Abstract
Following previous studies on delivery potential and immune response of chickens given Newcastle disease vaccine with gums, this study was conducted to evaluate the protective ability of vaccines delivered with plant gums against clinicopathological features of Newcastle disease (ND). Processed gums from incised trunks of Cedrela odorata and Khaya senegalensis trees were combined with ND vaccine in ratio 2:2:1 and administered at 21 days to white leghorn cockerels after weaning of maternal antibodies. The birds were grouped into gum-vaccine-oral (GVOR), vaccine-oral (VOR), gum-vaccine-ocular (GVOC), vaccine-ocular (VOC), gum-oral (GOR), gum-ocular (GOC), no-gum-no-vaccine/challenged (NGNV/C), no-gum-no-vaccine/unchallenged (NGNV/U). Vaccination was boosted with the same preparation at day 42 while birds were challenged with live ND virus (KUDU strain) at day 84. Clinical signs (Dullness, Diarrhoea, Paralysis, Torticollis) Post infection (Pi), terminal weakness, gross and histology lesions were scored on a severity scale from absent (0-), mild (1+) to moderate (2+) and severe (3+). Scores were assigned a quantitative score of 0, 10, 20, 30 respectively. Clinical signs scores for the 5 week Pi were subjected to Friedman test to assess the significance of severity among the groups. The test was significant at 1% significance level which implies that the clinical signs ranked highest in the NGNV/C, followed by the Gum alone groups, the vaccine alone groups and the gum-vaccine groups irrespective of route. Moribund birds subsequently euthanized were seen in the GOR and GOC group at 21% each and at 57% in NGNV/C group alone. No signs were seen in the NVNG/U group. Grossly, mild to moderate lesions were seen in all groups except GVOR and NGNV/U. At histology, pulmonary congestion, acute pneumonia, cecal tonsilar haemorrhages, gliosis and neuronophagia were present at different proportions in all groups except the GVOR and NGNV/U. Overall, lesion severity was least in the gum-vaccine groups while the oral groups had less lesion score compared to the ocular. From this study, phytogenic mucoadhesives polymers used hold immense potential as a delivery agent capable of improving protection against clinicopathologic features of Newcastle disease in previously vaccinated birds.
1 Introduction
Newcastle disease is an infectious often fatal viral disease of poultry. It is endemic in most countries of the world with seasonal or sporadic epizootic spikes since first reported in England [Citation1,Citation2]. It is caused by Avian Paramyxovirus 1 (APMV-1) of the genus-Avulavirus in the family Paramyxoviridae which was formerly alongside grouped with other nine serotypes (APMV 2–9) into genus Rubulavirus until recently [Citation1,Citation3]. It is also responsible for major economic losses in both commercial and backyard flocks because outbreaks often reach 100% mortality in virulent strains [Citation4].
Broadly, there are 3 classifications of APMV-1 strains namely Velogenic (high virulence), Mesogenic (intermediate virulence) and Lentogenic (low virulence) although an overlap of these strain classes exists in the field [Citation4]. Severity of the disease often follows the virulence of the infecting strain amongst other factors such as host immune status, co-infections with other agents, age of host, environmental stress and endemicity [Citation5,Citation6].
Generally, non-specific signs of the disease could include depression, greenish diarrhoea, prostration and oedema of the head including the wattles, paralysis, torticollis and other respiratory signs. In layer flocks, drop to complete stoppage of egg production, small, spotted or soft shelled eggs have been reported to indicate the presence of the virus [Citation7]. However grossly, post mortem lesions could range from severe eyelid oedema, haemorrhages in the intestinal tracts (duodenum, jejunum and ileum), caeca tonsils, proventriculus, perithymic areas to necrohemorrhagic plugs in the ceca and small intestine. Splenic congestion, enlargement, stippled appearance extending from the capsule into the parenchyma could also be noticed in other strains [Citation4]. Catarrhal or caseous exudates may be present from associated secondary bacterial infection [Citation8]. Birds in lay could develop egg yolk peritonitis. The ovarian follicles may be flaccid and degenerative in growing pullets [Citation8,Citation9].
