Abstract
Semen cryopreservation is a well-established procedure used in veterinary assisted reproduction technology applications. We investigated damaging effects of cryopreservation on the structural and ultrastructural characteristics of bull sperm induced at different temperatures and steps during standard cryopreservation procedure using transmission (TEM) and scanning electron microscopy. We also examined the effect of cryopreservation on sperm DNA and chromatin integrity. Five healthy, fertile Friesian bulls were used, and the ejaculates were obtained using an artificial vagina method. The semen samples were pooled and diluted in a tris-yolk fructose (TYF) for a final concentration of 80 × 106 spermatozoa/ml. The semen samples were packed in straws (0.25 ml), and stored in liquid nitrogen (−196°C). Samples were evaluated before dilution, just after dilution (at 37°C), at 2 h and 4 h during equilibration, and after thawing (37°C for 30 s in water bath). In association with step-wise decline in motility and viability, our results showed that the plasma membrane surrounding the sperm head was the most vulnerable structure to cryo-damage with various degrees of swelling, undulation, or loss affecting about 50% of the total sperm population after equilibration and freezing. Typical acrosome reaction was limited to 10% of the spermatozoa after freezing. We also observed increased number of mitochondria with distorted cristae (15%). Chromatin damage was significantly increased by cryopreservation as evident by TEM (9%). This was mainly due to DNA breaks as confirmed by Sperm Chromatin Structure Assay (SCSA) (8.4%) whereas the chromatin structure was less affected as evaluated microscopically by toluidine blue staining. We concluded that, using standard cryopreservation protocol, the most pronounced damage induced by cryopreservation is observed in the plasma membrane. Further improvement of cryopreservation protocols should thus be targeted at reducing plasma membrane damage. Acrosomal, mitochondrial and chromatin damage are also evident but appear to be within acceptable limits as discussed.
1 Introduction
Semen cryopreservation is a well-established procedure used in human and veterinary assisted reproduction technology (ART) applications. Over the last 50 years, it was used for genetic improvement of beef and dairy cattle. It is also used to control venereal diseases and facilitate management of cattle herd fertility. In human, it is usually associated with male fertility preservation which is usually required prior to cancer therapy [Citation1].
Spermatozoa are characterized by plasma membrane fluidity and low water content which make it more resistant to cryo-damage compared to other cell types [Citation2]. However, cryopreservation have been shown to induce deleterious changes of sperm structure and function [Citation3]. This involves thermal stress due to the change in temperature during cooling, freezing and thawing as well as the osmotic stress caused by addition of high concentrations of cryoprotective agents and by crystallization [Citation4]. This results in protein denaturation, shrinkage and irreversible membrane collapse [Citation3]. Therefore, phospholipids and cryoprotective agents, as well as optimal dilution, equilibration and cooling procedures are required to avoid cold shock, reduce crystallization and minimize sperm damage.
Conventional sperm evaluation parameters used in AI centers for sire semen evaluation and post-thawing assessment of cryopreserved semen are usually limited to examining post-thawing motility and morphology. Evaluation of sperm motility either subjectively or by computerized sperm analysis has been used as the main parameter to determine sperm quality and predict fertility in humans and animals [Citation5,Citation6]. However, motility shows a high degree of biological variability and in some cases are found to be fair measures of fertility. For example, variation in sperm motility from 60 to 90% had very low correlation to pregnancy rates in swine [Citation7]. In cattle and sheep, a wide variation (about 20%) in post-thaw motility percentage was not correlated to the fertility [Citation8]. Therefore, it is important to use more efficient tests for better evaluation.
Several structural and ultrastructural sperm components have been reported to be affected by cryopreservation, however their relative importance for routine semen evaluation is not clear. For example, acrosome and plasma membrane integrity are critical for the process of acrosome reaction (AR) and their damage may cause premature AR leading to reduced fertilizing capacity [Citation9]. Mitochondria provide ATP which determines sperm motility [Citation10] and are also vulnerable to cryo-damage. In addition, DNA damage has been recognized as an important indicator of sperm quality and has a great clinical significance in assessment of sperm selection in human [Citation11]. However, in cattle, the effect of cryopreservation on sperm chromatin integrity has not been extensively examined. Assessment of sperm DNA damage is widely determined using Sperm Chromatin Structure Assay (SCSA) which require assessment by flow cytometry and therefore not routinely used [Citation12]. Instead, Toluidine Blue (TB) test is a less expensive staining that is used to estimate chromatin integrity which has been shown to be correlated to SCSA results [Citation13], and therefore may be a good evaluation tool for post thawing sperm quality.
