Abstract
Canine cranial cruciate ligament rupture (CrCLR) is a very common pathology. Surgical stabilization is the first choice treatment, although it does not fully eliminate the increased risk of osteoarthritis. This preliminary study was carried out to explore whether a newly formulated joint health supplement would benefit metabolic, clinical and radiographic changes in dogs with CrCLR surgically treated with tibial plateau leveling osteotomy (TPLO). Besides chondroitin sulfate and glucosamine hydrochloride, the studied supplement contained anti-inflammatory and antioxidant ingredients, the main ones being N-palmitoyl-D-glucosamine (Glupamid®) and quercetin. It was thus intended to target not only chondrodegenerative components of osteoarthritis, but also post-injury inflammatory response and oxidative stress of joint tissues. Thirteen dogs underwent TPLO and were randomly allocated to treatment (n = 6) and control groups (n = 7), the former receiving the joint supplement for 90 days. Lameness and radiographic osteoarthritis changes were scored before (i.e., baseline) and at 30 and 90 days post-surgery. Synovial fluid samples were collected from injured stifles at the same time points. Levels of representative metabolites were measured by proton nuclear magnetic resonance spectroscopy in a blinded fashion. In the metabolomic analysis, special attention was paid to lactate, due to its emerging recognition as a key marker of inflammation. In the last time period (from the 30th to the 90th day), lameness improved by a factor of 2.3 compared to control dogs. No significant difference was observed in the radiographic osteoarthritis score between groups. In the first postoperative month, lactate and creatine levels significantly dropped in treated compared to control dogs. Compared to surgery alone, combining the joint supplement with TPLO resulted in a trend to a better clinical outcome in the later time interval but did not influence osteoarthritis radiographic progression. A significantly better rebalance of joint microenvironment in the early time interval (baseline – 30 days) was shown by metabolomic analysis, thus suggesting that the study supplement could limit ongoing inflammatory responses.
1 Introduction
Rupture of the cranial cruciate ligament (CrCLR) is one of the most common orthopaedic problems in dogs and an important cause of hindlimb lameness and osteoarthritis (OA) [Citation1]. Surgery is the gold standard to stabilize the stifle [Citation2]. Among several techniques used to provide joint stability, tibial plateau leveling osteotomy (TPLO) is currently the most preferred [Citation2,Citation3], especially in medium- to large-breed dogs. Nonetheless, and like other surgical procedures [Citation4,Citation5], TPLO neither reduces nor halts progression of OA [Citation6–Citation9]. Therefore, the recommended treatment for CrCLR should also target secondary OA. Although the mechanisms leading to OA following ligament injury have not been fully established, chondrodegeneration and inflammatory changes are both considered important contributors [Citation9–Citation11]. Dietary supplements for joint health have gained increased recognition as valuable management options within the combined therapy for OA [Citation12]. The efficacy of joint supplements is clearly formulation-dependent, relying mainly on their respective composition (i.e., ingredients used, relative amounts, excipients, purity, etc). Since the 1990s, a wide variety of compounds with different chemical structures, bioavailability, mechanism of action and degree of purity have been introduced in the veterinary market as chondroprotective agents, i.e. substances specifically aimed to rebalance the metabolism of degenerating cartilage, by boosting the synthetic pathways (pro-anabolic effects) while inhibiting degradative responses (anti-catabolic effects) [Citation13,Citation14]. More recently, a new class of joint supplements has been developed, able not only to support or enhance the articular intrinsic repair capability (chondroprotection sensu strictu), but also exert anti-inflammatory and analgesic effects and improve the intrarticular oxidative status [Citation12,Citation15,Citation16]. The joint supplement used in the present study as an add-on/complementary treatment belongs to this latter class. In this formulation, classical chondroprotective compounds (chondrointin sulfate and glucosamine) are combined with an antioxidant and anti-inflammatory agent to target both OA-associated inflammation and pain. N-palmitoyl-d-glucosamine and quercetin are the main constituents responsible for the latter activities, largely due to their anti-inflammatory, antioxidant and pain relieving effects [Citation17–Citation19].
Metabolomics is the “omic” technique aimed to study metabolic OA profiling (metabolome), and is considered a promising strategy to follow disease progression and evaluate the effect of disease-modifying therapies [Citation20–Citation22]. One of the most attractive high-throughput technologies for global screening of joint metabolites is high resolution proton nuclear magnetic resonance spectroscopy (1H NMR) [Citation23,Citation24]. It is usually performed on synovial fluid samples, since metabolic joint products are most likely to accumulate earlier and at higher concentrations compared to other biological fluids (e.g., serum, urine) [Citation25,Citation26]. In evaluating the anti-inflammatory effects of the study supplement, particular attention was paid to synovial changes in lactate, since increased levels of this metabolite have been related to the degree of inflammation in metabolomic studies of joint disease [Citation23,Citation27].