Histologically, depletion of mononuclear cells from the spleen, extensive fibrin deposits around the periarteriolar lymphoid sheaths, considerable destruction of lymphoid areas in the ceca and intestine with deposit of necrotic materials are often observed. In some cases, lymphoid depletion of the bursa and thymus might be present as well as ulcers in the small intestine. In the central nervous system, focal neuronal degeneration, gliosis, presenting with a non-suppurative encephalomyelitis are the main reported lesions [Citation10,Citation11].
Major control measures aside proper biosecurity involves the use of vaccines with recommended schedules. However, major constraint with existing vaccine protocols in developing nations with endemic ND burden involves short duration and inconsistent protective humoral response. The latter is associated with live attenuated vaccines administered orally or intraocular, while invasiveness/ease of administration and unwanted reactions such as egg drop are seen with parenteral oil based vaccines. Newer recombinant vaccines also have low coverage and are cost prohibitive. Hence these shortfalls have resulted in disharmonized or abuse of vaccine protocols among farmers [Citation9,Citation12].
Therefore the need to optimize antigenic uptake and elicit protective immune responses along mucosa surfaces with reduction of clinical signs and associated mortality resulting in economic losses influenced the purpose of this research. This study was aimed at evaluating the clinicopathologic features of challenged chickens previously vaccinated with ND vaccine using natural mucoadhesive biopolymers such as gums from plants. This was following recent reports by Emikpe et al. [Citation13] who reported strong mucoadhesive property of gums obtained from Cedrela odorata and Khaya senegalensis trees when used as vaccine vehicles with a suspected immunopotentiating action when investigated using ex-vivo means. Further, Oyebanji et al. [Citation14] reported higher, faster serum and mucosal humoral responses in an in vivo model with challenged chickens previously vaccinated against Newcastle disease using these gums as delivery vehicle. Studies have also shown these gums to contain polysaccharides such as arabinose, d-galactose, l-rhamnose, d-galacturonic acid and 4-O-60 methyl-d-glucoronic acid [Citation15,Citation16]. Conversely, in a separate report, Wiahenya, [Citation17] reported reduced morality and clinical symptoms of NDV infection as a results of using acemannan (a mannose derived polysaccharide) containing Aloe secundiflora in broilers. Hence this study attempts to evaluate the protection against clinical signs and pathologic lesions of Newcastle disease in chickens previously vaccinated using Cedrela odorata and Khaya senegalensis gum vehicle and subsequently exposed to a field viral agent.
2 Materials and methods
2.1 Ethical approval
All international protocols concerning animal studies were followed for this in vivo study.
2.2 Vaccine reconstitution and experimental groupings
Following recommended dilution for the gum delivery agent [Citation14], NDV Lasota (BN:183888) vaccine was combined with 1 in 8 bench dilution of 400 mg of purified and lyophilized Cedrela odorata and Khaya sengalensis gums (1:1) dissolved in 8 mL distilled water. This above mixture was the dilution needed to vaccinate exactly 200 birds giving 2 drops of constituted gum-vaccine preparation using 3 mL and 1 mL rubber pipettes per bird through the oral and ocular routes respectively as follows:
| • | Group A: Gum vaccine oral (GVOR) | ||||
| • | Group B: Vaccine oral (VOR) | ||||
| • | Group C: Gum vaccine ocular (GVOC) | ||||
| • | Group D: Vaccine ocular (VOC) | ||||
| • | Group E1: Gum alone oral (GOR)
| ||||||||||
| • | Group F1: No Gum No Vaccine/Challenged (NGNV/C)
| ||||||||||
Each vaccine vial was reconstituted as stated for the treatment groups. Birds were randomly grouped along the treatments into a number of 36 birds each and the NGNV/C and NGNV/U had 18 birds each.