During standard cryopreservation procedure, different processing steps are involved, namely, dilution of semen at 37°C with Tris-based diluents containing egg yolk and glycerol, followed by cooling to 4°C and equilibration. Rapid freezing is then performed to avoid crystallization. It is also not clear how each processing step contributes to the overall damage observed post-thawing. Understanding when the damage is induced can help in improvement of the most critical steps in the future.
Therefore, this study aims to 1) screen several structural and ultrastructural assessment tools to evaluate sperm quality during and after cryopreservation (plasma membrane and acrosome integrity, mitochondrial structure, chromatin damage, in addition to motility, viability and morphological abnormalities) 2) define which tests are more valuable for routine use, and 3) identify the damage caused by each processing step during cryopreservation (before dilution, after dilution, 2 h equilibration, 4 h equilibration and after thawing).
2 Materials and methods
All chemicals were purchased from (Sigma Pharmaceuticals, UK) unless otherwise stated.
2.1 Collection and selection of semen samples
Ejaculates were collected from five healthy, fertile Friesian bulls, 4–8 years old, raised at the international livestock management training center, Skha, Kafr El-Sheikh, Egypt. Semen was collected twice a week for 5 weeks. The bulls were kept under standard conditions of feeding and management. Semen was collected using an artificial vagina (Neustadt/Aisch, Müller, Nürnberg, Germany) pre-warmed to 42°C. The percentage of progressive motility for each sample was determined subjectively by two experienced researchers using a phase contrast microscope with 200× magnification. Ejaculates with ≥70% motility were selected for cryopreservation experiments.
2.2 Cryopreservation procedures
Semen was cryopreserved using standard production procedures in our AI centers according to Chen et al. [Citation14] with some modifications. Briefly, semen was gradually diluted at 37°C with Tris-yolk fructose (TYF) extender containing 30.28 mg/mL tris aminomethane, 16.75 mg/mL citric acid anhydrous, 12.5 mg/mL fructose, 7% (v/v) glycerol, 20% (v/v) egg yolk, 100 IU/mL penicillin and 100 µg/mL streptomycin. The extension rate was 1 semen: 20 extender to bring the sperm concentration to 80 × 106 sperm/mL. Diluted semen samples were kept at 4°C in a cooling chamber for 4 h as an equilibration period then automatically filled in 0.25 mL French straws (IVM technologies, L’ Aigle, France), placed 4 cm above liquid nitrogen for 10 min then frozen in liquid nitrogen (−196°C) as described by Salisbury et al. [Citation15]. Samples were evaluated before dilution, just after dilution (at 37°C), at 2 h and 4 h during equilibration, and after thawing (37°C for 30 s in water bath).
2.3 Assessment of sperm progressive motility
Percentage of progressive sperm motility in each semen sample (10 µL) was determined using phase contrast microscope (Olympus, Tokyo, Japan) supplied with a warm stage adjusted to 37°C.
2.4 Assessment of sperm viability and abnormalities
A smear from diluted semen was made on a glass slide and was stained by eosin (1.67%) and nigrosin (10%) stain [Citation16]. A total of 300 sperm were examined in each sample at 400× under light microscope (Olympus). The number of dead spermatozoa (red stained) were counted. The number of sperm cells bearing head and tail morphological abnormalities were also recorded as previously described [Citation17].
2.5 Scanning electron microscope evaluation of semen samples
To assess the structural damage induced by each step of cryopreservation, sperm samples were examined by a scanning electron microscopy. Samples (3 replicates) were centrifuged at 500×g for 20 min, and the sperm pellets were collected. Samples were fixed in a solution containing 2.5% (w/v) buffered glutaraldehyde and 2% (w/v) paraformaldehyde in 0.1 M sodium phosphate buffer (Sorensen buffer) pH 7.4 at 4°C overnight [Citation18]. Fixed sperm pellets were then washed three times for 15 min each in 0.1 M sodium phosphate and treated in 2% sodium phosphate buffered osmium tetroxide pH 7.4 for 90 min. Pellets were finally washed in 0.1 M sodium phosphate buffer pH 7.4 and dehydrated in an increasing gradient of ethanol. Three drops of 100% acetone were added to the pellets on small glass plates before gluing them onto the specimen stubs of the microscope. The specimens obtained after the acetone had evaporated were coated with gold-palladium membranes and observed using a scanning electron microscope (Jeol JSM-6510 L.V). The microscope was operated at 30 kV. Only the central areas of the glass plates were examined (100 sperm per sample per replicate). The occurrence of detached and cracked heads and damage in the tail region was examined.
