Abstract
Implantation is one of the most critical steps in mammalian reproduction and implantation failure constitutes a major cause of infertility in both animals and humans. The mechanism of implantation is exclusively under the control of ovarian steroids progesterone and oestrogen whose actions are mediated in a complex phenomenon that involves a number of cytokines and growth factors. According to a plethora of literature on implantation in mammalian species, prominent of these cytokines and growth factor playing crucial roles in implantation include integrin, osteopontin, integrin, insulin-like growth factor and leukaemia inhibitory factor. Others are cluster domain 44, hyaluronan system and many non-adhesive molecules such as glycoprotein mucin 1. In this review, the specific roles played by these molecules are expatiated. Generally, they function as adhesive molecules that facilitate attachment of ligands/proteins on the trophectoderm to their respective receptors on endometrial luminal epithelia or vice versa. Sometimes, they also function as signalling molecules that enhance communication between implanting blastocyst and receptive endometrium. This is of particular importance in embryo culture and embryo transfer where in vitro derived blastocyst unlike the in vivo condition, is not exposed to these substances and hence, their absence may be partly responsible for the low implantation rate observed in the surrogate. Appreciation of the roles played by these cytokines, growth factors and molecules as revealed in this review will spur further research on these topics, facilitate their inclusion in embryo culture media (if positively required) and are considered as vital aspect while developing strategies to improve fertility.
1 Introduction
Sub-fertility is a pervasive problem affecting both human and animal species. In humans, available evidence suggests that pregnancy loss predominantly during pre-implantation and the first few weeks of pregnancy is one of the major causes of subfertility. According to the global statistics presented by Boivin and Bunting [Citation1], an estimate of over 72 million women between the ages of 20–40 years are infertile. In domestic animals, subfertility is a limitation to animal production and is one of the main issues for dairy cows selected for milk production [Citation2]. An estimate of 60% pregnancy loss occurs in dairy cattle, with a significant number observed during early stages of embryo development [Citation3]. Fertilization rates in cattle are around 90%, however, one-third of embryos fail to survive the first 18 days of pregnancy [Citation4]. This implies that reproductive losses in dairy cows due to early embryo death are 3–4 times greater than losses due to fertilization failure. In spite of almost 100% fertilization rate in sheep [Citation2], only 60–80% of the fertilized eggs proceed to live birth, while higher percentage of these losses occur before day 18 of pregnancy [Citation5]. Collectively, this suggests that implantation failure constitutes a major source of pregnancy loss and infertility in both human and animal subjects.
The early stage of pregnancy is thus termed ‘a critical period’ because of the high risk of embryo loss. One event known to occur during this critical period is blastocyst implantation to the maternal endometrium. Implantation is a complex process and has been generally acknowledged as the most critical step in mammalian reproduction. In primate and human, embryo stays momentarily in the oviduct before being transported to the uterus between 72 and 96 h post fertilization, readily prepared for implantation. In domestic ruminants, definitive implantation is achieved by adhesion of the mononuclear trophoblast cells to the endometrial luminal epithelium and formation of syncytia by the fusion of trophoblast binucleate cells with the luminal epithelium. The protracted period of peri-implantation embryo has made the ruminant especially sheep a unique model for classical study on molecular mechanism of implantation in mammalian species generally [Citation6].
The contact between the embryo and the maternal tissue soon after fertilization is crucial for subsequent development and survival of the embryo in utero because it creates the medium of interaction between the two entities and also generates the platform that eventually leads to the formation and development of placenta [Citation7] which is necessary to facilitate the exchange especially of micronutrients and gases from the mother to the conceptus.
Endometrial cells undergo cyclic renewal, differentiation and eventually apoptosis and shedding (in primate) as well as secretory with the primary purpose of allowing implantation of a viable embryo in a conceptive cycle. Many of these physiological processes depend on the timely expression of cell adhesion, bridging and signalling molecules as well as disappearance of others (such as non-adhesive molecules) which maintain tissue micro-architecture by mediating cell-to-cell and cell-to-substratum attachments that constitutes endometrial remodelling. In other words, endometrial remodelling is a prerequisite for the uterus to attain structural and functional capacity during implantation. This remodelling occurs only during the receptive phase of reproductive cycle [Citation8] and this period is termed ‘window of receptivity’ when attachment of blastocyst to the maternal endometrium is physiologically possible [Citation9].
Window of receptivity in mammalian species is exclusively under the influence of ovarian steroids, progesterone and oestrogen [Citation10–Citation12]. High level of oestrogen at ovulation causes uterine cell proliferation while subsequent increase progesterone during dioestrus/pregnancy suppresses proliferation and causes cell differentiation in preparing the uterus for receptivity [Citation13]. In addition to the steroids, a plethora of other molecules, including macromolecules, growth factors and pro-inflammatory cytokines mediate and modulate the actions of these steroid hormones to bring about the required changes in the uterine extracellular matrix [Citation14]. Among these cytokines/growth factors playing crucial roles in implantation are integrins (ITG), osteopontin (OPN), insulin-like growth factor (IGF) and leukaemia inhibitory factor (LIF). Others are hyaluronan (HA) system, cluster domain 44 (CD44), and many other non-adhesive molecules such as glycoprotein mucin 1 (MUC1). To date, the list is inexhaustible and continues to grow, however, the molecular mechanism underlying the phenomenon of implantation still remains, in the word of Aplin [Citation9], elusive. For the purpose of simplicity in this review, they are better classified as (i) adhesive and bridging molecules for those that initiate the visible actual attachment observed, (ii) signalling molecules that induce the transcription and translation of other genes and proteins that initiate communication/interaction between the receptive maternal endometrium and implantation-competent blastocyst and then (iii) the protective non-adhesive molecules on the endometrium that have to be removed before implantation could occur.
As reproductive biologists persist in their continued effort to understand the mechanisms underlying implantation process for a better development of strategies towards improving its success rate, our present understanding on the mechanism of implantation is still far from being complete. The objective of this review is to highlight the roles of ITG, OPN, IGF, LIF, HA system, CD44 and the non-adhesive MUC1 as mediators of implantation in mammalian species.
2 Methodology
The preliminary search strategy involved using the Unites States National Library of Medicine (https://www.ncbi.nlm.nih.gov/pubmed/) while matching the word implantation with cytokines, growth factors and adhesive molecules. The plenty of papers generated each time were selected based on their relevance to the subject matter of this review by going through the titles and abstract. These were read one by one and key references from them were also reviewed to generate a broad knowledge on the roles of ITG, OPN, IGF, LIF, HA, CD44 and MUC1 as mediators of implantation in mammalian species. Other relevant textbooks on the subject were also consulted to come up with a broad knowledge which has been summarised in the discussion that follows. It is noteworthy to state that the aforementioned mediators are not the only cytokines, growth factors or macromolecules involved in implantation, they are selectively chosen based on compelling evidence that suggests their synergistic and interlinked interactions during implantation from bodies of previous studies on this subject as summarized in and subsequently illustrated in .
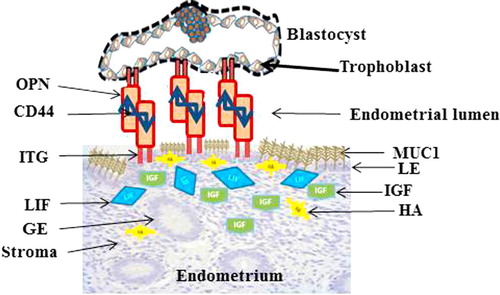
Table 1 Sources and roles of cytokines, growth factors and macromolecules mediating and modulating implantation process in mammalian species.