This preliminary study was carried out to explore metabolic, clinical and radiographic outcomes in dogs with spontaneous ligament rupture surgically treated with TPLO and supplemented or not with a joint health supplement. Our working hypothesis is that addition of the study joint supplement to surgery may benefit joint metabolome, lameness and OA radiographic progression during the study period, in light of the purported chondroprotective and anti-inflammatory activities of said formulation.
2 Materials and methods
2.1 Animals
The study was designed as a 90-day, open-label, controlled preliminary trial, using an untreated group as control. Medium to large-breed client-owned dogs with naturally occurring unilateral CrCLR referred for TPLO were enrolled. The following inclusion criteria had to be met: (i) complete unilateral CrCLR, with no contralateral stifle problems as assessed clinically and radiographically; (ii) acute ligament injury, i.e., occurring less than 20 days before entering the study as determined by information provided by the owner or referring veterinarian; (iii) body weight between 15 and 55 kg; (iv) age between 12 months and 8 years; (v) mild to moderate stifle OA, corresponding to scores 1 and 2 on a 4-point radiographic grading system [Citation28]. Exclusion criteria were any abnormality in the complete blood count and serum biochemical profile, any history or evidence of previous stifle joint surgery, and other orthopaedic and neurologic diseases. Additional exclusion criteria were concomitant meniscal lesions (as confirmed by arthroscopic meniscal inspection before TPLO procedure), and concurrent treatment with non-steroidal anti-inflammatory drugs and corticosteroids. All owners gave informed consent before enrolment of their dog and approval from the local ethical committee was obtained. Breed, gender, age, body weight and clinical history of each dog were recorded.
2.2 Surgery
All surgeries were performed by the same surgeon (F.M.M.) according to the TPLO technique as described by Slocum and Slocum [Citation29] and modified by Pozzi et al. [Citation30] without meniscal release. The hind limb was clipped and prepared for surgery in a routine manner. A skin incision was made medially from approximately the distal fourth of the femur to the proximal third of the tibia. Stifle arthroscopy was performed before TPLO in all procedures. The jig was not applied [Citation31] and a tibial osteotomy was made using an 18 mm, 24 mm or 30 mm biradial saw blade. The proximal tibial segment was then rotated according to Slocum’s recommendations on the basis of the tibial plateau angle calculated from radiographs obtained prior to surgery. All osteotomies were stabilized with one or two anti-rotational Kirschner wires placed from the tibial crest directed caudo-distally to exit the caudal cortex of the proximal fragment. The osteotomy was secured by either a 3.5-mm SLOCUM TPLO plate, 3.5-mm small SLOCUM TPLO plate, 3.5-mm TPLO SYNTHES locking compression plate, 3.5-mm broad TPLO SYNTHES locking compression plate, 3.5-mm SECUROS TPLO plate or 3.0/3.5-mm FIXIN T Support, based on the size and temperament of the dog. The K-wires were removed after application of the bone plate. Postoperative prophylaxis consisted of cefadroxil (20 mg/kg q12h PO for 5 days), carprofen (3 mg/kg q24h PO for 7 days) and tramadol if needed (3 mg/kg q6h PO days 1–3). A modified Robert Jones was applied for 48 h postoperatively. All owners received written instructions concerning postoperative care and follow-up examinations. Instructions for postoperative confinement and the 8-week rehabilitation period were based on TPLO course recommendations [Citation32].
2.3 Treatment
After TPLO, dogs were randomly allocated into two groups according to a simple randomization procedure. Group C (control) received no further treatment. Group T (treated) received the joint health nutraceutical,Footnote1 administered daily at a dose of 1 tablet/25 kg b.w./PO for 90 days, starting on the day after surgery. Each tablet had the following composition: low molecular weight normosulfated chondroitin sulfate (NSCS 5/20 patented fraction), 200 mg; glucosamine hydrochloride, 300 mg; N-palmitoyl-d-glucosamine (Glupamid®), 100 mg; quercetin, 75 mg; Vitamin E, 50 mg; ω3 essential fatty acids, 50 mg.