2.6 Transmission electron microscope (TEM) evaluation of semen samples
At different steps of sperm dilution and cryopreservation, sperm samples from 3 replicates were processed for TEM as described by Oliveira et al. [Citation19] with some modifications. Briefly, samples (500 μL) were centrifuged and resuspended in a fixative solution composed of 4% glutaraldehyde in Dulbecco’s modified phosphate buffered saline for 2 h at 4°C. Samples were then washed and post-fixed in 1% osmium tetroxide for 1 h at room temperature. Fixed samples were then dehydrated in an ethanol gradient, treated with propylene oxide and embedded in Epon resin (Epon 812; Fluka Chemie, Switzerland) and ultrathin-sectioned (60–70 nm) for TEM. Ultrathin sections were observed at 80 kV using a JEOL 2100 TEM at 80 kV. The sperm ultrastructure was examined at the head region (n = 100 per sample) to examine the plasma membrane, acrosome and nucleus morphology. The mid-piece in 100 sperm per sample was also examined to investigate the morphology of the mitochondria. According to our subsequent observations, it was decided to classify the ultrastructure of the plasma membrane into: intact, slightly swollen, swollen or lost; acrosome: typical, atypical, or lost; mitochondria and chromatin material: intact or damaged.
2.7 Determination of chromatin integrity
2.7.1 Toluidine blue staining and microscopic evaluation
Toluidine blue staining was performed as previously described [Citation13] with some modification. Smears obtained during different steps of semen cryopreservation (3 replicates) were fixed in ethanol–acetic acid (3:1, v/v) for 1 min and 70% ethanol for 3 min. Smears were hydrolyzed for 20 min in 4 mM Chloridric acid, rinsed in distilled water and air-dried. One droplet of 0.025% toluidine blue in Mcllvaine buffer (sodium citrate-phosphate) pH 4.0 was placed over each smear and then cover slipped. Smears were evaluated with light microscopy at 1000× magnification. The percentage of chromatin damage was estimated by evaluating 300 cells on each smear. Spermatozoa stained as green to light blue were considered to have normal chromatin while those stained dark blue to violet were considered to have damaged chromatin.
2.7.2 Sperm chromatin structure assay (SCSA)
The SCSA was applied following the procedure described before [Citation20]. Briefly, 200 µL of diluted semen from each sample at each time point/step (3 replicates) was diluted to a concentration of 1 × 106 sperm/mL with a buffer composed of 0.01 M Tris–HCl, 0.15 M NaCl and 1 mM EDTA (pH 7.4) and treated with an acid detergent solution (pH 1.2) containing 0.1% Triton X-100, 0.15 mol/l NaCl and 0.08 N HCl for 30 s. Spermatozoa were then stained with 6 µg/mL purified acridine orange (AO) in a phosphate-citrate buffer, pH 6.0. Cells were analysed using a FACSort flow cytometer (Becton Dickinson, San Jose, CA, USA), equipped with an air-cooled argon ion laser. A total of 10,000 events were accumulated for each measurement at a flow rate of 200–300 cells/s. Acridine orange that is intercalated in double-stranded DNA emits green fluorescence, whereas AO associated with single-stranded DNA emits red fluorescence. The extent of DNA denaturation was expressed in terms of DFI, which is the ratio of red to total (red plus green) fluorescence intensity.
2.8 Statistical analysis
Mean percentages of motility, viability, and sperm cell abnormalities from 5 independent repeats were analyzed using analysis of variance (ANOVA) by SAS [Citation21] computer program. When main effects were significant, pairwise comparison between means were examined by Duncan’s Multiple Range Test. Proportions (p) of plasma membrane integrity and acrosome, tail and mitochondrial damage as well as chromatin integrity were analyzed using logistic regression in SPSS version 21 (IBM). Overall effect was determined by Omnibus tests of model coefficients, and dummies comparison was performed using Wald test based on 95% confidence interval (95% CI) of odds ratio (OR). 95% CI not including zero and P values less than .05 were considered significant. Reference group was set to be freshly diluted semen at 37°C.
3 Results
3.1 Structural changes induced by semen processing and cryopreservation
Raw and freshly diluted semen samples maintained at 37°C exhibited similar motility and viability. After initiation of cooling and during the equilibration period, significant reduction in sperm motility (P < .05) was associated with a significant decrease in sperm viability and increase in the percentage of sperm abnormalities (mainly coiled and bent tails). These figures were further exacerbated after the freeze-thaw step (). The sperm cell abnormalities after freezing also included cracked tails and detached heads as shown by scanning electron microscopy ().
