Abstract
Single embryo culture is essential for culturing embryos derived from few oocytes obtained from elite cows through ultrasonography guidance. Bovine in vitro fertilization (IVF) and individual embryo culture is a challenge as it generally leads to impaired embryo development. In this study, we explored the embryonic development and the sex ratio of IVF-derived bovine embryo cultured individually in chemically defined two-step culture medium. Total 63 cumulus-oocyte complexes were collected, in vitro matured, in vitro fertilized and the resultant fertilized oocytes were randomly cultured individually (4 trials, 15–16 oocytes each) in microdrops of 5 µL of a chemically defined two-step culture medium. Blastocysts were counted in every trial (n = 32, 50.79%) and all of them were used for both genomic DNA and total RNA extraction, cDNA synthesis and PCR using specific primers for GAPDH, GDP6, XIST and SRY genes. Results showed significant difference in expression of XIST (positive expression in 11 blastocysts) and SRY (positive expression in 21 blastocysts) mRNAs, P < .05. This result supports the hypothesis of sexual dimorphism among the pre-implantation in vitro produced embryos and provides an efficient medium for single bovine embryos in vitro production.
1 Introduction
Single bovine embryo in vitro culture is a challenge but it is essential for culturing embryos derived from oocytes where obtained from elite cows through ultrasonography guidance. Several researchers reported the beneficial effect of group culture medium for bovine in vitro produced embryos [Citation1–Citation5], while the demand for improving single embryo culture is essentially needed for accurate study of the early embryonic development [Citation6,Citation7], and for toxicity screening [Citation8–Citation11]. Therefore, extensive research was performed to improve single embryo culture through co-culturing with somatic [Citation10,Citation12] or embryonic cells [Citation13].
We have previously shown that in vitro embryonic development and the yield of cloned, transgenic embryos and viable calves were increased by using a two-step chemically defined medium for IVF-derived bovine embryos [Citation14–Citation18].
In the current study, we explored the embryonic development and the sex ratio of IVF-derived bovine embryo cultured individually in a chemically defined two-step culture medium using genomic DNA marker and RT-PCR.
2 Materials and methods
2.1 Chemicals
Unless otherwise stated, all chemicals and hormones were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA).
2.2 Oocyte collection and in vitro maturation (IVM)
Ovaries were collected from a slaughterhouse into NaCl solution 0.9% at 30–33 °C and transported to the laboratory within 2–3 h. Cumulus-oocyte complexes (COCs) from follicles 2–8 mm in diameter were aspirated using an 18-gauge needle attached to a 10 mL disposal syringe. The COCs with evenly-granulated cytoplasm and enclosed by more than three layers of compact cumulus cells were selected, washed three times in HEPES-buffered tissue culture medium-199 (TCM-199; Invitrogen, Carlsbad, CA, USA), supplemented with 10% FBS, 2 mM NaHCO3, and 1% penicillin–streptomycin (v/v). For IVM, COCs were cultured in four-well dishes (30–40 oocytes per well; Falcon, Becton-Dickinson Ltd., Plymouth, UK) for 24 h in 450 μL TCM-199 supplemented with 10% FBS, 0.005 IU/mL FSH (Antrin, Teikoku, Japan), 100 µM Cysteamine and 1 μg/mL 17β-estradiol at 39 °C in a humidified atmosphere of 5% CO2.