2.3 Live virus for challenge test
Live Newcastle disease viral stock (KUDU strain) was acquired from the National Veterinary Research Institute, Vom Jos, Nigeria. The virus was inoculated into specific pathogen free (SPF) 9–11 day old embryonated chicken eggs to determine Embryo Lethal Dose (ELD) 50 dose as well as the titre. Allantoic fluid from the eggs with known titre per mL (105.5 per ELD) at 3.5 ELD50 was used for the challenge experiments at day 42 post booster vaccination.
2.4 Clinical features, gross, histopathology and scoring
Post infection, clinical signs; terminal weakness (euthanized and recorded as mortality), gross and histopathology lesions were recorded and scored as described by Emikpe et al. [Citation18] with modifications. Daily, presence or absence of clinical signs (dullness, diarrhoea, paralysis, and torticollis) with degree of severity was scaled as absent (0-); mild (1+), moderate (2+) and severe (3+). These scales were then assigned a score of 10 degrees such that 0− is assigned score of 0; 1+ assigned score of 10; 2+ assigned score of 20 and 3+ assigned score of 30. Scores for each clinical parameter were summed up as the collective clinical score for each group on a weekly basis. Gross lesions and histopathological were also scored as described above for lesions observed in main organs at the termination of experiment. Terminal weakness (euthanized and recorded as mortality) observed were recorded and percentage determined on a weekly basis. Post-infection, tissues were harvested and routinely processed for histologic examination. Briefly, samples were fixed in 10% formalin before trimming and sectioning for histology. Formalinized samples were dehydrated in grades of alcohol concentrations, cleared in xylene, and embedded in molten wax. On solidifying, sections of 5 µm thick were made with microtome (Leica RM2125 RTS), floated in water bath and incubated at 60°C for 30 min. The sectioned tissue was again cleared in grades of xylene and dehydrated in alcohol graded concentrations while sections were stained with routine hematoxylin and eosin stains. Examination of stained sections was done using light microscope (Olympus CH, XSZ107BN; 072351) while photomicrographs were taken with Ucmos series microscope camera Mu900 and Toupview 3.2 image software.
2.5 Statistical analysis
Friedman test (MINITAB version 16), a nonparametric alternative to the randomized complete block experiment was used to assess the significance of the clinical signs from the eight experimental groups for the five week observatory period post infection.
3 Results
The scored clinical signs, gross and histopathological lesion severity were presented in the bar charts (–). Post infection clinical signs observed were dullness, greenish watery diarrhoea, leg paralysis, torticollis, and terminal weakness recorded as mortality. From the clinical score in the treatment groups as presented in the Friedman test results () and illustrated in , severity of clinical signs ranked highest in the No-gum-No-Vaccine/Challenged group, followed by the Gum alone groups irrespective of route, then the vaccine alone groups and finally, the gum-vaccine groups irrespective of route. The No-gum-No-Vaccine/Unchallenged group showed least severity being the control uninfected.