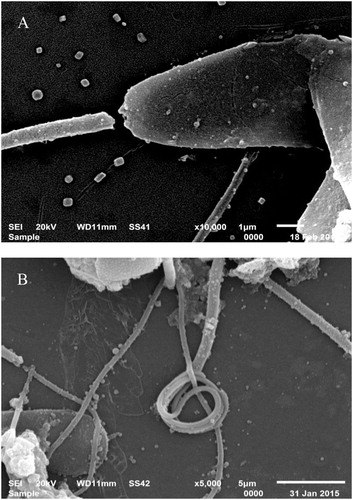
Table 1 The effect of different processing steps of semen cryopreservation on sperm motility, viability and morphological abnormalities examined by light microscopy (mean percentage ± SEM, n = 5).
3.2 Ultrastructural changes induced by semen processing and cryopreservation
3.2.1 Plasma membrane (PM) damage
Examination of sperm cells using TEM revealed that the PM, particularly surrounding the sperm head, was remarkably affected, and this was clearly visible under TEM () and the effect varied at different processing steps of cryopreservation (). Stages of semen cooling, equilibration with cryoprotectants and freezing did not affect the number of spermatozoa with slight swelling in PM compared to diluted semen at 37°C (P > .1). Marked swelling significantly increased after 4 h equilibration (P = .005) and further after freezing (P < .0001). Freezing, but not cooling or equilibration, also resulted in a complete loss of PM in a significant number of spermatozoa (P = .032).
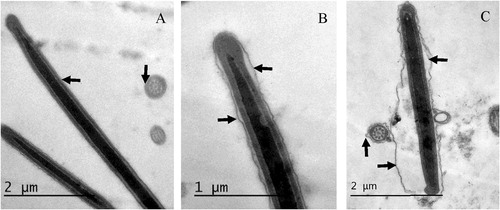
Table 2 The effect of different processing steps of semen cryopreservation on sperm plasma membrane (PM) and acrosome reaction (AR) examined by transmission electron microscopy.
3.2.2 Acrosome damage
Different acrosomal patterns were observed and classified into; 1) Intact Acrosome: where sperm heads exhibited intact acrosomal membrane surrounding the acrosomal ground substance, 2) Acrosome Reaction (AR): a swelling of acrosomal ground substance with vesicles of fused plasma and outer acrosomal membranes and 3) Atypical AR: sperm head presenting swelling of acrosomal ground substance dispersed under the swollen outer acrosomal membrane. Few sperm cells showed completely absent acrosome ( and ).
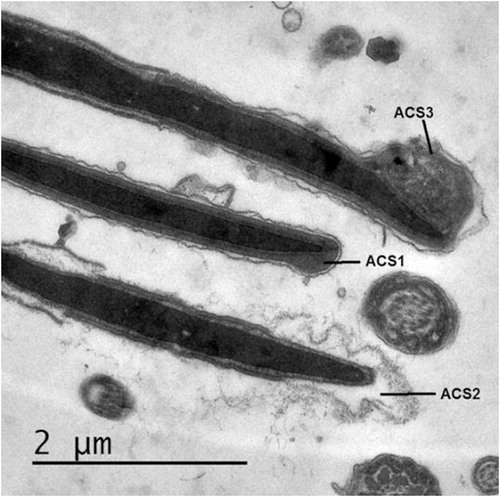
In addition, freezing significantly increased typical acrosome reaction compared to diluted semen (37°C) (P = .002) and also increased atypical AR (P = .046) and tended to increase the probability of complete loss of acrosome (P = .91). Cooling and equilibration did not induce significant changes in the acrosomal structure.
3.3 Ultrastructural changes in the mitochondria
By TEM examination of sperm mid-piece, we investigated the morphology of the mitochondria. In fresh semen, mitochondria appeared normal with a membrane space of variable width and clearly visible cristae (). After cryopreservation, mitochondrial damage was observed in the form of increased number of mitochondria with distorted cristae. Damaged mitochondria appeared vacuolated with narrowed membrane space ( and ). The proportions of affected spermatozoa in each processing step are shown in . Cryopreservation had no significant effect on mitochondrial integrity during cooling and equilibration with cryoprotectants, whereas freezing increased mitochondrial damage to 15% of the sperm population.
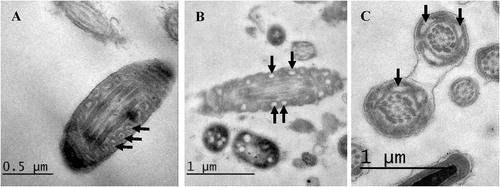
Table 3 The effect of different processing steps of semen cryopreservation on sperm mitochondrial damage examined by transmission electron microscopy.