2.3 Sperm preparation, in vitro fertilization (IVF) and in vitro culture of embryos (IVC)
Motile spermatozoa were purified and selected using the Percoll gradient method [Citation19]. Briefly, spermatozoa were selected from the thawed semen straws by centrifugation on a Percoll discontinuous gradient (45–90%) for 15 min at 1500 rpm. The 45% Percoll solution was prepared with 1 mL of 90% Percoll (Nutricell, Campinas, SP, Brazil) and 1 mL of capacitation-TALP (Nutricell) [Citation20]. The sperm pellet was washed two times with capacitation-TALP by centrifugation at 1500 rpm for 5 min. The active motile spermatozoa from the pellet used for insemination of matured oocyte (At 24 h of IVM). Following maturation, COCs were randomly distributed in 30 μL microdrops of IVF-TALP medium (Nutricell) and were then inseminated (day 0) with 1–2 × 106 spermatozoa/mL for 18 h. Each microdrop contained two COCs and overlaid with mineral oil at 39 °C in a humidified atmosphere of 5% CO2. In vitro culture (IVC) dishes were labelled and numbered to specifically monitor the individually cultured embryos. Presumptive zygotes were denuded and cultured individually in 5 µL two-step defined culture medium (first 5 days with stage-1 medium then transferred to the later stage medium [Citation16]) overlaid with mineral oil and incubated at 39 °C in an atmosphere of 5% O2, 5% CO2 and 90% N2. Cleavage and blastocyst rates were recorded on days 2 and 7, respectively.
2.4 Sex determination with genomic DNA and expression of sex-specific mRNA transcripts in single embryo by RT-PCR
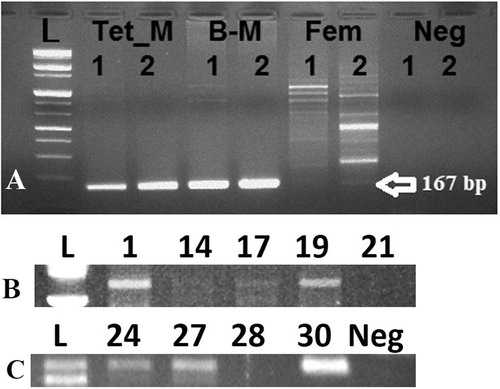
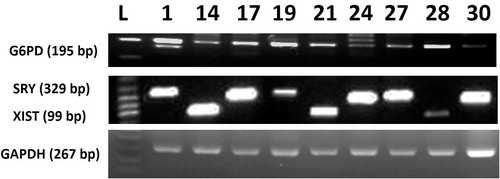
Each blastocyst was washed in TALP medium then transferred into 5 μL of diethylpyrocarbonate (DEPC) treated water (Invitrogen) and stored at −80 °C. Individual embryos were transferred into 100 μL Trizol reagent and mixed very well then were divided (50 μL each) into genomic DNA or total RNA extraction procedures according to specific kits (Qiagen, Valencia, CA, USA) according to the manufacturer’s instruction [Citation14,Citation21]. Specific primers were used to amplify Y-specific segment from the extracted genomic DNA by PCR (). Different somatic cell lines were used [from transgenic male cow (Tet_M); brain of male cow (B-M); and from a female cow (Fem)] to examine the primers efficiency for Y-specific segment amplification. Reverse transcription was carried out at 50 °C for 50 min. Individual RT reaction was performed using random hexamer and superscript TM III reverse transcriptase (Invitrogen) in a 20 μL reaction. 10 ng cDNA subjected to reverse transcription-polymerase chain reaction (RT-PCR) using Maxime PCR PreMix kit-i-starTaq (Intron Biotech., Seoul, Republic of Korea) and 1 pM of forward and reverse primers. Duplex PCR was used for identification of SRY and XIST in the same cDNA sample. Primer sequences, annealing temperatures and approximate size of the amplified fragments are listed in . The PCR amplification carried out for one cycle of denaturation at 95 °C for 5 min and subsequent 42 cycles with denaturation at 95 °C, annealing for 30 s, extension at 72 °C for 45 s and final extension at 72 °C for 5 min. and then ten μL of PCR products were fractioned on 1% agarose gel (Intron Biotech.) and stained with RedSafe TM (Intron Biotech.).
Table 1 Primers used for reverse transcription PCR (RT-PCR) and genomic PCR analysis.
2.5 Statistical analysis
The percentage of male to female embryos was analyzed by Pearson’s chi-squared test. Value of P < .05 was considered statistically significant.