3 Integrins
ITGs are members of a larger family of cell adhesion protein believed to have major roles in cellular processes such as differentiation, motility and attachment, apoptosis and cell survival [Citation15]. Roles of ITG in implantation are hypothesized to be either through ITG attachment of cells to the extracellular matrix [Citation16] or ITG initiating a signalling transduction from the embryo to the extracellular matrix (ECM) leading to the transcription and translation of genes critical for implantation [Citation17]. The two major players of implantation, maternal endometrium and blastocyst reportedly expressed ITG [Citation18,Citation19]. Therefore, conceptualising a role for ITG during implantation is reasonable.
ITG undergoes dynamic temporal and spatial patterns of expression on endometrial cells during the menstrual cycle and in the early stages of human pregnancy [Citation20]. There are many isoforms of ITG in mammals, however, only three isoforms α1β1, α4β1 and αVβ3 are found to be particularly involved in implantation, with αVβ3 seemingly playing more conspicuous roles than others [Citation21]. In human, these isoforms were shown to be expressed in the endometrium during the window of implantation (day 20–24) when the endometrium is structurally and physiologically conducive to implanting blastocysts. On day 16 of bovine oestrous cycle, ITG αvβ3 and the oestrogen receptor are detected in uterine environment as molecular markers for the adhesion and signalling [Citation22]. ITG subunits are detected at sites of attachment between uterine epithelial cells and trophectoderm on Days 12–15 of swine pregnancy [Citation23]. Expression of ITG αVβ3 at the foeto-maternal interface in many species including sheep, pigs, baboon and human during blastocyst attachment and implantation further substantiates the concept that ITG is an adhesion as well as bridging molecule during the process of implantation [Citation24–Citation27].
On the contrary, several conditions that interfere with expression of ITG in the endometrium culminate into implantation failure. The blockade of the ITG αVβ3 inhibits implantation in mouse [Citation28]. ITG expression was also significantly reduced in human glandular epithelial cells and endometrial lumen as well as stromal cells of the hydrosalpinx group when compared with those of the control group [Citation29,Citation30], sequel to which is infertility in the hydrosalpinx group. In addition, some treatments in IVF protocol such as ovarian stimulation may adversely affect the expression of ITG which may partly responsible for low success outcome. For instance, all the three variants of ITG (α1β1, α4β1 and αVβ3) were reduced in glandular endometrium coupled with a reduced expression of the αVβ3 in the luminal epithelium after ovulation induction with gonadotropin [Citation31]. This was partially restored with administration of GnRH agonist and not GnRH antagonist in mice [Citation32].
Synthetic ITG ligands are currently been explored in other fields of research. Novel synthetic cyclic ITG αVβ3 binding peptide ALOS4 was reported to initiate antitumor activity in mouse melanoma models thorough inhibition of cell migration [Citation33]. Considering the importance of ITG in implantation, reduced or absence of ITG in surrogate endometrium may be detrimental to the success outcome of assisted conception. The question is, ‘Is it possible to compensate for the reduced ITG in the endometrium through exogenous addition of synthetic ITG in the embryo transfer media or intrauterine infusion?’ Further studies are warranted to determine the possible roles of ITG as a media component for embryo culture and embryo transfer since it is not included presently.
4 Osteopontin
Osteopontin (OPN) otherwise called secreted phosphoprotein 1 is an ECM proteins/cytokine capable of undergoing extensive phosphorylation, glycosylation and cleavage to yield molecular mass variants ranging from 25 to 75 kDa [Citation34]. It has multiple functions by binding cell surface receptors to mediate cell-cell adhesion and cell-ECM communication as well as cell migration.
OPN is hypothesised to play significant roles in mammalian implantation in a number of ways. Firstly, OPN is a component of histotroph required for adhesion and signal transduction at the uterine-placental interface resulting into conceptus attachment [Citation35]. Secondly, it is a gene product expressed by uterine stroma as it decidualizes in response to conceptus invasion, and thirdly, as a constituent of resident placental and uterine immune cells that regulates immune cells behaviour and cytokine production [Citation36,Citation37]. All mammalian uteri including human contain endometrial glands that secrete substances comprising enzymes, growth factors, cytokines, lymphokines, hormones, transport proteins and other substances altogether refer to as histotroph [Citation38]. Histotroph plays a role in conceptus nourishment, production of maternal pregnancy recognition signals, immuno-tolerance of semi-allograft embryo, blastocyst attachment and implantation as well as placentation [Citation38,Citation39].
Uterine gland secretion is active and support pregnancy in human during the first trimester (10 weeks) of gestation [Citation40]. Previous studies with ewes in which uterine glands were epigenetically ablated (known as uterine gland knockout, UGKO ewes) by neonatal progestin exposure confirmed that histotroph is required to maintain pregnancy during peri-implantation period [Citation41,Citation42]. OPN, a key component of histotroph is distinctly absent in uterine secretion of UGKO ewe such that UGKO ewes are infertile due to failure to support pregnancy at the very early stage [Citation43]. In sow, OPN was detectable at the maternal-placenta interface from day 25 and remained elevated till day 85 [Citation44]. Localisation of OPN at the point of maternal-placenta interface is suggestive of ITG interaction with its receptors on the conceptus and uterus to promote conceptus development and signalling. In vitro modelling of early implantation with human endometrial cells (Ishikawa) and mouse or human embryos or ligand-coated beads showed that OPN of epithelial origin binds the receptor ITG αvβ3 at the maternal surface to support adhesion during the early stages of implantation [Citation45].
Non-interaction of ITG αvβ3 and OPN at the foetal-maternal interface in ruminants especially bovine was reported by Kimmins et al. [Citation46]. On the contrary, other workers reported co-localisation of both OPN and ITG αvβ3 in sheep uterus and trophectoderm to induce adhesion between endometrial luminal epithelium and trophectoderm during period of implantation [Citation24,Citation47,Citation48] while a summary of these works has been reviewed elsewhere [Citation49]. There are limited studies of these cytokines in cattle possibly due to size of the animal. Certainly, further studies are required to clarify this ambiguity as regards simultaneous expression of OPN and ITG αvβ3 at the foetal-maternal interface during implantation in ruminants.
5 Insulin-like growth factor
According to Clemons [Citation50], the insulin-like growth factor (IGF) family includes the two ligands-IGF1 and IGF11, their receptors-IGF1R and IGFIIR and six binding proteins (insulin-like growth factor binding protein, IGFBP1-6). IGFs have structural (50%) homology with pro-insulin and hence the name insulin-like growth. IGF system is extremely complex and functions in a wide variety of physiological and pathological conditions in tissues of various types. It may promote differentiation and migration in some cells while inhibiting apoptosis in some other cell type. Besides, the binding of IGFs to their respective receptors may elicit intracellular signalling cascade using Gi-coupled receptor or Mitogen Activated Protein Kinase (MAPK) [Citation51] and may involve α5β1 integrin [Citation52].
IGFs form a part of the body immune response mechanism and may be produced in response to endotoxins [Citation53], however, they are reported to be involved in foetal and placental development [Citation54]. During oestrous cycle, IGFs and their binding proteins are expressed in the cow oviduct [Citation55]. IGF1, IGF11 and IGF1-R are also expressed in sheep endometrium during oestrous cycle as well as in early pregnancy [Citation56]. There is an increase in foetal hepatic IGFI, IGFBP-2, -3 and -4 concentrations during gestation in sheep [Citation57] while high concentrations of IGF1 and 11 are detectable in human maternal circulation during early pregnancy [Citation58]. These data suggest endocrine roles of IGFs in regulation of placental and foetal growth.
Reproduction is a secondary characteristic and occurs essentially when the primary metabolic requirement has been fulfilled. IGF system also seems to be a link between nutrition and reproduction. The study of Fenwick et al. [Citation59] demonstrated the effect of negative energy balance on level of circulating IGF1 after calving that may impede embryo development and causing embryo mortality in dairy cow. In related study, low concentration of IGF1 of live heifer calves indicated the survivability of calf post partum in dairy [Citation60]. Collectively, it could be inferred that IGFs are key metabolic signalling molecules that may be used as indicator/marker of metabolic stress in domestic farm animals [Citation61]. Stress is a limitation to attainment of full reproductive potential in mammalian species.