2.4 Synovial fluid sample collection and preparation
Synovial fluid samples (0.5 mL each) were aseptically collected by arthrocentesis from the affected stifle joints before surgery (baseline visit, V0), and on days 30 and 90 after TPLO (V30 and V90, respectively). Samples were filtered using membranes of 0.8 µm porosity, centrifuged at approximately 17,000 g for 15 min in order to separate the cells and stored at −80 °C. The main metabolites present in canine synovial fluid samples were studied by 1H NMR spectroscopy, in order to investigate potential differences between treated and control groups. This technique allows a one-shot, high-throughput multicomponent analysis of biological fluids with the advantage of not requiring sample pre-treatment [Citation23,Citation24,Citation33]. Briefly, 420 µL of each sample were mixed with 280 µL of 0.23 M solution of 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS) in deuterium oxide (Sigma-Aldrich Co, St. Louis, USA) and then poured into 5 mm diameter 1H NMR tubes. DSS was used as a standard reference, both to calibrate the chemical shift scale (δ = 0.00 ppm) and to quantify the synovial fluid metabolites.
2.5 Proton NMR spectroscopy
All measurements were performed on a Bruker Avance III spectrometer (Bruker, Karlsruhe, Germany), operating at 400.13 MHz for 1H observation and a temperature of 298.13 K, equipped with a BBI 5 mm direct detection probe incorporating a z axis gradient coil. For each sample, after a 5-min waiting period for temperature equilibration, a standard one-dimensional 1D zgpr, cpmgpr (Carr–Purcell–Meiboom–Gill spin-echo sequence, CMPG) and 2D 1H J-resolved NMR spectra were recorded, using the standard presaturation sequence for the water signal suppression. Spectra were obtained by the following conditions: zgpr pulse program, 32 K time domain, spectral width of 20.0264 ppm (8000 Hz), p1 (F1 channel-90° 1H transmitter pulse) 8.68 μs, pl1 −1.00 db (decibel), 16 repetitions; cpmgpr pulse program 32 K time domain, spectral width 20.0264 ppm (8000 Hz), p1 8.9 μs, pl1 −1.00 db (decibel), 256 repetitions; jresgppsqf pulse program 8 K time domain, spectral width 11.9851 ppm (4795.396 Hz), pl1 −1 db (decibel), 32 repetitions for 128 experiments. NMR data were processed using TopSpin 2.1 (Bruker) and visually inspected using Amix 3.9 (Bruker). The spectral region studied in all spectra was between 0.0 and 6.0 ppm. The metabolites (qualitative analysis) were assigned on the basis of 1D (cpmgpr) and 2D (1H Jresolved) NMR spectra analysis and compared with published data [Citation33–Citation36]. Qualitative and quantitative analysis of spectra was limited to those low molecular weight components which were most easily detectable because of their high concentration, or due to their resonance occurring in areas of the spectrum free of other signals. The presence of high molecular weight components (e.g., hyaluronic acid), producing background resonance broadened between 3 and 4 ppm; overlap with signals arising from other low molecular weight metabolites was minimized by particular devices, such as the initial sample filtration, application time of the excitation pulse and relaxation time. The relative concentration of each metabolite was obtained by calculating in the zgpr acquired spectra the integrals of the areas selected from time to time, referenced to a known concentration of DSS (prepared in such a proportion as to be comparable with that of metabolites present in the spectrum). From the concentrations obtained, relating to the number of protons in the molecule, absolute concentrations of metabolites were calculated as ratio of the molar concentration of DSS, and subsequently of the dilution factor used to prepare the sample [Citation37]. All samples were processed by a technician who was blind to treatment. In view of the primary purpose of the study, only the most representative of all identifiable resonances for the joint compartment were analyzed. Particularly, synovial fluid levels of nine metabolites which are considered to give information about inflammatory and degradative status of the joint (lactate, pyruvate, acetate, N-acetyl groups, glucose, glycerol, choline, creatine and alanine) were quantified in each sample and compared between groups.
2.6 Orthopaedic and radiographic examinations
Each dog was examined for hindlimb lameness according to a previously described scoring system (), ranging from 1 to 5 [Citation38]. Lameness was assessed before surgery (V0) and on days 30 and 90 after TPLO (V30 and V90, respectively). At the same time points, orthogonal radiographs (caudocranial and mediolateral views) of the affected stifle joint were obtained. For the mediolateral view; the stifle was flexed at 90 degrees [Citation29]. An OA score for each stifle was determined as previously described [Citation28] by evaluating joint structures (patella, femur, tibia, and surrounding soft tissues) for 32 specific radiographic features, including but not limited to osteophytes, enthesiopathy, subchondral cystic lesions and soft tissue thickening (). Each feature was weighted equally and scored as 0 = absent, 1 = mild, 2 = moderate or 3 = severe, thus giving an absolute OA score for each stifle ranging from 0 to 96.
Table 1 Lameness scoring system.
Table 2 Radiographic osteoarthritis score (0 = absent, 1 = mild, 2 = moderate or 3 = severe for each feature, with an absolute 0–96 score for each stifle).