3.4 Effect of cryopreservation on DNA and chromatin integrity
In fresh semen samples, normal sperm head containing normal nucleus with homogenous condensed chromatin surrounded by intact plasma membrane and acrosome were noted using TEM. In contrast, an increased percentage of spermatozoa showing signs of nuclear damage and loss of chromatin integrity could be seen in frozen-thawed samples (). This was evident in about 3–4% in semen samples prior to freezing (P > .05), and increased to 9% in frozen thawed semen (P > .05) when assessed by TEM (). Numerically, similar proportions were obtained using SCSA however the difference became statistically significant due to the bigger sample size (sperm numbers 10,000 per sample). Microscopic evaluation of TB staining resulted in numerically lower numbers of spermatozoa detected with chromatin damage compared to TEM and SCSA (). Statistically, it was more likely to find chromatin damages spermatozoa in frozen-thawed samples as compared to diluted or cooled samples (P < .05)
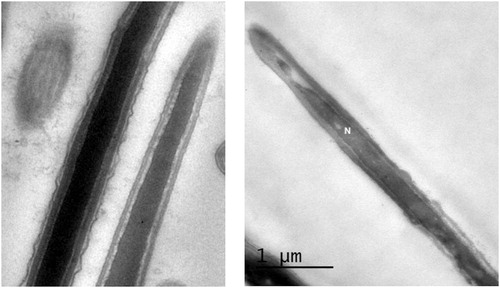
Table 4 The effect of different processing steps of semen cryopreservation on sperm DNA damage examined by transmission electron microscopy, TB staining and SCSA.
4 Discussion
The present study investigated the ultrastructural and DNA damage associated with each step of bull semen processing and cryopreservation, as well as the effect on sperm motility, viability and morphological abnormalities. We used the classical slow-freezing technique to cryopreserve bull spermatozoa for this study, which involves the use of Tris-based extender, 7% glycerol and 20% hen’s egg yolk. This procedure is routinely used for research and production of dairy and beef bull semen straws in our laboratory. However, it must be addressed that currently there is no gold standard technique for semen cryopreservation either in humans or animals, and different techniques are being constantly evaluated and optimized for better results.
The findings of the present study showed a step-wise reduction in sperm motility and a loss of sperm viability of about 50% after thawing. We also observed increased number of sperm cell abnormalities mainly in the form of bent and coiled tail, detached and/or damaged heads. These were evident by scanning electron microscopy and were significantly increased by cooling and further increased by freezing. These morphological abnormalities have been previously linked to cold shock and were observed when cold shock was induced in bull semen by cooling and storing in the absence of egg yolk, and was associated with reduced in vitro fertilization rate [Citation22].
Functionally, although motility and viability were reduced, tris-egg-yolk-glycerol protected semen preserve 50% viable sperms available after thawing which is sufficiently acceptable for AI or in vitro fertilization in our laboratory and worldwide. However, as highlighted in the introduction, partial damage of spermatozoa with retaining viability and motility is the main issue which requires further research focus because it may reduce fertilizing ability, or may result in detrimental effects on post-fertilization embryo development.
Therefore, we further examined the damage induced in step-by-step manner during cryopreservation using TEM. We demonstrated that the plasma membrane (PM) was the most affected structure. Equilibration with cryoprotectants for 4 h induced severe swelling or complete loss of the PM of about 18% of the sperm population. This was further exaggerated by the freeze-thawing procedure (to a total of 50%). Damage of PM was predominant in the head region. This is in line with previous observations in cryopreserved human sperm [Citation23]. This observed swelling is most probably caused by changes in the extracellular osmotic pressure which creates a situation in which a cell will attempt to attain equilibration by either gaining or losing water. Spermatozoa with slight swelling were observed in all samples (∼15–20%). This could be partially due to fixation and handling of the sperm specimens for TEM and also accounts for cryo-damage.
Sperm PM is known to regulate many sperm functions. Sperm PM regulates sodium, potassium [Citation24] and calcium [Citation25] ion exchange which critically regulates motility and mitochondrial functions respectively. Sperm PM also possess receptors which mediate sperm-zona binding and fusion, like PH-20 [Citation26] and other receptors which regulate sperm capacitation [Citation27]. Intact PM is also necessary for fusion with the outer acrosomal membrane and induction of acrosome reaction [Citation28]. Although evidence of repair mechanisms of damaged PM in the female reproductive tract have been shown, by e.g. heat shock protein 70 which improves membrane fluidity and enhances sperm binding to oviductal epithelial cells and capacitation [Citation29], the impact of a 50% reduction in sperm population with intact PM described here requires further research attention. Since this damage is initiated during equilibration, optimized protocols focusing on reducing plasma membrane damage through modification of cryoprotectants or cooling speed should be targeted.