The use of IGF in assisted reproductive technology is well documented. A reasonable number of studies have shown that IGF1 especially with oestrus cow serum can facilitate embryo development and increase blastocyst rate [Citation62], possibly by increasing embryo signalling via MAPK [Citation63]. In vitro culture of oocyte, luteal and follicular cells and embryo are important aspect of assisted reproductive technology. The morphological and functional characteristics of cultured luteal cells are retained in media containing insulin and luteal angiogenesis is also facilitated with IGF1 [Citation64,Citation65]. IGF1 produces anti-apoptotic effect in regressing porcine corpus luteum [Citation66] possibly through stimulation of progesterone secretion as was observed in cultured luteal cells obtained from early pregnant subject [Citation67].
Inclusion of IGF11 to embryo culture media improves blastocyst rate and blastocyst hatching in vitro in mouse [Citation68]. Addition of 100 ng IGF-I per mL of embryo culture media shortens the transition from the morula to the blastocyst stage and increases the proportion of blastocysts and hatched blastocysts on day 13 [Citation62]. One of the major limitations of assisted conception is failure of the blastocyst to implant. A recent study has shown that IGF1 improves attachment of mouse blastocysts to Ishikawa cells in vitro [Citation69], thus indicates the potential of IGF1 to enhance adherence of implantation-competent blastocysts in the surrogate. Summarily, in spite of several reports of beneficial inclusion of IGF in experimental studies, the clinical application of these findings to improve results of assisted conception in animal and human has not been optimised.
6 Leukaemia inhibitory factor
Leukaemia inhibitory factor (LIF) belongs to a group of cytokines known as interleukin-6 (IL-6) family. Other members in the group are IL-6 and IL-11. Receptors for LIF are LIF receptor alpha (LIFRα), LIF receptor beta (LIFRβ) and glycoprotein gp130 [Citation70].
The LIF receptor is expressed during secretory and post ovulatory phases of the oestrous cycle and is restricted to the luminal epithelium [Citation71]. The associated signal-transducing component of the LIF receptor, gp130 is also expressed in both the luminal and glandular epithelium throughout the mestrual cycle; however, maximal expression of LIF was reportedly expressed during the secretory phase at which time implantation occurs [Citation72]. The coexistence of a high level of LIF protein, LIF receptor and gp130 on day 4 of gestation in gravid uterus further emphasizes the importance of LIF in blastocyst implantation in mouse and LIF possible involvement in signalling between the foetus and the endometrium [Citation73]. The role of LIF in implantation was clearly demonstrated in studies using LIF knockout model in which a LIF null female mice exhibited failure of implantation that was restored on LIF administration [Citation74]. Such implantation failure was partly attributed to profound disturbance of normal luminal epithelial and stromal differentiation during early pregnancy in LIF-null mice which was characterised by non-development of apical pinopods, increased glycocalyx and failure of endometrial cell decidualization during the peri-implantation period [Citation75]. In addition, absence of LIFR to activate Janus kinase-signal transducer and activation of transcription 3 (Jak-Stat3) signalling pathway in the LE that could have induced receptivity in the LE [Citation76].
At early stage of pregnancy, LIF has also been detected in uterus of mouse [Citation77], rabbit [Citation78], sheep [Citation79], western spotted skunk [Citation80] and uterine flushing in human with good prognosis for implantation success [Citation81]. Uterine expression of LIF in human is proposed to play significant role in embryo implantation, possibly through an autocrine/paracrine interaction between LIF and its receptor at the luminal epithelium [Citation72]. Similar effect is proposed in rabbit [Citation82]. To buttress this line of thought, low expression of endometrial LIF at the proliferative phase of the cycle has been associated with infertility primarily due to implantation failures [Citation83]. This makes LIF one of the candidate genes/molecules to be considered when investigating infertility in human especially with those implantation failures after embryo transfer [Citation84]. Progesterone and IFNt were shown to regulate expression of LIF and its receptor LIFR in sheep GE and trophectoderm during the period of implantation. This was also associated with increased phosphorylation of signalling molecules STAT3 and MAPK3/1 protein [Citation79] as well association of LIFR and gp130 forming a functional heterodimer in the uterus during the attachment reaction to direct LIF signalling [Citation85].
These results indicate that LIF is involved in blastocyst implantation to the endometrium. This is possible through generation of paracrine signalling that complements the endocrine signal between the mother and the conceptus [Citation86] or through the indirect local effect of LIF on other implantation-related cytokines produced by the endometrium or conceptus.
7 Hyaluronan system
Hyaluronan, otherwise known as hyaluronic acid or hyaluronate (HA) is a unique high molecular weight anionic members of a group of macromolecules called glycosaminoglycans (GAGs) that constitute major components of the ECM in all animal tissues [Citation87]. At low concentrations, HA is ubiquitous in the body tissue and fluid. It is detectable in tendon, muscle, joint, uterus and cartilage with more than 50% of total HA body content existing in the skin where it keeps the dermis moisturized [Citation88]. It is also present in various fluids and tissues of the reproductive tract, in amounts that vary from one mammalian species to another. In human follicular fluid, HA concentrations range between 48 and 72.8 ng/mL with significant variation in fluid with fertilized and unfertilized oocytes [Citation89]. In the serum, HA level gradually increases as the pregnancy proceeds with the highest concentration (about 100.4 ng/mL) occurring during labour [Citation90]. There is a high concentration of HA in the foetal circulation and amniotic fluid. In mice, the HA content of the uterus is 4053.0 ± 651.4 ng/g during dioestrus [Citation91].
HA is produced by three trans-membrane enzymes hyaluronan synthases (HAS1, HAS2 and HAS3) and systematically degraded by hyaluronidase (HYALs). It is also capable of altering cell structure and function by binding to its receptor, mainly cluster domain 44 (CD44) and receptor for HA mediated motility (RHAMM) [Citation92,Citation93]. Many roles of HA in mammalian reproduction that include cumulus expansion and oocyte maturation, sperm-oocyte interaction, cervical ripening and dilation as well as development of embryo have been reviewed elsewhere [Citation94]. HA increases about five to six folds in mouse uteri on the day of implantation with potential to support attachment from observation of an embryo cultured on HA-coated tissue culture plates [Citation95].
During the early stage of its discovery, HA was originally thought of fulfilling the functions of space filling and tissue hydration alone. Evidence in the last two decades implies that HA is involved in diverse physiological processes in the body. The issue of finding a suitable embryo transfer media partly brought HA into the scope of reproductive biotechnology research. According to available data, HA seems to be beneficial in assisted reproductive technologies involving in vitro fertilization (IVF) and embryo transfer (ET). Its use becomes attractive since HA is a naturally occurring substance. HA is the only non-sulphated GAG that has been detected in various segments of the mammalian reproductive tract including oviduct and uterus [Citation96,Citation97] and expressed at different stages of embryo development [Citation98]. If HA is produced by the two principal partakers of implantation (endometrium and embryo), then, conception of HA roles in implantation is rational. Besides, HA is up-regulated in endometrial stroma at the time of implantation in human [Citation99] and enhancement of implantation in many clinical trials in human using HA-supplemented media for embryo transfer (ET) supports this line of thought [Citation100–Citation102].