2.7 Data analysis
The mean, median and standard deviation (SD) values of demographics were used for descriptive purposes. The mean, median and standard error (SE) values of 1H NMR synovial metabolites, OA and lameness scores were calculated at all time points. For the statistical analysis of the effect SE was preferred to SD since it better represents the variability of the means. The generalized linear mixed model for repeated measures was used to analyze mean global differences between the two groups. A Kruskall-Wallis post hoc test with Bonferroni-Holm P correction for family-wise error was performed to analyze single differences between groups at study intervals (preoperative and at one and three months postoperative). Due to the ordinal-level nature of the orthopaedic evaluation (i.e., 5-point scoring system) Wilcoxon and Kruskal-Wallis tests were used for analyzing the treatment effect and the difference between groups, respectively. Data were analyzed using SAS v9.2 (SAS Institute, Cary, NC, USA). The significance threshold was set at 0.05. Exact P values are reported.
3 Results
Thirteen dogs were enrolled in the study. Demographic characteristics are summarized in . All dogs had acute lameness lasting between 7 and 20 days. There was no significant difference between groups in terms of body weight (P = 0.60), gender distribution (P = 0.72) and age (P = 0.87) ().
Table 3 Demographics of the enrolled dogs by group.
3.1 Synovial fluid metabolites
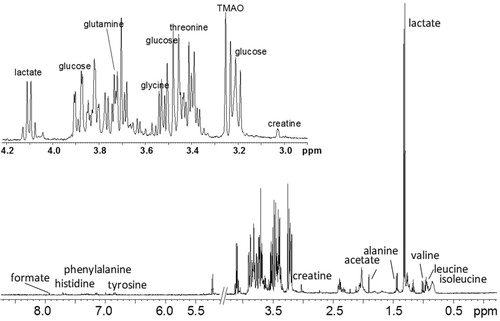
In total, 39 1H NMR spectra were acquired (13 dogs × 3 time points). Relevant peaks, related to the studied metabolites, in a typical synovial fluid cpmgpr 1D spectrum, are shown in . Of the analyzed metabolites, lactate and creatine showed significant differences between groups and are detailed below.
3.1.1 Lactate
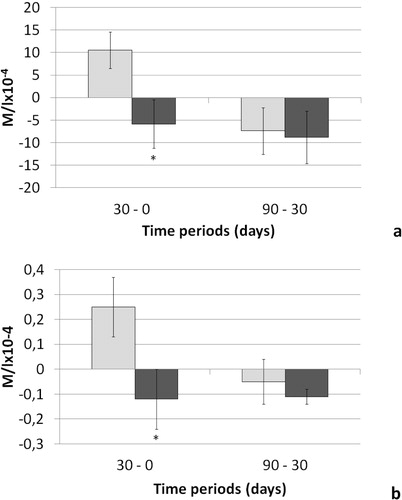
Lactate was detected in the spectral range of 4.0–4.2 ppm. The mean delta of lactate synovial fluid concentration between baseline and V30, i.e. one month after surgery, was negative in T dogs (−5.86 M/l × 10−4) and positive in C dogs (10.51 M/l × 10−4) (). In this time interval the difference between groups was statistically significant (P = 0.03), while it was not in the later one (−7.40 M/l × 10−4 and −8.85 M/l × 10−4 for the C and T groups, respectively, ).
3.1.2 Creatine
Creatine was detected in the spectral range of around 3.3 ppm. As observed for lactate, the mean delta concentration for creatine in the first post-operative month (V0-V30) was statistically significantly different between groups (P = 0.04, ). In particular, the mean delta of creatine synovial fluid concentration was negative in group T dogs (−0.12 M/l × 10−4) and positive in C group dogs (0.25 M/l × 10−4, ). In the second time interval (V30-V90), mean delta values failed to show any significant difference between groups (−0.05 M/l × 10−4 and −0.11 M/l × 10−4 in the C and T groups, respectively, ).
3.2 Radiographic findings and lameness score
There was no significant difference in the mean OA scores over time by group () either in terms of total score (P = 0.70) or on each stifle component score (for single P values see ).
Table 4 Radiographic osteoarthritis scores at study time points.
The mean lameness score significantly improved over time, regardless of treatment (P = 0.011 and P = 0.010 for C and T groups, respectively, ). There was no significant difference between groups (P = 0.60, ). Thirty days after surgery, 3/6 (50%) and 4/7 (57%) dogs in the T and C groups, respectively, scored 1 on the lameness scoring system, corresponding to “stands and walks normally”. At the following visit (V90) of those dogs that could have further improved, 2/3 (67%) of the T group and 1/3 (33%) of the C group actually showed a lower lameness score (i.e., score = 1). Finally, in the last time period (V30-V90), the improvement achieved by treated dogs was 2.3-fold higher compared to control dogs ().