Capacitation and acrosome reaction processes are essential for fertilization and have to occur in timely manner. Researchers have suggested that in mammals, for successful fertilization, AR should occur at the vicinity of the mature cumulus oocyte complex [Citation30]. Premature AR will thus result in reduced fertilization rates. Here, we have shown that cryopreservation resulted in reduced number of spermatozoa bearing normal acrosome. However, the induced typical AR is minimal and only 10% were observed after thawing. Complete AR associated with complete loss of outer acrosomal membrane was evident in only 6% of post-thawing spermatozoa. Cooling and equilibration did not affect physiological AR. We have demonstrated that cryopreservation significantly increased percentage of atypical AR to 19% compared to 9% before cooling. Similarly, 28% atypical AR was reported in frozen-thawed bovine semen [Citation19]. This type of abnormal morphology was attributed to degeneration associated with sperm cell death [Citation31]. When cryopreserved semen is used for in vitro fertilization during ART, standard procedure including centrifugation of the sample through percol gradient is essential to separate motile spermatozoa from cryoprotectants and dead cells. However, this process has been shown to further decrease the percentage of sperm cells bearing normal acrosome and increase atypical AR [Citation19].
Equally important, we observed altered mitochondrial morphology suggesting reduced mitochondrial functions in 15% of frozen-thawed spermatozoa. Mitochondrial function and ability to produce sufficient ATP have been correlated to sperm motility and hyperactivity [Citation32] and are also linked to higher fertilization potential in vitro [Citation33]. However, mitochondrial membrane potential after cryopreservation was found to vary between bulls and also among different ejaculates from the same bull [Citation34].
Furthermore, the effect of cryopreservation on sperm DNA integrity is particularly important, since this DNA damage has been shown to affect genes that are crucial for fertilization and early embryo development [Citation35]. Induced DNA damage in murine sperms has been shown to persist after ICSI fertilization and no repair could be observed with DNA synthesis in the zygote [Citation36]. This may explain the relation between sperm DNA damage and unexplained recurrent spontaneous abortion [Citation37]. Several physiological, mechanical and chemical causes can lead to damage of sperm chromatin. In human, aging [Citation38], inter-individual variation [Citation39], pollution [Citation40] and other metabolic disturbances have been shown to impact chromatin integrity, which is a factor that can substantially affect fertility in men, rather than by basic sperm parameters. The mechanism of the generation of DNA fragmentation and chromatin damage after cryopreservation due to the mechanical effect of crystallization or chemical damage induced by cryoprotectants has not been clearly elucidated, but mainly attributed to oxidative stress [Citation41] and activation of caspases [Citation11].
The extent and impact of sperm chromatin damage are dependent on the method of evaluation. Percentage of sperms with chromatin defects showed significant negative correlation with fertilization rate following ICSI [Citation42] and to embryo quality and pregnancy outcomes following in vitro fertilization [Citation43] when evaluated using chromomycin A3 or Diff-Quik kit respectively. These correlations were not observed when aniline blue and acridine orange staining was used for assessment [Citation42]. This may explain the presence of controverting reports regarding the effect of cryopreservation on sperm DNA integrity demonstrated by different research results. Some examples have been previously reviewed [Citation44]. Here we found that the DNA damage estimated by SCSA was comparable to that observed by TEM (∼8%). However, microscopic evaluation of chromatin damage by TB showed significantly lower values (∼4%). This is consistent with some reports in human [Citation45] and may be attributed to underestimation due to indistinct staining intensities of TB and heterogeneous staining of slides which make evaluation subjective and experience dependent [Citation46].
In human, risk for infertility increased when DNA fragmentation index assessed by SCSA was higher than 20% with normal standard semen parameters (motility, morphology, concentration) or higher than 10% with one abnormal parameter [Citation47]. In ovine, it has been shown that a pronounced decline in PR was observed when percentages of decondensed have reached thresholds of 10.5–30% [Citation48]. Therefore, the chromatin damage reported here (∼8%) appears to be within safe limits in regards to its impact on fertility.
5 Conclusions
Using standard cryopreservation protocol with glycerol and egg-yolk protection in tris-based extenders, we noticed that dilution, cooling and equilibration with cryoprotectants do not induce ultrastructural defects despite reducing sperm motility and viability and increasing sperm abnormalities. The most pronounced damage was observed in the plasma membrane structure where more swollen and lost membranes occurred after the freeze-thawing step. Minor acrosomal, mitochondrial and chromatin damage were also evident after freezing. We highlighted that reducing damage in sperm plasma membrane, mitochondria and DNA should be assessed and targeted during equilibration and at freezing, before most of the damage is initiated, for improving cryopreservation protocols of bull semen used for AI. This can also be applied during sire selection using TEM and SCSA as described above.