As much as there is a plethora of data suggesting beneficial roles for HA in human embryo implantation, the mechanism through which HA promotes implantation still remains ambiguous. It is generally proposed to be attributed to early stages of implantation facilitating apposition and attachment of the trophectoderm to the maternal endometrium [Citation103]. A possible mechanism of HA involvement in implantation may also be through the ability of HA to promote angiogenesis [Citation104], a process which is fundamental to embryonic development [Citation105]. Other known roles of HA including facilitation of cell adhesion, cell to cell matrix and HA mediated signalling [Citation106,Citation107], induction of heat shock protein and the suppression of apoptosis by low molecular weight HA are all processes essential for embryonic development and implantation [Citation108]. Besides, HA or its related protein has been closely linked with low molecular weight cytokines or growth factors such as prostaglandins, IGF, epidermal growth factor and LIF [Citation109], which have been individually implied to be involved in embryo implantation. Moreover, sheep endometrial cell culture treated in vitro with low molecular weight HA upregulated transcript expression of LIF, CD44, IGF but reduced MUC1 expression into the culture media [Citation110].
Despite these studies indicating beneficial roles of HA in embryo development, many other studies have failed to find a positive influence of HA inclusion in embryo transfer media on pregnancy rate [Citation102,Citation111]. The result of a recent study clearly demonstrated the need for HA clearance at the foetal-maternal interface in sheep for successful implantation to occur [Citation112]. More studies are required to clarify this ambiguity on the role of HA inclusion in embryo transfer media and implantation.
8 Cluster domain 44 (CD44)
CD44 is a single-pass trans-membrane glycoprotein located on the surface of most vertebrate cells [Citation113]. It has been detected in various segments of the reproductive tract in bovine, ovine, mouse, mare and human under normal physiological [Citation96,Citation97,Citation114,Citation115] or pathological conditions such as neoplastic endometrium in human [Citation116]. CD44 is also detected during early stages of embryo development in mouse, bovine and human [Citation117,Citation118]. The specific roles of CD44 at the blastocyst-endometrial interface during implantation were demonstrated by the study of Illera et al. [Citation119] where intra-uterine administration of anti-CD44 impeded implantation in the rabbit, while no effect was seen in control rabbits with intra-peritoneal administration of the same antibodies.
The signalling properties of CD44 embrace physiological processes like oocyte maturation and implantation [Citation120]. It is a major receptor for HA [Citation121] and also a receptor for OPN [Citation122]. OPN, as a primary ligand of ITG binds to cell surface integrins primarily αVβ3 heterodimer expressed by trophectoderm and uterus to promote cell-cell attachment and cell spreading [Citation47]. ITG has been acknowledged to bridge the gaps between the ITG family in the maternal endometrium and trophoblast, an event that is critical for initiation of initial attachment during implantation [Citation123]. In the scheme of attachment cascade, CD44 is proposed to play a crucial role of activating the OPN which unites ITG in the trophectoderm and the endometrial luminal epithelia via the ITG αvβ3 ().
In vitro maturation of oocytes is widely used for in vitro fertilization. The localisation of CD44 in the cumulus cells which produces HA matrix of the cumulus oophorus [Citation124] is suggestive of HA-CD44 induced signalling during oocyte maturation. The mechanism is that HA-CD44 interaction regulates the tyrosine phosphorylation of Connexin 43 (the major gap protein found in the cumulus oophorus) leading to closure of the gap junctions and subsequent upregulation of maturation promotion factor activity [Citation120]. The latter brings about resumption of meiotic division in the oocytes. This line of thought is corroborated by the earlier work of Schoenfelder and Einspanier [Citation125] in which HA and its receptor CD44 were reportedly involved in maturation of bovine oocyte.
It should be reiterated that many studies on HA-CD44 signalling have focussed on cancer. HA-CD44 signalling in cancer [Citation106,Citation126], though well-documented in the literature is beyond the scope of this review. However, HA-CD44 signalling is also observed under physiological conditions. HYAL2 reportedly caused increased phosphorylation of MAPK1/3 signalling in bovine embryos [Citation127]. A recent study also showed induction of signalling by HA in human placenta through MAPK1/3 and Phospho inositol 3 kinase signalling pathways; an event that enhanced trophoblast growth and invasion possibly through placenta angiogenesis [Citation128]. Even though the latter study did not show clearly that the signalling was through HA binding to CD44, it is likely to be through HA-CD44 because CD44 is the major receptor for HA and earlier studies have shown expression of CD44 in the human endometrium [Citation96] and trophoblast [Citation117] where it was proposed to play a significant role in placenta angiogenesis [Citation129]. Collectively, it may be concluded that CD44 is germane to implantation process specifically and/or as receptors for other key cytokines OPN and HA already shown to be involved in implantation cascades.
9 Glycoprotein mucin 1 (MUC1)
Mucins encompass a family of highly glycosylated and high molecular weight (>250 kDa) glycoproteins expressed on the epithelia surface subsequent to its production by epithelial tissue [Citation130]. There are about 15 variants of mucin in mammals, however, MUC1 is the most widely distributed in reproductive tract [Citation130,Citation131]. MUC1 expression has been reported in endometrial luminal epithelia in most mammals that including human, sheep, horse, pig and rabbit [Citation132]. MUC1 is a non-adhesive molecule whose regulation, expression and functions with regards to implantation vary according to species. In sheep, endometrial MUC1 expression is cyclically regulated by both oestrogen and progesterone in vivo and in vitro, and directly down regulated by interferon tau treatment in vitro [Citation133]. Interferon tau is an agent of maternal recognition of pregnancy produced by the blastocyst to abrogate luteolysis in ruminants [Citation134].
In some animals like mice, rats, pigs, rabbits and ruminants, MUC1 expression at the uterine luminal epithelium is attributed to non-receptivity of the uterus and continued expression during the period of implantation will hysterically hinder the access of receptors to their ligands [Citation6]. Therefore, in these species, it is regarded as an anti-adhesive molecule that inhibits successful interaction between the maternal endometrium and the implanting embryo. In sheep, MUC1 is up-regulated in the LE of a progesterone-dominant endometrium observed during the luteal phase. This property is in consonance with its protective function against pathogens because there is likelihood of reduced maternal immune response during this period. Henceforth, for a successful implantation to occur, MUC1 expression in the endometrial epithelia has to be cleared. This down-regulation also coincides with loss of progesterone receptor in the endometrial epithelia [Citation135]. Removal of MUC1 from the epithelial surface at implantation sites is accomplished locally through signals apparently produced by the blastocyst [Citation136]. In addition it is successively followed with up-regulation in the expression of other adhesive molecule like OPN and ITG in the LE [Citation24]. In this context, MUC1 down-regulation in the endometrial epithelia can be used as a marker of endometrial receptivity in these species.
Human and mouse have the same haemochorial mode of placentation. For obvious ethical reason, our current understanding on human embryo implantation is based mostly on experiments derived from animal models especially mice, even though there is inverse expression of MUC1 in the endometrium during implantation in the two species. In a clear contrast to mice, MUC1 expression is highest at the time when the human endometrium is receptive, however, it is systematically removed from the apical endometrial epithelia in a paracrine pattern by the embryo just at the time of implantation [Citation137]. MUC1 is regarded as inherent constituent of secretory endometrium and its down-regulation during this period is associated with recurrent miscarriage in human [Citation138].
In mare, MUC1 does not inhibit implantation. MUC1 protein is expressed at foeto-maternal interface throughout the period of gestation. Therefore, the protracted period of implantation in this species is not attributable to adhesive property of MUC1 observed in the LE and the trophoblast before implantation [Citation139]. In the sow, MUC1 expression is also unique. MUC1 protein was detected in the attachment and inter-attachment region of the endometrium between day 13 and 24 of gestation [Citation140] and was found to be down-regulated during the time of implantation in this species [Citation23,Citation141].
It is concluded that MUC1 plays crucial roles in successful implantation and embryo survival, possibly through establishment of stromal decidualization and its down-regulation either locally at the region of implantation sites or generally along the entire endometrial luminal epithelia that is essential to allow ligands on the trophoblast gain access to their respective receptors on the endometrial epithelia and vice versa. Altogether, this implies that MUC1 expression and regulation in endometrial epithelia during implantation are species-specific.