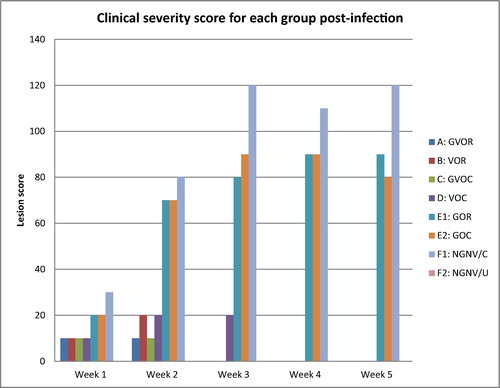
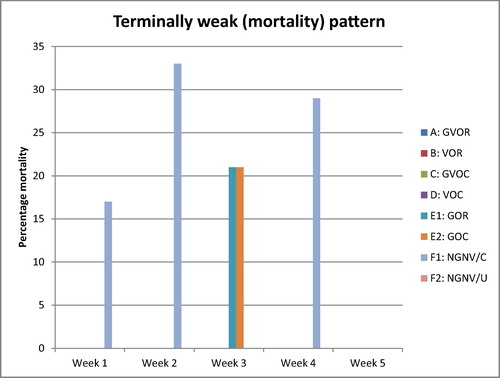
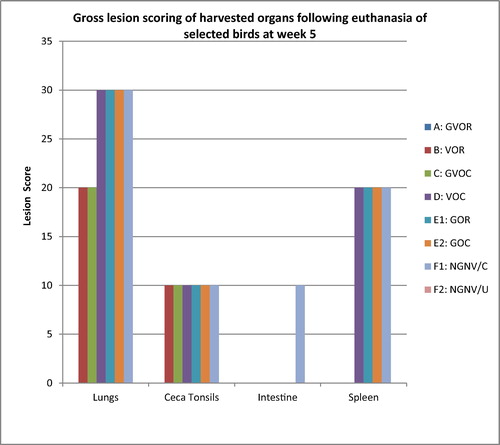
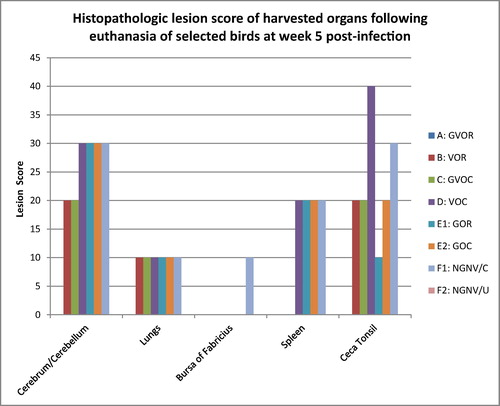
Table 1 Results of Friedman test for severity of observed clinical signs.
Further, in detail, first week post infection (PI), greenish diarrhoea was observed in all groups except the no gum-no-vaccine-unchallenged (NGNV/U) group. These became moderately severe in the vaccine alone group by the 2nd week and more severe in the gum alone and no-gum-no-vaccine/challenged (NGNV/C) groups throughout the PI period. By the day 9, diarrhoea sign had subsided in the Gum vaccine oral group (GVOR), day 15 in the vaccine oral group (VOR), and in the gum vaccine ocular groups (GVOC), however, it continued in the vaccine ocular (VOC) group till 3rd week before it subsided while it was present throughout the 5 weeks PI in gum alone and unvaccinated infected groups. Consequently, dullness was observed in the gum alone flock irrespective of the route as well as in the NGNV/C group throughout PI period beginning from 2nd week PI. Also, torticollis and leg paralysis followed a similar pattern in these groups.
Grossly, at termination of experiment, main organs that had visible lesions grossly across the groups with the exception of NGNV/U were the lungs, ceca tonsils, intestines (jejunum and ileum) and spleen. In the lungs, congestion and focal areas of consolidation were most severe in the vaccine ocular (VOC), gum oral (GOR), gum ocular (GOC), and no-gum-no-vaccine/challenged (NGNV/C), moderately severe in the vaccine oral (VOR) and gum-vaccine ocular (GVOC) groups while absent in the gun-vaccine oral (GVOR) and no-gum-no-vaccine/Unchallenged (NGNV/U) group. In the ceca tonsils, mild petechial haemorrhages were seen in all groups except the GVOR and NGNV/U groups. In the intestine, mild petechial to echymotic haemorrhages were seen only in the NGNV/C group. In the spleen, No lesions were observed for the GVOR, VOR, GVOC and NGNV/U groups. However, moderate splenomegaly with hyperplastic follicles were seen in the VOC, GOR, GOC and NVNG/C groups.
At histology, acute interstitial pneumonia, parabronchial infiltration of heterophils and mild pulmonary congestion were observed in all groups except the GVOR and NGNV/U groups (). In the cerebrum, severe gliosis, neuronal cell body necrosis and lymphocytic perivascular cuffing were observed in the VOC, GOR, GOC and NGNC/C groups, moderate lesions in VOR and GVOC while these lesions were absent in the GVOR and NGNV/U groups. In the spleen, moderate necrosis and depletion of lymphoid follicles were observed in the VOC, GOR, GOC and NGNV/C groups while these findings were absent in the GVOR, VOR, GVOR and NGNV/U groups. In the ceca tonsils, severe haemorrhagic necrosis with lymphoid depletion were observed in the VOC and NGNV/C groups while the lesions were moderate in VOR, GVOC, GOC and mild in the GOR groups. Bursa of Fabricius presented with mild lymphocytic depletion of follicles and disruption of follicular architecture only in the NGNV/C group.