Competing interests
There is no conflict of interest to declare.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- A.K.NangiaS.A.KriegS.S.KimClinical guidelines for sperm cryopreservation in cancer patientsFertil Steril100201312031209
- Y.AgcaJ.GilmoreM.ByersE.J.WoodsJ.LiuJ.K.CritserOsmotic characteristics of mouse spermatozoa in the presence of extenders and sugarsBiol Reprod67200214931501
- P.F.WatsonThe causes of reduced fertility with cryopreserved semenAnim Reprod Sci60–612000481492
- E.ChantlerJ.V.Abraham-PeskirSignificance of midpiece vesicles and functional integrity of the membranes of human spermatozoa after osmotic stressAndrologia3620048793
- C.L.BarrattM.J.TomlinsonI.D.CookePrognostic significance of computerized motility analysis for in vivo fertilityFertil Steril601993520525
- J.L.VossB.W.PickettE.L.SquiresStallion spermatozoal morphology and motility and their relationships to fertilityJ Am Vet Med Assoc1781981287289
- M.L.BroekhuijseE.SostaricH.FeitsmaB.M.GadellaThe value of microscopic semen motility assessment at collection for a commercial artificial insemination center, a retrospective study on factors explaining variation in pig fertilityTheriogenology771466–14792012e1463
- L.G.Sanchez-PartidaD.P.WindsorJ.EpplestonB.P.SetchellW.M.MaxwellFertility and its relationship to motility characteristics of spermatozoa in ewes after cervical, transcervical, and intrauterine insemination with frozen-thawed ram semenJ Androl201999280288
- F.M.FleschB.M.GadellaDynamics of the mammalian sperm plasma membrane in the process of fertilizationBiochim Biophys Acta14692000197235
- S.M.LuoH.SchattenQ.Y.SunSperm mitochondria in reproduction: good or bad and where do they go?J Genet Genomics402013549556
- J.NovotnyN.AzizR.RybarJ.BrezinovaV.KopeckaR.FilipcikovaRelationship between reactive oxygen species production in human semen and sperm DNA damage assessed by sperm chromatin structure assayBiomed Pap Med Fac Univ Palacky Olomouc Czech Repub1572013383386
- M.BungumP.HumaidanM.SpanoK.JepsonL.BungumA.GiwercmanThe predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination IVF and ICSIHum Reprod19200414011408
- J.ErenpreissK.JepsonA.GiwercmanI.TsarevJ.ErenpreisaM.SpanoToluidine blue cytometry test for sperm DNA conformation: comparison with the flow cytometric sperm chromatin structure and TUNEL assaysHum Reprod19200422772282
- Y.ChenR.H.FooteC.TobbackL.ZhangS.HoughSurvival of bull spermatozoa seeded and frozen at different rates in egg yolk-tris and whole milk extendersJ Dairy Sci76199310281034
- G.W.SalisburyN.L.VanDemarkJ.R.LodgePhysiology of reproduction and artificial insemination of cattle2nd ed.1978W.H. Freeman and Co.San Francisco
- S.I.MoskovtsevC.L.LibrachMethods of sperm vitality assessmentSpermatogenesis: methods and protocols20131319
- A.G.MenonJ.C.ThundathilR.WildeJ.P.KastelicH.W.BarkemaValidating the assessment of bull sperm morphology by veterinary practitionersCan Vet J522011407408
- J.K.MorrisA formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopyJ Cell Biol2719651A149A
- L.Z.OliveiraV.F.Hossepian de LimaM.A.LevenhagenR.M.SantosT.I.AssumpcaoJ.O.JacominiTransmission electron microscopy for characterization of acrosomal damage after Percoll gradient centrifugation of cryopreserved bovine spermatozoaJ Vet Sci122011267272
- I.TsarevM.BungumA.GiwercmanJ.ErenpreisaT.EbessenE.ErnstEvaluation of male fertility potential by Toluidine Blue test for sperm chromatin structure assessmentHum Reprod24200915691574
- SAS: SAS/StatUser’s guide static’s, Ver., 6.064th ed.2004SAS Institute Inc.Cary, NC
- N.DuchaT.SusilawatiAulanni'amS.WahyuningsihM.PangestuUltrastructure and fertilizing ability of Limousin bull sperm after storage in CEP-2 extender with and without egg yolkPak J Biol Sci152012979985
- W.J.ZhuX.G.LiuCryodamage to plasma membrane integrity in head and tail regions of human spermAsian J Androl22000135138
- R.D.Peralta-AriasC.Y.VivenesM.I.CamejoS.PineroT.ProverbioE.MartinezATPases, ion exchangers and human sperm motilityReproduction1492015475484