10 Conclusions
Finally, cytokines, growth factors and macromolecules are all chemical messengers that mediate intercellular communication whose biological actions are mediated locally by specific receptors. They have been associated with many functions in the body including injury, inflammation, immune response and implantation. Pro-inflammatory mediators are produced in response to inflammation very similar to what are observed during implantation [Citation142]. Apart from the aforementioned cytokines and growth factors discussed in this review, there are many more that partake in implantation in mammalian species. Indeed, there are repertoires of genes ‘working behind the veil’ in a molecular template to bring about the actual observable event of cellular adhesion of the trophoblast to the maternal endometrium during mammalian implantation [Citation143–Citation145] and the aforementioned have been shown to play significant roles.
For implantation to be fruitful, the blastocyst must be implantation competent, while the endometrium has to be receptive. Certainly, the different modes of implantation across many mammalian species dictate different molecular mechanisms to be involved. Suitable universal markers and mediators of implantation have proved difficult to be identified partly because very few morphological or molecular correlates of the receptive/implantation states are common to all species. No single reliable universal marker that is strictly restricted only to the receptive phase is yet to be identified across board. Therefore, a combination of markers/mediators as done in this study seems logical.
The Assisted reproductive technology (ART) has contributed significantly towards improving animal and human fertility. Embryo culture is an emerging technical component of ART. However, the in vitro environment, no matter the level of simulation, the immediate macro-environment remains sub-optimal when compared to the in vivo condition. In the latter, the embryo is innately exposed to arrays of growth factors, cytokines and macromolecules some of which are the subject matter of this review. The high rate of implantation failure associated with embryo transfer in assisted conception may possibly be due to absence of some of these cytokines/growth factors. It is hoped that a clear understanding of the roles played by these cytokines and growth factors in mammalian implantation as revealed in this review will motivate further research on these topics to unravel some unresolved ambiguity on their roles in implantation. Such endeavours will facilitate their inclusion in embryo culture media (if found positive) and elevate them as a vital aspect to be considered along with steroid functions while developing strategies to improve fertility or investigating infertility in mammals.
Competing interests
There are no competing interests.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- J.BoivinL.BuntingJ.A.CollinsK.G.NygrenInternational estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical careHum Reprod226200715061512
- M.G.DiskinD.G.MorrisEmbryonic and early foetal losses in cattle and other ruminantsReprod Dom Anim432008260267
- M.G.DiskinJ.J.MurphyJ.M.SreenanEmbryo survival in dairy cows managed under pastoral conditionsAnim Reprod Sci963–42006297311
- L.D.DunneM.G.DiskinJ.M.SreenanEmbryo and foetal loss in beef heifers between day 14 of gestation and full termAnim Reprod Sci581–220003944
- C.J.AshworthMaternal and conceptus factors affecting histotrophic nutrition and survival of embryosLivest Prod Sci442199599105
- T.E.SpencerG.A.JohnsonF.W.BazerR.C.BurghardtImplantation mechanisms: insights from the sheepReproduction12862004657668
- J.D.AplinA.T.FazleabasS.R.GlasserL.C.GiudiceThe Endometrium Molecular, Cellular, and Clinical Perspectivessecond ed.2008Informa UK LtdUnited States
- D.D.CarsonI.BagchiS.K.DeyA.C.EndersA.T.FazleabasB.A.LesseyEmbryo implantationDev Biol22322000217237
- J.D.AplinEmbryo implantation: the molecular mechanism remains elusiveReprod Biomed Online1362006833839
- S.TranguchD.F.SmithS.K.DeyProgesterone receptor requires a co-chaperone for signalling in uterine biology and implantationReprod Biomed Online1420073948 Spec No. 1
- W.G.MaH.SongS.K.DasB.C.PariaS.K.DeyEstrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantationProc Natl Acad Sci USA1005200329632968
- S.OzturkR.DemirParticular functions of estrogen and progesterone in establishment of uterine receptivity and embryo implantationHistol Histopathol259201012151228
- A.E.KingH.O.CritchleyOestrogen and progesterone regulation of inflammatory processes in the human endometriumJ Steroid Biochem Mol Biol1202–32010116126
- H.WangS.K.DeyRoadmap to embryo implantation: clues from mouse modelsNat Rev Gen732006185199
- E.RuoslahtiN.A.NobleS.KagamiW.A.BorderIntegrinsKidney Int Suppl441994S17S22
- A.P.MaartensN.H.BrownAnchors and signals: the diverse roles of integrins in developmentCurr Top Dev Biol1122015233272
- B.A.LesseyJ.T.ArnoldParacrine signaling in the endometrium: integrins and the establishment of uterine receptivityJ Reprod Immunol391–21998105116
- B.A.LesseyIntegrins and the endometrium: new markers of uterine receptivityAnn N Y Acad Sci8281997111122
- Y.SunB.DaiY.WuL.YangP.LiuZ.WangCarbon disulfide exposure at peri-implantation disrupts embryo implantation by decreasing integrin beta3 expression in the uterine tissue of pregnant miceChem Biol Interact20622013126133
- B.LesseyA.CastelbaumC.BuckY.LeiC.YowellJ.SunFurther characterization of endometrial integrins during the menstrual cycle and in pregnancyFertil Steril6231994497506
- M.J.IlleraP.L.LorenzoY.T.GuiS.A.BeylerK.B.ApparaoB.A.LesseyA role for alphavbeta3 integrin during implantation in the rabbit modelBiol Reprod6832003766771
- S.KimminsL.A.MacLarenCyclic modulation of integrin expression in bovine endometriumBiol Reprod615199912671274
- J.A.BowenF.W.BazerR.C.BurghardtSpatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivoBiol Reprod555199610981106
- G.A.JohnsonMUC-1, integrin, and osteopontin expression during the implantation cascade in sheepBiol Reprod6532001820828
- J.A.BowenF.W.BazerR.C.BurghardtSpatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vitroBiol Reprod5621997409415
- A.T.FazleabasS.C.BellS.FlemingJ.SunB.A.LesseyDistribution of integrins and the extracellular matrix proteins in the baboon endometrium during the menstrual cycle and early pregnancyBiol Reprod5621997348356
- H.LinX.WangG.LiuJ.FuA.WangExpression of alphaV and beta3 integrin subunits during implantation in pigMol Reprod Dev7411200713791385
- M.IlleraE.CullinanY.GuiL.YuanS.BeylerB.LesseyBlockade of the alphaVbeta3 integrin adversely affects implantation in the mouseBiol Reprod62200012851290
- A.P.F.FlintG.E.LammingH.J.StewartAbayasekara D. The role of the endometrial oxytocin receptor in determining the length of the sterile oestrous cycle and ensuring maintenance of luteal function in early pregnancy in ruminantsPhilos Trans R Soc Lond B Biol Sci3441994291304