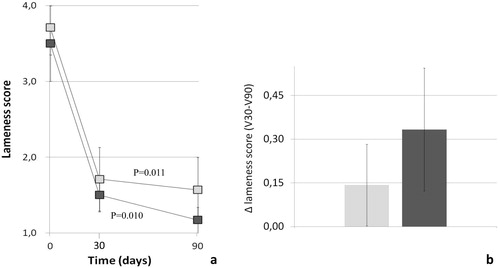
Table 5 Lameness scores at study time points.
4 Discussion
This work is the first exploratory study evaluating the effects of combining a joint health supplement with TPLO in dogs with naturally-occurring unilateral CrCLR. The findings suggest that the supplement utilized favoured a rapid rebalance of the joint microenvironment, mainly on the inflammatory side. Consistent with the metabolic aspect of the present study’s hypothesis, the decrease in synovial fluid concentrations of both lactate and creatine was significantly higher in the treated compared to control dogs during the first month after surgery. Lactate is an end-product of anaerobic glycolysis and its synovial fluid levels are increased during canine OA [Citation27,Citation34] and human joint inflammation [Citation39], probably the result of a predominantly anaerobic metabolism due to increased oxidative stress and low-grade inflammation. The development of inflammation following CrCLR is confirmed by the significant increase of a variety of pro-inflammatory cytokines [Citation40] and the occurrence of histological signs of inflammation [Citation41]. The effect on lactate observed here thus suggests an early anti-inflammatory effect exerted by the study supplement. This effect is consistent with previous findings showing that combining a supplement similar to the one used here with reconstruction surgery significantly limited the increase of lactate compared with surgery alone [Citation33].
To the best of our knowledge this is the first observation of a rebalancing effect on the levels of creatine in synovial fluid. Creatine is considered an intermediate of muscle energy metabolism that increases during OA, both in horses and dogs [Citation34,Citation42]. The significantly higher decrease that we found in the treated group compared to controls would be consistent with an eventual rebalancing effect on muscle metabolism. Taken together, the results on synovial fluid metabolites suggest that, when associated with TPLO, the supplement used here has the potential to speed up rebalance of the joint microenvironment compared to surgery alone, particularly in terms of inflammation. The study joint supplement consisted of a mixture of feed materials (Regulation (EU) No 68/2013 on the Catalogue of feed materials and following amendments), principally normosulfated low molecular weight chondroitin sulfate, N-palmitoyl-d-glucosamine and quercetin. N-palmitoyl-d-glucosamine (Glupamid®) is a congener of the parent aliamide molecule palmitoylethanolamide, a broad-acting anti-inflammatory and neuroprotective lipid mediator [Citation43]. It is the amide of palmitic acid and glucosamine and is thought to exert not only the characteristic chondroprotective effects of glucosamine [Citation12] but also the anti-inflammatory/anti-nociceptive activities of aliamides [Citation44,Citation45]. In particular, N-palmitoyl-d-glucosamine down-modulates mast cell behavior – the so called ALIA (Autacoid Local Injury Antagonism) mechanism [Citation46]. In this context it is rather interesting, as synovial mast cell hyperreactivity is viewed as a potential new target for the treatment of joint inflammation and pain [Citation11].
In an experimental model of OA, N-palmitoyl-d-glucosamine significantly relieved joint pain [Citation17]. The studied supplement also contains quercetin, an anti-oxidant flavonoid able to significantly limit oxidative damage in canine chondrocytes [Citation47], protect joints from inflammation [Citation19], inhibit overproduction of reactive oxygen species and reduce pro-inflammatory cytokines [Citation18]. When co-micronized with palmitoylethanolamide, quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain models [Citation48]. Conceivably, N-palmitoyl-d-glucosamine and quercetin contained in the supplement used here may be responsible for the early anti-inflammatory effect observed in treated dogs compared to control, as reflected by changes in synovial fluid levels of lactate and creatine during the first postoperative month. Unfortunately this effect did not correspond to a better radiographic outcome in the treated compared to control dogs. The hypothesis of a favorable influence of the study supplement on OA progression was thus not supported by these findings. This could depend, in part, on the longer times usually required to achieve structurally (radiographic) detectable changes after TPLO surgery [Citation8,Citation28,Citation49,Citation50] and also after administration of joint supplements [Citation12]. As radiographic analysis only visualizes the effect on bony structures, short-term changes would not have been detected. Longer study duration and/or larger sample size could have helped in revealing the difference.
A statistically significant reduction in lameness score was observed in both the treated and control groups. This finding is consistent with previous observations concerning improvement in joint function starting 4–6 weeks after TPLO [Citation51]. It is, however, interesting to note that during the last observation period (i.e., V30-V90) the improvement achieved by treated dogs was 2.3-fold higher compared to control dogs. In other words, this functional improvement was 130% higher with respect to control dogs. Although not reaching statistical significance, the magnitude of this difference could suggest an adjunctive clinical benefit of the supplement over surgery and support, in principle, our hypothesis.