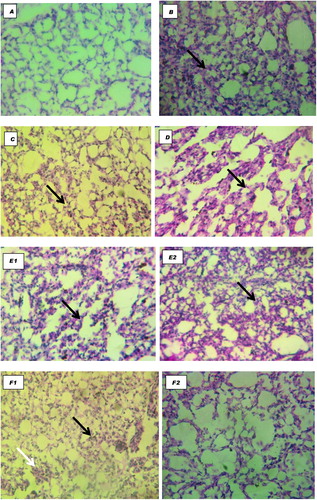
In summary, the severity of clinical signs, gross and histopathology changes could be graded from least severe to the most severe as follows:
| • | GVOR < NGNV/U < GVOC < GOR < VOR < VOC < GOC < NGNV/C. | ||||
4 Discussion
This study evaluates clinical signs and pathological changes observed in chickens challenged with a virulent field strain of Newcastle disease virus after prior and booster vaccinations with Newcastle disease vaccine delivered using gums from Cedrela odorata and Khaya senegalensis through the oral and ocular routes. From this study, it was evident that the vaccine used gave100% protection with regards to terminal weakness leading to mortality among vaccinated groups irrespective of the route or delivery agent used. This validates the effectiveness of the vaccine used as well as the protection offered by both routes against mortality induced by Newcastle disease in birds. Protective capacities of vaccination through oral and ocular routes in chickens have already been reported in literature [Citation19]. This is owing to the large surface area of the mucosa surface exposed to constant irritation and adapted for antigenic sampling and effector mechanisms [Citation14,Citation20].
However, significant difference exists in severity of clinical signs and post mortem lesions observed as the oral groups fared better than the ocular groups while the gum-vaccine groups showed lesser degree of clinical signs/post mortem lesions compared to the vaccine alone groups. This is especially true in the GVOR group where mild greenish diarrhoea was only observed for a duration of nine days post-infection (PI) without any other sign or post mortem lesion compared to GVOC, vaccine alone or gum alone groups where moderate to severe diarrhoea extended for longer duration. This observed degree of severity of clinical signs and absence/lesser degree of post mortem lesions in oral groups (GVOR, VOR and GOR) as against the ocular (GVOC, VOC, GOC) highlights the effect of route of administration. This was evident by moderate to mild lungs lesions such as congestion and acute pneumonia marked by presence of inflammatory cells present in the ocular groups but absent in the oral groups. This disparity might not be unconnected with larger surface area for antigenic induction, processing and more diffusely associated lymphoreticular structures with effector sites in the oral groups (gastrointestinal tract) compared to the ocular groups [Citation21].
The larger surface area of the oral route with more inductive and effector sites in the gastrointestinal mucosa i.e. more follicles with specialized M-cells, Peyer’s’ patches, ceca tonsils may help to elicit higher immune response than the ocular route with fewer surface area for antigen induction/effector. This might account for the preferential protection offered by the oral route compared to the ocular one. This report is however at variance with reports by Okwor et al. [Citation19] which favoured the intraocular route to the oral route mostly citing possible destruction of vaccine antigens by gastrointestinal secretions as probable reasons for reduced optimization of uptake and subsequent immune response. Hence, phytogenic gum used in this study might have mitigated the destruction of vaccine antigens thereby optimizing uptake, response and subsequent reduced clinicopathologic feature following challenge by field virus.