- L.LiB.F.XuQ.J.ChenX.X.SunEffects of hydrosalpinx on pinopodes, leukaemia inhibitory factor, integrin beta3 and MUC1 expression in the peri-implantation endometriumEur J Obstet Gynecol Reprod Biol15122010171175
- K.ThomasA.J.ThomsonV.SephtonC.CowanS.WoodG.VinceThe effect of gonadotrophic stimulation on integrin expression in the endometriumHum Reprod17120026368
- H.C.RuanX.M.ZhuQ.LuoA.X.LiuY.L.QianC.Y.ZhouOvarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in miceHum Reprod2110200625212529
- S.YacobovichL.TuchinskyM.KirbyT.KardashO.AgranyoniE.NesherNovel synthetic cyclic integrin alphavbeta3 binding peptide ALOS4: antitumor activity in mouse melanoma modelsOncotarget73920166354963560
- Y.KariyaM.KannoK.Matsumoto-MoritaM.KonnoY.YamaguchiY.HashimotoOsteopontin O-glycosylation contributes to its phosphorylation and cell-adhesion propertiesBiochem J4631201493102
- G.A.JohnsonOsteopontin: roles in implantation and placentationBiol Reprod695200314581471
- K.A.DunlapD.W.EriksonR.C.BurghardtF.J.WhiteK.M.ReedJ.L.FarmerProgesterone and placentation increase secreted phosphoprotein one (SPP1 or osteopontin) in uterine glands and stroma for histotrophic and hematotrophic support of ovine pregnancyBiol Reprod7952008983990
- G.A.JohnsonT.E.SpencerR.C.BurghardtF.W.BazerOvine osteopontin: I. Cloning and expression of messenger ribonucleic acid in the uterus during the periimplantation periodBiol Reprod6141999884891
- F.W.BazerG.WuG.A.JohnsonJ.KimG.SongUterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewesBiol Reprod856201110941107
- J.FilantT.E.SpencerUterine glands: biological roles in conceptus implantation, uterine receptivity and decidualizationInt J Dev Biol582–42014107116
- G.J.BurtonA.L.WatsonJ.HempstockJ.N.SkepperE.JauniauxUterine glands provide histiotrophic nutrition for the human fetus during the first trimester of pregnancyJ Clin Endocrinol Metab876200229542959
- A.C.GrayF.F.BartolK.M.TaylorA.A.WileyW.S.RamseyT.L.OttOvine uterine gland knock-out model: effects of gland ablation on the estrous cycleBiol Reprod6222000448456
- C.GrayF.W.BazerT.E.SpencerUterine glands: development biology and function during pregnancyAnn Rev Biomed Sci3200185126
- C.A.GrayR.C.BurghardtG.A.JohnsonF.W.BazerT.E.SpencerEvidence that absence of endometrial gland secretions in uterine gland knockout ewes compromises conceptus survival and elongationReproduction12422002289300
- J.E.GarlowH.KaG.A.JohnsonR.C.BurghardtL.A.JaegerF.W.BazerAnalysis of osteopontin at the maternal-placental interface in pigsBiol Reprod6632002718725
- Y.J.KangK.ForbesJ.CarverJ.D.AplinThe role of the osteopontin-integrin alphavbeta3 interaction at implantation: functional analysis using three different in vitro modelsHum Reprod2942014739749
- S.KimminsH.C.LimL.A.MacLarenImmunohistochemical localization of integrin alpha V beta 3 and osteopontin suggests that they do not interact during embryo implantation in ruminantsReprod Biol Endocrinol2200419
- G.A.JohnsonR.C.BurghardtT.E.SpencerG.R.NewtonT.L.OttF.W.BazerOvine osteopontin: II. Osteopontin and alpha(v)beta(3) integrin expression in the uterus and conceptus during the periimplantation periodBiol Reprod6141999892899
- J.KimD.W.EriksonR.C.BurghardtT.E.SpencerG.WuK.J.BaylessSecreted phosphoprotein 1 binds integrins to initiate multiple cell signaling pathways, including FRAP1/mTOR, to support attachment and force-generated migration of trophectoderm cellsMatrix Biol2952010369382
- G.A.JohnsonR.C.BurghardtF.W.BazerOsteopontin: a leading candidate adhesion molecule for implantation in pigs and sheepJ Anim Sci Biotechnol51201456
- D.R.ClemmonsInsulin-like growth factor binding proteins and their role in controlling IGF actionsCytokine Growth Factor Rev8119974562
- T.McKinnonC.ChakrabortyL.M.GleesonP.ChidiacP.K.LalaStimulation of human extravillous trophoblast migration by IGF-II is mediated by IGF type 2 receptor involving inhibitory G protein(s) and phosphorylation of MAPKJ Clin Endocrinol Metab868200136653674
- L.M.GleesonC.ChakrabortyT.McKinnonP.K.LalaInsulin-like growth factor-binding protein 1 stimulates human trophoblast migration by signaling through alpha 5 beta 1 integrin via mitogen-activated protein Kinase pathwayJ Clin Endocrinol Metab866200124842493
- N.BriardF.DadounG.PommierN.SauzeY.LeboucC.OliverIGF-I/IGFBPs system response to endotoxin challenge in sheepJ Endocrinol16432000361369
- I.Martin-EstalR.G.de la GarzaI.Castilla-CortazarIntrauterine growth retardation (IUGR) as a novel condition of insulin-like growth factor-1 (IGF-1) deficiencyRev Physiol Biochem Pharmacol1702016135
- T.Swangchan-UthaiS.W.WalshS.L.AlexanderZ.ChengM.A.CroweA.C.EvansComparison of mRNA for IGFs and their binding proteins in the oviduct during the peri-oestrous period between dairy heifers and lactating cowsReproduction14232011457465
- K.R.StevensonR.S.GilmourD.C.WathesLocalization of insulin-like growth factor-I (IGF-I) and -II messenger ribonucleic acid and type 1 IGF receptors in the ovine uterus during the estrous cycle and early pregnancyEndocrinology1344199416551664
- B.De VrijerM.L.DavidsenR.B.WilkeningR.V.AnthonyT.R.H.RegnaultAltered placental and fetal expression of IGFs and IGF-binding proteins associated with intrauterine growth restriction in fetal sheep during early and mid-pregnancyPediatr Res6052006507512
- F.A.HillsJ.EnglishT.ChardCirculating levels of IGF-I and IGF-binding protein-1 throughout pregnancy: relation to birthweight and maternal weightJ Endocrinol14821996303309
- M.A.FenwickS.LlewellynR.FitzpatrickD.A.KennyJ.J.MurphyJ.PattonNegative energy balance in dairy cows is associated with specific changes in IGF-binding protein expression in the oviductReproduction135120086375
- J.S.BrickellM.M.McGowanD.U.PfeifferD.C.WathesMortality in Holstein-Friesian calves and replacement heifers, in relation to body weight and IGF-I concentration, on 19 farms in EnglandAnimal38200911751182
- D.C.WathesMechanisms linking metabolic status and disease with reproductive outcome in the dairy cowReprod Domest Anim47Suppl. 42012304312
- G.A.PalmaM.MullerG.BremEffect of insulin-like growth factor I (IGF-I) at high concentrations on blastocyst development of bovine embryos produced in vitroJ Reprod Fertil11021997347353
- A.Q.BonillaM.OzawaP.J.HansenTiming and dependence upon mitogen-activated protein kinase signaling for pro-developmental actions of insulin-like growth factor 1 on the preimplantation bovine embryoGrowth Horm IGF Res2122011107111
- P.J.O'ShaughnessyD.C.WathesCharacteristics of bovine luteal cells in culture: morphology, proliferation and progesterone secretion in different media and effects of LH, dibutyryl cyclic AMP, antioxidants and insulinJ Endocrinol10431985355361