The principal limitation of the present study is its small sample size, largely due to challenges in recruitment and strict inclusion/exclusion criteria. For example, this factor might have prevented uncovering findings such as other synovial metabolite differences. A further limitation is the absence of synovial fluid sampling from the contralateral healthy stifle. Although ethical and technical issues (both considered mandatory) prevented arthrocentesis of healthy joints, the diseased to healthy stifle ratio/subtraction could have minimized intrasubject variability. Last, but not least, is the relatively short duration of the observation period (3 months) chosen to encourage owner compliance. These caveats notwithstanding, we show that associating the study dietary supplement for joint health with TPLO results in a significantly better metabolic outcome within the joint compartment at one month post-surgery, suggesting an early down-modulation of inflammatory responses. Moreover, a trend to better clinical outcome in the later time interval was also observed. Clearly, studies on larger sample sizes and longer durations will be needed to confirm these initial observations. Nevertheless, these preliminary findings might have interesting implications in the management of CrCLR if one considers that low grade inflammation may worsen or even precede related chondrodegeneration [Citation52,Citation53] and is considered a risk factor for some postoperative TPLO complications [Citation54].
5 Conclusions
This study provides the first evidence that, when combined with TPLO, a joint supplement containing N-palmitoyl-D-glucosamine and quercetin (besides chondroprotective substances) results in an early improvement of the joint inflammatory microenvironment. Although not statistically significant, the more than twofold improvement in lameness score during the last observation period (30–90 day interval) points to a possible functional advantage of the add-on nutraceutical treatment. Given the non-pharmacologic nature of the study supplement and these interesting, albeit preliminary, findings, use of joint health nutraceuticals targeting inflammation besides chrondrodegeneration should be considered in the combined treatment to canine OA.
Competing interests
Alda Miolo is an employee of Innovet Italia Srl. No conflicts of interest have been declared by other authors.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors would like to thank Dr. Cristian Negri (University of Salento) for help in collecting and organizing the 1H NMR data. We are indebted to Stephen D. Skaper (Department of Pharmaceutical and Pharmacological Sciences, University of Padua) for critical reading and English proofreading of the manuscript, and to Carlo Schievano (Innovative Statistical Research SRL, Padua) for the statistical analysis. The authors are grateful to Innovet Italia Srl for kindly providing the study supplement.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
1 Condrostress®, Innovet Italia Srl, Milan, Italy.
References
- G.HarasenCanine cranial cruciate ligament rupture in profileCan Vet J442003845846
- S.M.BerghC.SullivanC.L.FerrellJ.TroyS.C.BudsbergSystematic review of surgical treatments for cranial cruciate ligament disease in dogsJ Am Anim Hosp Assoc502014315321
- F.M.DuerrK.W.MartinM.RishniwR.H.PalmerL.E.SelmicTreatment of canine cranial cruciate ligament disease. A survey of ACVS Diplomates and primary care veterinariansVet Comp Orthop Traumatol272014478483
- J.F.InnesD.BaconC.LynchA.PollardLong-term outcome of surgery for dogs with cranial cruciate ligament deficiencyVet Rec1472000325328
- J.M.JohnsonA.L.JohnsonCranial cruciate ligament rupture. Pathogenesis, diagnosis and postoperative rehabilitationVet Clin North Am Small Anim Pract231993717733
- J.BoddekerS.DruenA.Meyer-LindenbergM.FehrI.NolteP.WefstaedtComputer-assisted gait analysis of the dog: comparison of two surgical techniques for the ruptured cranial cruciate ligamentVet Comp Orthop Traumatol2520121121
- C.R.HurleyD.L.HammerS.ShottProgression of radiographic evidence of osteoarthritis following tibial plateau leveling osteotomy in dogs with cranial cruciate ligament rupture: 295 cases (2001–2005)J Am Vet Med Assoc230200716741679
- J.A.LinebergerD.A.AllenE.R.WilsonT.A.TobiasL.G.ShaikenJ.T.ShiromaComparison of radiographic arthritic changes associated with two variations of tibial plateau leveling osteotomyVet Comp Orthop Traumatol120051317
- S.SuttonA.ClutterbuckP.HarrisT.GentS.FreemanN.FosterThe contribution of the synovium, synovial derived inflammatory cytokines and neuropeptides to the pathogenesis of osteoarthritisVet J17920091024
- E.J.ComerfordK.SmithK.HayashiUpdate on the aetiopathogenesis of canine cranial cruciate ligament diseaseVet Comp Orthop Traumatol2420119198
- M.FuscoS.D.SkaperS.CoaccioliA.PaladiniG.VarrassiDegenerative joint diseases and neuroinflammationPain Pract172017522532
- Y.HenrotinC.SanchezM.BalligandPharmaceutical and nutraceutical management of canine osteoarthritis: present and future perspectivesVet J1702005113123
- R.McLaughlinManagement of chronic osteoarthritic painVet Clin North Am Small Anim Pract302000933949
- P.S.McNamaraS.C.BarrA.IdouraineL.LippielloEffects of an oral chondroprotective agent (Cosequin) on cartilage metabolism and canine serumVet Comp Orthop Traumatol10199761
- B.S.BealeUse of nutraceuticals and chondroprotectants in osteoarthritic dogs and catsVet Clin North Am Small Anim Pract342004271289
- C.L.AragonE.H.HofmeisterS.C.BudsbergSystematic review of clinical trials of treatments for osteoarthritis in dogsJ Am Vet Med Assoc2302007514521
- Costa B, Comelli F, Miolo A, della Valle MF. Effect of Glupamid (N-palmitoyl-d-glucosamine) on knee osteoarthritis pain. In: Proceedings 3rd WVOC (World Veterinary Orthopedic) Congress 2010, Bologna, Italy, p. 553–554.