Further, the better protection offered by the addition of the gum to the vaccine given through the ocular route as against the vaccine alone along the same route supports the suspected immunopotentiating property of the gum [Citation14]. This could be mediated either through a slow antigen release ensuring prolonged antigenic exposure and stimulation or through an induction of a faster recall-memory response. This could ensure faster destruction of challenge live viruses since the birds were infected through the ocular route. Additionally, if the gum enhances immunologic memory induction, the principle of common mucosal immunity would assist in ensuring fairly even protection along mucosal effector sites thus reducing viral replication. Thus, under field conditions, protection along the ocular route could be maximized, by the addition of gums although the precise mechanism still remains to be evaluated.
Generally, the observed reduction in clinico-pathological features observed in the gum-vaccine groups might be related to the humoral systemic and mucosal responses to previous booster vaccination as suggested and demonstrated in our previous studies [Citation13,Citation14]. These studies showed a higher and faster peak humoral (mucosal and systemic) response (using HI and ELISA) [Citation14] in challenged chickens after prior and booster oral NDV vaccinations when gum vehicle was employed. Thus, this further highlights the importance of the gum vehicle in infection immune response when previously included in vaccine preparation.
Darabighane and Nahashon [Citation22] reported similar reduction of clinico-pathologic changes from other studies using plant parts. The report highlighted several studies about improved humoral, cellular responses as well as reduced clinical signs and lesions associated with different diseases as attributable to polysaccharides either present in plants used or given alone e.g. acemannan. In this study, as earlier stated, Cedrela odorata and Khaya senegalensis contain abundant polysaccharides. Thus, these polysaccharides may play a pivotal role in immunomodulation mechanisms observed leading to reduced clinical signs and pathologic changes in the gum-vaccine groups. Studies to validate this are presently underway.
Notably, presence of lung lesions and absence of marked lesions in the central nervous system, proventriculus and intestines of groups showing lesions might be attributed to the tropism of the infecting virus [KUDU] strain. Such reported preferential predilection of strains was reported by Wambura et al. [Citation23] who reported preferential tropism of I-2 NDV vaccine strain in lungs than V4 strain which favoured the intestine. Either way, the inclusion of gums in the oral delivery of Newcastle disease vaccine ensured protection against the challenge virus which seems to show more preference for the lungs.
5 Conclusions
In conclusions, the use of phytogenic mucoadhesives as delivery agents for Newcastle disease vaccines in chickens holds novel potentials in reduction of clinical and subsequent pathologic features associated with ND thus reducing economic losses usually observed with the disease. Further research studies are underway to evaluate the mechanism employed by gum vehicle in proffering better protection from clinical and pathologic changes of Newcastle disease observed in the study and immune response pattern observed from previous studies.
Competing interests
The authors of this manuscript have no competing interest.
Funding statement
We wish to acknowledge the Centre for Control and Prevention of Zoonoses (CCPZ), Faculty of Veterinary Medicine, University of Ibadan for the Mac Arthur post-graduate research grant awarded for the laboratory phase of this project.
Acknowledgement
The authors of project wish to acknowledge the support of clinical, anatomic pathology, and avian medicine laboratory workers and students that assisted with the procedures needed to complete this work.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- Alexander DJ. Newcastle disease and other avian Paramyxoviridae infections. In: Diseases of poultry, 10th Ed. BW. Calnek with HJ. Barnes, CW. Beard, LR. McDougald & YM. Saif, eds. Mosby-Wolfe, London 1997, p. 541–70.
- Health BC, Lindsey MS, McManus KP, Claxon PD. Newcastle disease vaccine for village chicken. Spadbrow P.D. (Ed). Australian Center for International Agricultural Research, Canberra, Australia 1991, p. 103.
- O.De LeeuwB.PeetersComplete nucleotide sequence of Newcastle disease virus: evidence for the existence of a new genus within the subfamily ParamyxovirinaeJ Gen Virol801999131136
- D.J.AlexanderNewcastle disease and other avian paramyxovirusesRev Sci Technol192000443462
- E.C.OkworD.C.EzeNewcastle disease in layers: preliminary studies on the stress associated with onset of lay and initiation of clinical diseaseAfr J Microbiol Res72013960965
- Newcastle disease. General diseases information sheet. Retrieved from http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/Disease_cards/NEWCAS-EN.pdf. World Organisation for Animal Health (OIE). [accessed 18:02:17].