- V.S.ChouhanS.S.DangiV.BabithaM.R.VermaS.BagG.SinghStimulatory effect of luteinizing hormone, insulin-like growth factor-1, and epidermal growth factor on vascular endothelial growth factor production in cultured bubaline luteal cellsTheriogenology847201511851196
- A.PtakM.KajtaE.L.GregoraszczukEffect of growth hormone and insulin-like growth factor-I on spontaneous apoptosis in cultured luteal cells collected from early, mature, and regressing porcine corpora luteaAnim Reprod Sci803–42004267279
- R.K.BaithaluS.K.SinghC.GuptaA.K.RajaA.SaxenaS.K.AgarwalInsulin stimulates progesterone secretion to a greater extent than LH in early pregnant buffalo luteal cells cultured in vitroAnim Reprod Sci1423–42013131136
- M.PantaleonH.JerichoG.RabnottP.L.KayeThe role of insulin-like growth factor II and its receptor in mouse preimplantation developmentReprod Fertil Dev151–220033745
- C.J.GreenS.T.FraserM.L.DayInsulin-like growth factor 1 increases apical fibronectin in blastocysts to increase blastocyst attachment to endometrial epithelial cells in vitroHum Reprod3022015284298
- C.MobergV.BourlevN.IlyasovaM.OlovssonEndometrial expression of LIF and its receptor and peritoneal fluid levels of IL-1alpha and IL-6 in women with endometriosis are associated with the probability of pregnancyArch Gynecol Obstet29222015429437
- L.AghajanovaLeukemia inhibitory factor and human embryo implantationUterus Hum Reprod10342004176183
- E.B.CullinanS.J.AbbondanzoP.S.AndersonJ.W.PollardB.A.LesseyC.L.StewartLeukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine paracrine function in regulating embryo implantationProc Natl Acad Sci USA937199631153120
- Z.M.YangS.P.LeD.B.ChenJ.CotaV.SieroK.YasukawaLeukemia inhibitory factor, Lif receptor, and Gp130 in the mouse uterus during early-pregnancyMol Reprod Dev4241995407414
- J.R.ChenJ.G.ChengT.ShatzerL.SewellL.HernandezC.L.StewartLeukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesisEndocrinology14112200043654372
- A.A.Fouladi-NashtaC.J.JonesN.NijjarL.MohametA.SmithI.ChambersCharacterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain miceDev Biol28112005121
- J.ChengG.RosarioT.V.CohenJ.HuC.L.StewartTissue-specific ablation of the LIF receptor in the murine uterine epithelium results in implantation failureEndocrinology1586201719161928
- L.Q.CaiY.J.CaoE.K.DuanEffects of leukaemia inhibitory factor on embryo implantation in the mouseCytokine1211200016761682
- C.Q.LiuY.YuanZ.X.WangEffects of leukaemia inhibitory factor on endometrial receptivity and its hormonal regulation in rabbitsCell Biol Int2510200110291032
- G.SongM.C.SatterfieldJ.KimF.W.BazerT.E.SpencerProgesterone and interferon tau regulate leukemia inhibitory factor receptor and IL6ST in the ovine uterus during early pregnancyReproduction13732009553565
- C.PassavantX.ZhaoS.K.DasS.K.DeyR.A.MeadChanges in uterine expression of leukemia inhibitory factor receptor gene during pregnancy and its up-regulation by prolactin in the western spotted skunkBiol Reprod6312000301307
- N.Ledee-BatailleG.Lapree-DelageJ.L.TaupinS.DubanchetR.FrydmanG.ChaouatConcentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantationHum Reprod1712002213218
- T.LeiZ.Q.YangT.XiaL.GanX.D.ChenJ.H.YuanStage-specific expression of leukaemia inhibitory factor and its receptor in rabbit pre-implantation embryo and uterine epithelium during early pregnancyReprod Domest Anim39120041318
- M.WuY.YinM.ZhaoL.HuQ.ChenThe low expression of leukemia inhibitory factor in endometrium: possible relevant to unexplained infertility with multiple implantation failuresCytokine6222013334339
- T.SteckR.GiessM.W.SuetterlinM.BollandS.WiestU.G.PoehlsLeukaemia inhibitory factor (LIF) gene mutations in women with unexplained infertility and recurrent failure of implantation after IVF and embryo transferEur J Obstet Gynecol Reprod Biol112120046973
- H.SongH.LimEvidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantationReproduction13122006341349
- K.LeducV.BourassaE.AsselinP.LeclercJ.LafondC.Reyes-MorenoLeukemia inhibitory factor regulates differentiation of trophoblastlike BeWo cells through the activation of JAK/STAT and MAPK3/1 MAP Kinase-signaling pathwaysBiol Reprod8622012
- I.AndersonThe properties of hyaluronan and its role in wound healingProf Nurse1742001232235
- S.E.ArmstrongD.R.BellRelationship between lymph and tissue hyaluronan in skin and skeletal muscleAm J Physiol Heart Circ Physiol2832002H2485H2494
- H.SaitoT.KanekoT.TakahashiS.KawachiyaT.SaitoM.HiroiHyaluronan in follicular fluids and fertilization of oocytesFertil Steril746200011481152
- H.KobayashiG.W.SunY.TanakaT.KondoT.TeraoSerum hyaluronic acid levels during pregnancy and laborObstet Gynecol9341999480484
- T.R.C.GomesC.VernaH.B.NaderSimões R.dos SantosJ.L.DreyfussJ.R.M.MartinsConcentration and distribution of hyaluronic acid in mouse uterus throughout the estrous cycleFertil Steril9222009785792
- J.NecasL.BartosikovaP.BraunerJ.KolarHyaluronic acid (hyaluronan): a reviewVet Med5382008397411
- B.P.TooleHyaluronan in morphogenesisSem Cell Devel Biol12220017987
- A.A.Fouladi-NashtaK.A.RaheemW.F.MareiF.GhafariG.M.HartshorneRegulation and roles of the hyaluronan system in mammalian reproductionReproduction15322017R43R58
- D.D.CarsonA.DuttJ.P.TangGlycoconjugate synthesis during early pregnancy: hyaluronate synthesis and functionDev Biol12011987228235
- A.M.AfifyS.CraigA.F.PaulinoTemporal variation in the distribution of hyaluronic acid, CD44s, and CD44v6 in the human endometrium across the menstrual cycleAppl Immunohistochem Mol Morphol1432006328333
- K.A.RaheemW.F.MareiK.MifsudM.KhalidD.C.WathesA.A.Fouladi-NashtaRegulation of the hyaluronan system in ovine endometrium by ovarian steroidsReproduction14552013491504
- W.F.MareiF.GhafariA.A.Fouladi-NashtaRole of hyaluronic acid in maturation and further early embryo development of bovine oocytesTheriogenology7832012670677
- L.SalamonsenS.ShusterR.SternDistribution of hyaluronan in human endometrium across the menstrual cycleCell Tissue Res30622001335340
- K.NakagawaC.TakahashiY.NishiH.JyuenR.SugiyamaY.KuribayashiHyaluronan-enriched transfer medium improves outcome in patients with multiple embryo transfer failuresJ Assist Reprod Genet2972012679685
- M.SvobodovaJ.BrezinovaI.ObornaJ.DostalM.KrskovaEmbryoGlue the transfer medium with hyaluronan in the IVF+ET programCeska Gynekol72120071519
- S.ChunJ.E.SeoY.J.RimJ.H.JooY.C.LeeY.H.KooEfficacy of hyaluronan-rich transfer medium on implantation and pregnancy rates in fresh and frozen-thawed blastocyst transfers in Korean women with previous implantation failureObstet Gynecol Sci5932016201207
- F.HambilikiE.LjungerP.O.KarlstromA.Stavreus-EversHyaluronan-enriched transfer medium in cleavage-stage frozen-thawed embryo transfers increases implantation rate without improvement of delivery rateFertil Steril945201016691673
- M.SlevinJ.KrupinskiJ.GaffneyS.MatouD.WestH.DelisserHyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathwaysMatrix Biol2620075868