- G.CarulloA.R.CappelloL.FrattaruoloM.BadolatoB.ArmentanoF.AielloQuercetin and derivatives: useful tools in inflammation and pain managementFuture Med Chem920177993
- N.HaleagraharaS.Miranda-HernandezM.A.AlimL.HayesG.BirdN.KetheesanTherapeutic effect of quercetin in collagen-induced arthritisBiomed Pharmacother9020173846
- M.LotzJ.Martel-PelletierC.ChristiansenM.L.BrandiO.BruyèreR.ChapurlatValue of biomarkers in osteoarthritis: current status and perspectivesPostgrad Med J902014171178
- R.PrioriR.ScrivoJ.BrandtM.ValerioL.CasadeiG.ValesiniMetabolomics in rheumatic diseases: the potential of an emerging methodology for improved patient diagnosis, prognosis, and treatment efficacyAutoimmun Rev12201310221030
- S.B.AdamsJr.L.A.SettonD.L.NettlesThe role of metabolomics in osteoarthritis researchJ Am Acad Orthop Surg2120136364
- J.M.DuffyJ.GrimshawD.J.GuthrieG.M.McNallyR.A.MollanP.L.Spedding1H-nuclear magnetic resonance studies of human synovial fluid in arthritic disease states as an aid to confirming metabolic activity in the synovial cavityClin Sci851993343351
- K.ShetS.M.SiddiquiH.YoshiharaJ.KurhanewiczM.RiesX.LiHigh-resolution magic angle spinning NMR spectroscopy of human osteoarthritic cartilageNMR Biomed252012538544
- T.HugleH.KovacsI.A.HeijnenT.DaikelerU.BaischJ.M.HicksSynovial fluid metabolomics in different forms of arthritis assessed by nuclear magnetic resonance spectroscopyClin Exp Rheumatol302012240245
- B.MickiewiczB.J.HeardJ.K.ChauM.ChungD.A.HartN.G.ShriveMetabolic profiling of synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritisJ Orthop Res3320157177
- A.Z.DamyanovichJ.R.StaplesA.D.ChanK.W.MarshallComparative study of normal and osteoarthritic canine synovial fluid using 500 MHz 1H magnetic resonance spectroscopyJ Orthop Res171999223231
- T.P.LazarC.R.BerryJ.J.deHaanJ.N.PeckM.CorreaLong-term radiographic comparison of tibial plateau leveling osteotomy versus extracapsular stabilization for cranial cruciate ligament rupture in the dogVet Surg342005133141
- B.SlocumT.D.SlocumTibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canineVet Clin North Am Small Anim Pract231993777795
- A.PozziM.P.KowaleskiD.ApeltC.MeadowsC.M.AndrewsK.A.JohnsonEffect of medial meniscal release on tibial translation after tibial plateau leveling osteotomyVet Surg352006486494
- K.I.SchmerbachC.K.BoeltzigU.ReifJ.C.WieserT.KellerV.GrevelIn vitro comparison of tibial plateau leveling osteotomy with and without use of a tibial plateau leveling jigVet Surg362007156163
- L.S.RomanoJ.L.CookSafety and functional outcomes associated with short-term rehabilitation therapy in the post-operative management of tibial plateau leveling osteotomyCan Vet J562015942946
- A.CrovaceL.LacitignolaF.P.FanizziA.MioloSurgery plus chondroprotection for canine cranial cruciate ligament (CCL) rupture. A proton NMR studyVet Comp Orthop Traumatol192006239245
- A.Z.DamyanovichJ.R.StaplesK.W.Marshall1H-NMR investigation of changes in the metabolic profile of synovial fluid in bilateral canine osteoarthritis with unilateral joint denervationOsteoarthr Cartil71999165172
- J.K.NicholsonP.J.FoxallM.SpraulR.D.FarrantJ.C.Lindon750 MHz 1H and 1H–13C NMR spectroscopy of human blood plasmaAnal Chem671995793811
- T.W.FanMetabolite profiling by one- and two-dimensional NMR analysis of complex mixturesProg Nucl Magn Reson Spectrosc281996161219
- W.E.HullMeasurement of absolute metabolite concentrations in biological samplesBruker Report219871518
