology of Pest des petits ruminant (PPR) virus. This may be accentuated in bacterial complicated viral pneumonia.
The findings further corroborate the assertion of Schawrz [Citation26] who suggested the application of anti-oxidants in the therapy of viral infections due to the generation of reactive metabolites [Citation30].
Dyer et al. [Citation31] also examined oxidative respiratory burst activity in lavage-procured bovine pulmonary alveolar macrophages where it was shown that non-stimulated alveolar macrophages released a minimal quantity of superoxide anion and had small amounts of glucose flux through the pathways of energy metabolism. This could be aggravated in severe pneumonic conditions and massive neutrophilic influx, as was observed from H2O2 generation, MDA and MPO levels in the pneumonic goats.
Furthermore, the impact of oxidative stress in virus infections could be related to the nature of viral replication in cells, growth and packaging of proteins [Citation30]. The changes in anti-oxidants and enzymes levels in the goats explain the reductive effects of free radicals in caprine pneumonia complex. The influence of ROS may have contributed to the severity of cellular injury in broncho-interstitial pneumonia and bronchopneumonia.
Thus periodic assessment of oxidative stress in small ruminants is important with supplementation of proper anti-oxidants as supportive treatment in pneumonic animals. In humans, Israel and Gougerot-Pocidalo [Citation32] already showed the influence of antioxidant molecules as good therapeutics.
The histological pattern of disease linked to the BALf may not have strengthened the attribution of causality. However, this study has further compared the histological findings with BALf redox changes in pneumonia and its contribution to the pathology and fatality in small ruminants.
5 Conclusions
Oxidative stress markers in BALf may serve as predictors of pneumonia especially in the early stages and there is a need to closely study their role in complicated pneumonia for control of caprine pneumonia complex. The management of caprine pneumonia should incorporate anti-oxidant supplementation to correct the imbalance in pro-oxidant and anti-oxidant levels.
Funding statement
This study was funded by the University of Ibadan Research Grant (SRG/FVM/2010/6A).
Author B has received the research grants (SRG/FVM/2010/6A) of the University of Ibadan.
Competing interests
All the authors declare that they have no conflict of interest.
Acknowledgement
We wish to appreciate Dr. J.M. Afolabi for his support during laboratory analysis of this work.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- B.O.EmikpeT.A.JarikreO.D.EyarefeRetrospective study of disease incidence and type of pneumonia in nigerian small ruminants in Ibadan, NigeriaAfr J Biomed Res162013107113
- T.A.JarikreB.O.EmikpeO.A.MorenikejiS.O.AkpaviePattern and associated risk factors of caprine pneumonia in NigeriaAsian Pac J Trop Dis62016179183
- S.SamarghandianA.BorjiR.AfshariM.B.DelkhoshA.GholamiThe effect of lead acetate on oxidative stress and antioxidant status in rat bronchoalveolar lavage fluid and lung tissueToxicol Mech Methods232013432436https://doi.org/10.3109/15376516.2013.777136
- Y.NaitoM.C.LeeY.KatoR.NagaiY.YoneiOxidative stress markers. Review articleJ Anti Aging Med720103644
- B.KalyanaramanTeaching the basics of redox biology to medical and graduate students: oxidants, anti-oxidants and disease mechanismsRedox Biol12013244257
- N.KatariaA.K.KatariaR.MaanA.K.GahlotEvaluation of oxidative stress in brucella infected cowsJ Stress Physiol Biochem620101931