- G.BreierAngiogenesis in embryonic development-a reviewPlacenta21Suppl. 12000S11S15
- B.P.TooleHyaluronan: from extracellular glue to pericellular cueNat Rev Cancer472004528539
- E.A.TurleyP.W.NobleL.Y.W.BourguignonSignaling properties of hyaluronan receptorsJ Biol Chem2777200245894592
- H.XuT.ItoA.TawadaH.MaedaH.YamanokuchiK.IsaharaEffect of hyaluronan oligosaccharides on the expression of heat shock protein 72J Biol Chem2771920021730817314
- A.T.PalaszH.Rodriguez-MartinezP.Beltran-BreñaS.Perez-GarneloM.F.MartinezA.Gutierrez-AdanEffects of hyaluronan, BSA, and serum on bovine embryo in vitro development, ultrastructure, and gene expression patternsMol Reprod Dev7312200615031511
- K.A.RaheemA.A.Fouladi-NashtaInfluence of Hyaluronan on molecular markers of implantation in sheepProc Society of Reproduction and Fertility2015St Catherine's CollegeOxford, UK
- J.H.CheckD.Summers-ChaseW.YuanK.SwensonD.HorwathM.Press“Embryo glue” does not seem to improve chances of subsequent pregnancy in refractory in vitro fertilization casesClin Exp Obstet Gynecol39120121112
- W.F.MareiD.C.WathesK.A.RaheemO.Mohey-ElsaeedF.GhafariA.A.Fouladi-NashtaInfluence of hyaluronan on endometrial receptivity and embryo attachment in sheepReprod Fertil Dev2016
- H.PontaL.ShermanP.A.HerrlichCD44: from adhesion molecules to signalling regulatorsNat Rev Mol Cell Biol4120033345
- A.-S.BergqyistM.YokooR.BageE.SatoH.Rodriguez-MartinezDetection of the hyaluronan receptors CD44 in the bovine oviductal epitheliumJ Reprod Dev5142005445453
- H.I.RodriguezA.J.StewartD.F.WolfeF.J.CaldwellM.HarrieE.M.WhitleyImmunolocalization of the hyaluronan receptor CD44 in the reproductive tract of the mareTheriogenology7522011276286
- A.M.AfifyS.CraigA.F.PaulinoR.SternExpression of hyaluronic acid and its receptors, CD44s and CD44v6, in normal, hyperplastic, and neoplastic endometriumAnn Di Path962005312318
- S.CampbellH.R.SwannJ.D.AplinM.W.SeifS.J.KimberM.ElsteinCD44 is expressed throughout pre-implantation human embryo developmentHum Reprod1021995425430
- C.FurnusA.ValcarcelF.DuloutA.ErrecaldeThe hyaluronic acid receptor (CD44) is expressed in bovine oocytes and early stage embryosTheriogenology609200316331644
- M.J.IlleraP.BermejoJ.HernandezA.GonzalezJ.C.IlleraThe effect of ant-CD44 on embryo implantation in rabbitsReprod Fertil Dev1622004190191
- E.SatoM.YokooMorphological and biochemical dynamics of porcine cumulus-oocyte complexes: role of cumulus expansion in oocyte maturationItal J Anat Embryol1102 Suppl. 12005205217
- C.M.IsackeH.YarwoodThe hyaluronan receptor, CD44Int J Biochem Cell Biol3472002718721
- R.ZoharN.SuzukiK.SuzukiP.AroraM.GlogauerC.A.McCullochIntracellular osteopontin is an integral component of the CD44-ERM complex involved in cell migrationJ Cell Physiol18412000118130
- G.A.JohnsonR.C.BurghardtM.M.JoyceT.E.SpencerF.W.BazerC.A.GrayOsteopontin is synthesized by uterine glands and a 45-kDa cleavage fragment is localized at the uterine-placental interface throughout ovine pregnancyBiol Reprod69120039298
- N.KimuraY.KonnoK.MiyoshiH.MatsumotoE.SatoExpression of hyaluronan synthases and CD44 messenger RNAs in porcine cumulus-oocyte complexes during in vitro maturationBiol Reprod662002707717
- M.SchoenfelderR.EinspanierExpression of hyaluronan synthases and corresponding hyaluronan receptors is differentially regulated during oocyte maturation in cattleBiol Reprod6912003269277
- B.P.TooleHyaluronan-CD44 interactions in cancer: paradoxes and possibilitiesClin Cancer Res1524200974627468
- W.F.MareiM.SalavatiA.A.Fouladi-NashtaCritical role of hyaluronidase-2 during preimplantation embryo developmentMol Hum Reprod1992013590599
- R.ZhuY.H.HuangY.TaoS.C.WangC.SunH.L.PiaoHyaluronan up-regulates growth and invasion of trophoblasts in an autocrine manner via PI3K/AKT and MAPK/ERK1/2 pathways in early human pregnancyPlacenta3492013784791
- R.GoshenI.ArielS.ShusterA.HochbergI.VlodavskyN.de GrootHyaluronan, CD44 and its variant exons in human trophoblast invasion and placental angiogenesisMol Hum Reprod291996685691
- M.BraymanA.ThathiahD.D.CarsonMUC1: a multifunctional cell surface component of reproductive tissue epitheliaReprod Biol Endocrinol220044
- J.D.AplinAdhesion molecules in implantationRev Reprod2219978493
- J.D.AplinMUC-1 glycosylation in endometrium: possible roles of the apical glycocalyx at implantationHum Reprod14Suppl. 219991725
- K.A.RaheemW.F.A.MareiB.K.CampbellA.A.Fouladi-NashtaIn vivo and in vitro studies of MUC1 regulation in sheep endometriumTheriogenology859201616351643
- K.A.RaheemAn insight into maternal recognition of pregnancy in mammalian speciesJ Saudi Soc Agric Sci16201516
- G.A.SurveyorS.J.GendlerL.PembertonS.K.DasI.ChakrabortyJ.JulianExpression and steroid hormonal control of MUC-1 in the mouse uterusEndocrinology1368199536393647
- L.H.HoffmanG.E.OlsonD.D.CarsonB.S.ChiltonProgesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epitheliumEndocrinology13911998266271
- M.MeseguerJ.D.AplinP.Caballero-CampoJ.E.O'ConnorJ.C.MartinJ.RemohiHuman endometrial mucin MUC1 is up-regulated by progesterone and down-regulated in vitro by the human blastocystBiol Reprod6422001590601
- J.D.AplinN.A.HeyT.C.LiMUC1 as a cell surface and secretory component of endometrial epithelium: reduced levels in recurrent miscarriageAm J Reprod Immunol3531996261266
- S.WilsherS.GowerW.R.AllenPersistence of an immunoreactive MUC1 protein at the feto-maternal interface throughout pregnancy in the mareReprod Fertil Dev2552013753761
- Q.RenS.GuanJ.FuA.WangTemporal and spatial expression of muc1 during implantation in sowsInt J Mol Sci116201023222335
- A.J.ZiecikA.WaclawikM.M.KaczmarekA.BlitekB.M.JalaliA.AndronowskaMechanisms for the establishment of pregnancy in the pigReprod Domest Anim46Suppl. 320113141
- A.RiceT.ChardCytokines in implantationCytokine Growth Factor Rev93–41998287296
- S.AltmäeJ.A.Martínez-ConejeroA.SalumetsC.SimónJ.A.HorcajadasA.Stavreus-EversEndometrial gene expression analysis at the time of embryo implantation in women with unexplained infertilityMol Hum Reprod1632010178187
- M.S.M.van MourikN.S.MacklonC.J.HeijnenEmbryonic implantation: cytokines, adhesion molecules, and immune cells in establishing an implantation environmentJ Leukoc Biol8512009419
- T.E.SpencerF.W.BazerConceptus signals for establishment and maintenance of pregnancyReprod Biol Endocrinol2200449
- J.BowenJ.HuntThe role of integrins in reproductionProc Soc Exp Biol Med22342000331343
- R.S.RobinsonG.E.MannT.S.GaddG.E.LammingD.C.WathesThe expression of the IGF system in the bovine uterus throughout the oestrous cycle and early pregnancyJ Endocrinol16522000231243
- E.B.CullinanS.J.AbbondanzoP.S.AndersonJ.W.PollardB.A.LesseyC.L.StewartLeukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantationProc Natl Acad Sci USA937199631153120
- J.D.AplinM.MeseguerC.SimonM.E.OrtizH.CroxattoC.J.JonesMUC1, glycans and the cell-surface barrier to embryo implantationBiochem Soc Trans29Pt 22001153156