- S.C.BudsbergM.S.BerghL.R.ReynoldsH.K.StreppaEvaluation of pentosan polysulfate sodium in the postoperative recovery from cranial cruciate injury in dogs: a randomized, placebo-controlled clinical trialVet Surg362007234244
- D.P.NaughtonR.HaywoodD.R.BlakeS.EdmondsG.E.HawkesM.GrootveldA comparative evaluation of the metabolic profiles of normal and inflammatory knee-joint synovial fluids by high resolution proton NMR spectroscopyFEBS Lett3321993221225
- Y.FujitaY.HaraY.NezuK.S.SchulzM.TagawaProinflammatory cytokine activities, matrix metalloproteinase-3 activity, and sulfated glycosaminoglycan content in synovial fluid of dogs with naturally acquired cranial cruciate ligament ruptureVet Surg352006369376
- J.G.BarrettZ.HaoB.K.GrafL.D.KaplanJ.P.HeinerP.MuirInflammatory changes in ruptured canine cranial and human anterior cruciate ligamentsAm J Vet Res66200520732080
- L.LacitignolaF.P.FanizziE.FranciosoA.Crovace1H NMR investigation of normal and osteoarthritic synovial fluid in the horseVet Comp Orthop Traumatol2120088588
- S.D.SkaperL.FacciP.GiustiGlia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediatorMol Neurobiol482013340352
- A.MioloP.BadinoR.BarberoG.ReGlupamid®: a novel nutraceutical approach to canine and feline osteoarthritisJ Vet Pharmacol Ther29Suppl. 12006202203
- G.ReR.BarberoA.MioloV.Di MarzoPalmitoylethanolamide, endocannabinoids and related cannabimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animalsVet J17320072332
- A.MioloP.BadinoR.BarberoG.ReEffetti di una nuova forma di glucosamina sulla degranulazione dei mastociti. Studio in vitro Effects of a new form of glucosamine on mast cell degranulation. An in vitro studyVeterinaria2120072328
- A.MioloQuercetina e artrosi: razionale di utilizzo ed evidenze sperimentali Quercetin and osteoarthritis: use rational and experimental evidencesAnnali Piante Officinali820043445
- D.BrittiR.CrupiD.ImpellizzeriE.GugliandoloR.FuscoC.SchievanoA novel composite formulation of palmitoylethanolamide and quercetin decreases inflammation and relieves pain in inflammatory and osteoarthritic pain modelsBMC Vet Res132017229
- K.K.AuW.J.Gordon-EvansD.DunningK.J.O'Dell-AndersonK.E.KnapD.GriffonComparison of short- and long-term function and radiographic osteoarthrosis in dogs after postoperative physical rehabilitation and tibial plateau leveling osteotomy or lateral fabellar suture stabilizationVet Surg392010173180
- J.F.InnesM.CostelloF.J.BarrH.RudorfA.R.BarrRadiographic progression of osteoarthritis of the canine stifle joint: a prospective studyVet Radiol Ultrasound452004143148
- S.A.CorrC.BrownA comparison of outcomes following tibial plateau levelling osteotomy and cranial tibial wedge osteotomy proceduresVet Comp Orthop Traumatol202007312319
- J.A.BleedornE.N.GreuelP.A.ManleyS.L.SchaeferM.D.MarkelG.HolzmanSynovitis in dogs with stable stifle joints and incipient cranial cruciate ligament rupture: a cross-sectional studyVet Surg402011531543
- J.P.LittleJ.A.BleedornB.J.SutherlandR.SullivanV.L.KalscheurM.A.RamakerArthroscopic assessment of stifle synovitis in dogs with cranial cruciate ligament rupturePLoS ONE92014e97329
- T.N.FreyM.G.HoelzlerT.D.ScavelliR.P.FulcherR.P.BastianRisk factors for surgical site infection-inflammation in dogs undergoing surgery for rupture of the cranial cruciate ligament: 902 cases (2005–2006)J Am Vet Med Assoc23620108894