- Y.M.SaifH.J.BarnesJ.R.GlissonA.M.FadlyL.R.McDougaldD.E.SwayneDiseases of poultry11th ed.2005Iowa State University PressAmes
- Afayoa M. Lesions and prevalence of Newcastle disease in chicken presented for necropsy at faculty of veterinary medicine, Makerere University. Dissertation submitted to the graduate school in partial fulfilment of the requirements for the award of Master of Science in veterinary pathology of Makerere University, http://makir.mak.ac.ug/handle/10570/2221; 2010 [accessed 01:02:17].
- E.C.OkworD.C.EzeThe annual prevalence of Newcastle disease in commercial chickens reared in South Eastern Savannah zone of NigeriaRes J Poult Sci320102326
- Spadbrow PD. Epidemiology of Newcastle disease and the economics of its control. Proceeding of the workshop on poultry as at tool in poverty eradication and promotion of gender equality, March 23–26, Tune Landboskole, Denmark; 1999. p. 1–6.
- S.B.OladeleP.A.AbduA.J.NokK.A.N.EsievoN.M.UsehPreliminary reports on neuraminidase, erythrocyte surface and free serum sialic acid concentrations in serum of healthy and Newcastle infected ChickensMed Vet Pays Trop552002265268
- O.E.OluwoleB.O.EmikpeB.O.OlugasaAttitude of poultry farmer towards vaccination against Newcastle disease and Avian influenza in Ibadan, NigeriaSokoto J Vet Sci102012512
- B.O.EmikpeV.O.OyebanjiM.A.OdeniyiA.M.SalaamA.O.OladeleT.A.JarikreEx-vivo evaluation of the mucoadhesive properties of Cedrela odorata and Khaya senegalensis gums with possible applications for veterinary vaccine deliverySpringerPlus520161289
- V.O.OyebanjiB.O.EmikpeA.O.OladeleM.A.OdeniyiA.SalamiO.I.OsowoleEvaluation of immune response in challenged chickens vaccinated with Newcastle disease vaccine using gums from Cedrela odorata and Khaya senegalensis as delivery agentsJ Immunoass Immunochem232016111
- G.N.de TroconisM.MartinezL.G.de PintoA.BhasasEstudio químico y espectroscó pico del polisacá rido de la goma de Cedrela odorataCiencia92001 235–3
- M.A.OdeniyiN.H.KhanK.K.PehRelease and mucoadhesion properties of diclofenac matrix tablets from natural and synthetic polymer blendsActa Polo Pharma – Drug Res722015 559-7
- R.WaihenyaM.MtamboG.NkwengulilaEvaluation of the efficacy of the crude extract of Aloe secundiflora in chickens experimentally infected with Newcastle disease virusJ Ethnopharmacol792002299304
- B.O.EmikpeP.N.TankoO.M.OniludeM.Y.SabriThe influence of dexamethasone treatment and successive road transport stress on the occurrence of caprine pneumonia in a hot humid tropical environmentVet World62013497501
- E.C.OkworD.C.EzeO.M.UzuegbuComparative studies on the oral and intraocular routes of administration of Newcastle disease vaccine, La Sota in adult chickensJ Agric Vet Sci320134851
- M.F.PeraltaM.G.M.DanelliA.VivasRediscovering the importance of Mucosal Immune System (MIS) in poultryAcad J Biotechnol420169195
- A.FixL.H.ArpMorphologic characterization of conjunctiva-associated lymphoid tissue in chickensJ Vet Res52199118521859
- B.DarabighaneS.N.NahashonA review on effects of Aloe vera as a feed additive in broiler chicken dietsAnn Anim Sci142014491500
- P.WamburaJ.MeersP.SpradbrowDetermination of organ tropism of Newcastle disease virus (Strain I-2) by virus isolation and reverse transcription-polymerase chain reactionVet Res Commun302006697706