- J.TriphanG.AumullerT.BrandenburgerB.WilhelmLocalization and regulation of plasma membrane Ca(2+)-ATPase in bovine spermatozoaEur J Cell Biol862007265273
- C.LalancetteV.DorvalV.LeblancP.LeclercCharacterization of an 80-kilodalton bull sperm protein identified as PH-20Biol Reprod652001628636
- M.ShimadaY.YanaiT.OkazakiN.NomaI.KawashimaT.MoriHyaluronan fragments generated by sperm-secreted hyaluronidase stimulate cytokine/chemokine production via the TLR2 and TLR4 pathway in cumulus cells of ovulated COCs, which may enhance fertilizationDevelopment135200820012011
- C.N.TomesMolecular mechanisms of membrane fusion during acrosomal exocytosisSoc Reprod Fertil Suppl652007275291
- N.Moein-VaziriI.PhillipsS.SmithC.AlminanaC.MasideM.A.GilHeat-shock protein A8 restores sperm membrane integrity by increasing plasma membrane fluidityReproduction1472014719732
- R.YanagimachiMammalian sperm acrosome reaction: where does it begin before fertilization?Biol Reprod85201145
- N.L.CrossS.MeizelMethods for evaluating the acrosomal status of mammalian spermBiol Reprod411989635641
- H.C.HoK.A.GranishS.S.SuarezHyperactivated motility of bull sperm is triggered at the axoneme by Ca2+ and not cAMPDev Biol2502002208217
- T.KasaiK.OgawaK.MizunoS.NagaiY.UchidaS.OhtaRelationship between sperm mitochondrial membrane potential, sperm motility, and fertility potentialAsian J Androl4200297103
- C.KossH.BollweinVariability in plasma membrane integrity, mitochondrial membrane potential and acrosomal status before and after cryopreservation of bull spermBerl Munch Tierarztl Wochenschr12120087377
- D.G.ValcarceF.Carton-GarciaM.F.RiescoM.P.HerraezV.RoblesAnalysis of DNA damage after human sperm cryopreservation in genes crucial for fertilization and early embryo developmentAndrology12013723730
- Y.YamauchiJ.M.RielM.A.WardPaternal DNA damage resulting from various sperm treatments persists after fertilization and is similar before and after DNA replicationJ Androl332012229238
- L.ZhangL.WangX.ZhangG.XuW.ZhangK.WangSperm chromatin integrity may predict future fertility for unexplained recurrent spontaneous abortion patientsInt J Androl352012752757
- R.RybarV.KopeckaP.PrinosilovaP.MarkovaJ.RubesMale obesity and age in relationship to semen parameters and sperm chromatin integrityAndrologia432011286291
- K.OleszczukA.GiwercmanM.BungumIntra-individual variation of the sperm chromatin structure assay DNA fragmentation index in men from infertile couplesHum Reprod26201132443248
- A.E.CalogeroS.La VigneraR.A.CondorelliA.PerdichizziD.ValentiP.AseroEnvironmental car exhaust pollution damages human sperm chromatin and DNAJ Endocrinol Invest342011e139143
- L.K.ThomsonS.D.FlemingR.J.AitkenG.N.De IuliisJ.A.ZieschangA.M.ClarkCryopreservation-induced human sperm DNA damage is predominantly mediated by oxidative stress rather than apoptosisHum Reprod24200920612070
- F.G.IranpourImpact of sperm chromatin evaluation on fertilization rate in intracytoplasmic sperm injectionAdv Biomed Res32014229
- R.S.TavaresA.F.SilvaB.LourencoT.Almeida-SantosA.P.SousaJ.Ramalho-SantosEvaluation of human sperm chromatin status after selection using a modified Diff-Quik stain indicates embryo quality and pregnancy outcomes following in vitro fertilizationAndrology12013830837
- M.Di SantoN.TarozziM.NadaliniA.BoriniHuman sperm cryopreservation: update on techniques, effect on DNA integrity, and implications for ARTAdv Urol20122012 854837
- K.R.ChohanJ.T.GriffinM.LafromboiseC.J.De JongeD.T.CarrellComparison of chromatin assays for DNA fragmentation evaluation in human spermJ Androl2720065359
- E.H.DuranT.GurganS.GunalpM.E.EnginsuH.YaraliA.AyhanA logistic regression model including DNA status and morphology of spermatozoa for prediction of fertilization in vitroHum Reprod13199812351239
- A.GiwercmanL.LindstedtM.LarssonM.BungumM.SpanoR.J.LevineSperm chromatin structure assay as an independent predictor of fertility in vivo: a case-control studyInt J Androl332010e221227
- T.KhalifaA.LymberopoulosChangeability of sperm chromatin structure during liquid storage of ovine semen in milk-egg yolk- and soybean lecithin-based extenders and their relationships to field-fertilityCell Tissue Bank142013687698