- T.O.OmobowaleA.A.OyagbemiO.A.OyewunmiO.A.AdejumobiChemopreventive effect of methanolic extract of Azadirachta indica on experimental Trypanosoma brucei induced oxidative stress in dogsPhcog Res72015249258
- P.K.PilaniaS.SolankiN.MohammedS.AsopaR.MaanA.JoshiOxidative stress in vis a vis gastrointestinalism and pneumonia in Marwari goatVet Res620135457
- S.A.MousaS.M.SolimanOxidant and Antioxidant Status in Pneumonic Goats with Special Reference to Bacterial EtiologyInt J Livest Res6520161523
- A.K.KatariaN.KatariaEvaluation of oxidative stress in sheep affected with peste des petits ruminantsJ Stress Physiol Biochem820127277
- T.A.JarikreB.O.EmikpeO.G.OhoreT.A.AkinremiS.O.AkpavieBronchoalveolar lavage fluid cellular and haematological changes in different types of caprine pneumoniaNiger J Physiol Sci.3120163136
- M.N.KhinM.Zamri-SaadThe effect of dexamethasone on immune responses of calves to intranasal exposures to live attenuated gdhA derivative of Pasteurella multocida B:2Pertanika J Trop Agric Sci332010205211
- A.G.GornallC.J.BardawillM.M.DavidDetermination of serum proteins by means of the biuret reactionJ Biol Chem1771949751766
- H.P.MisraI.FridovichThe role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutaseJ Biol Chem247197231703175
- D.J.JollowJ.R.MitchellN.ZampaglioneJ.R.GilletteBromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolitePharmacology111974151169
- E.BeutlerO.DuronB.M.KellyImproved method for the determination of blood glutathioneJ Lab Clin Med611963882888
- B.MannervikThe isozymes of glutathione transferaseAdv Enzymol Relat Areas Mol Biol571985357417
- R.VarshneyR.K.KaleEffects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomesInt J Radiat Biol581990733743
- S.F.WolffFerrous ion oxidation in the presence of ferric ion indicator xylenol orange for measurement of hydrogen peroxidesMethods Enzymol2331994182189
- Y.XiaJ.L.ZweierMeasurement of myeloperoxidase in leukocyte-containing tissuesAnal Biochem24519979396
- N.P.TankoB.O.EmikpeM.Y.SabriChanges in haematological parameters and oxidative stress response of goats subjected to road transport stress in a hot humid tropical environmentComp Clin Path252016285293
- S.KayaO.MerhanC.KacarA.ColakK.BozukluhanDetermination of ceruloplasmin, some other acute phase proteins, and biochemical parameters in cows with endometritisVet World9201610561062
- J.S.LewisM.HoustonJ.AndersonIncreased levels of glutathione in bronchoalveolar lavage fluid from patients with asthmaAm Rev Respir Dis147199314611464
- M.DhimanJ.G.Estrada-FrancoJ.M.PandoF.J.Ramirez-AguilarH.SprattS.Vazquez-CorzoIncreased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with Chagas′ diseaseClin Vaccine Immunol162009660666
- B.SchmekelS.E.KarlssonM.LindenC.SundströmH.TegnerP.VengeMyeloperoxidase in human lung lavage. I. A marker of local neutrophil activityInflammation141990447454
- K.B.SchwarzOxidative stress during viral infection: a reviewFree Radic Biol Med211996641649
- L.GilG.MartinezR.TapanesO.CastroD.GonzalezL.BernardoOxidative stress in adult dengue patientsAmer J Trop Med Hyg712004652657
- A.D.RomeroN.L.GuerreroM.A.Gotor-LazaroE.Roche-ColladoOxidative stress and infectious pathologyAn Med Interna121995139149
- C.NisbetG.F.YarimS.O.GumusovaZ.YaziciInvestigation of the antioxidative metabolism in sheep with peste des petits ruminantsActa Vet (Beograd)572007351356
- E.PeterhansOxidants and antioxidants in viral diseases: Disease mechanisms and metabolic regulationJ Nutr1271997962S965S
- R.M.DyerS.ErneyP.SpencerC.E.BensonOxidative metabolism of the bovine alveolar macrophageAm J Vet Res501989448454
- N.IsraelM.A.Gougerot-PocidaloOxidative stress in human immunodeficiency virus infectionCell Mol Life Sci531997864870