3 Results & Discussion
Cleavage rate of embryos was 90.4% (n = 57/63) and the blastocyst ratio was 50.79% (n = 32/63). This result clearly clarify that chemically defined medium significantly improved the development of single bovine embryos when compared with undefined medium, such as modified synthetic oviductal fluid (mSOF) reported in previous studies [Citation1,Citation4,Citation5,Citation22] that showed detrimental effects of single embryo culture in undefined medium conditions. Among the 32 studied blastocysts, genomic DNA analysis for Y-chromosome specific STS marker BovY4 showed clearly positive bands in 19 blastocysts while it was faintly amplified in embryo #17 (). This result might indicate the minute amount of isolated genomic DNA from blastocyst by the current protocol or the differences in cell numbers between the isolated blastocysts [Citation14] that will affect the yield of the isolated genomic DNA. On the other hand, RT-PCR showed expression of mRNA transcripts of GAPDH and G6PD in all examined blastocysts. Moreover, significant difference in sex ratio was observed (P = .02); XIST mRNA was expressed in 11 blastocysts (34.4%), SRY was expressed in 21 blastocysts (65.6%). is representing the expression of SRY and XIST mRNA transcripts in nine embryos randomly selected from the resultant blastocysts. This result might indicate a sexual dimorphism and difference in tolerance of blastocysts of different sexes. A result coincides with Pegoraro et al. [Citation23] who also observed that the percentage of male embryos was increased over female ones when embryos were co-cultured with feeder cells. Moreover, Mittwoch [Citation24] demonstrated that XY embryos can grow faster than XX embryos in vitro. We showed that both genomic DNA a RNA can be isolated from single blastocysts, however due to the little amount of resultant DNA, we recommend improving the protocol of amplification of such minute amount of genomic DNA to give satisfactory results. Other researchers improved the DNA amplification used different kind of PCR protocol such as loop-mediated isothermal amplification (LAMP) [Citation25,Citation26].
The current study supports the beneficial effects of two-step defined culture medium for single IVF-derived bovine embryo culture which can reach ∼50% blastocyst formation and provides a cues for further studying the sexual dimorphism of early embryo in vitro development in bovine.
Competing interests
The author declares no conflicts of interest regarding the publication of this paper.
Acknowledgement
The authors extended their appreciation to Dr. Woojae Choi for his technical help.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- I.DonnayA.Van LangendoncktP.AuquierB.GrisartA.VansteenbruggeA.MassipEffects of co-culture and embryo number on the in vitro development of bovine embryosTheriogenology47199715491561
- T.FujitaH.UmekiH.ShimuraK.KugumiyaK.ShigaEffect of group culture and embryo-culture conditioned medium on development of bovine embryosJ Reprod Dev522006137142
- M.HoelkerF.RingsQ.LundN.GhanemC.PhatsaraJ.GrieseEffect of the microenvironment and embryo density on developmental characteristics and gene expression profile of bovine preimplantative embryos cultured in vitroReproduction1372009415425
- C.L.KeeferS.L.SticeA.M.PaprockiP.GoluekeIn vitro culture of bovine IVM-IVF embryos: cooperative interaction among embryos and the role of growth factorsTheriogenology41199413231331
- I.SalvadorA.Cebrian-SerranoD.SalamoneM.A.SilvestreEffect of number of oocytes and embryos on in vitro oocyte maturation, fertilization and embryo development in bovineSpanish J Agric Res92011744752
- M.B.WheelerE.M.WaltersD.J.BeebeToward culture of single gametes: the development of microfluidic platforms for assisted reproductionTheriogenology68Suppl 12007S178S189
- A.MurilloM.MuñozD.Martín-GonzálezS.CarroceraA.Martínez-NistalE.GómezLow serum concentration in bovine embryo culture enhances early blastocyst rates on day-6 with quality traits in the expanded blastocyst stage similar to BSA-cultured embryosReprod Biol172017162171
- P.E.J.BolsL.G.F.GoovaertsE.P.A.JE.M.L.PetroA.LangbeenNew applications for bovine IVP technology: from single oocyte culture to toxicity screeningAnim Reprod92012388394
- I.G.GoovaertsJ.L.LeroyE.P.JorssenP.E.BolsNoninvasive bovine oocyte quality assessment: possibilities of a single oocyte cultureTheriogenology74201015091520
- I.G.F.GoovaertsJ.L.M.R.LeroyA.LangbeenE.P.A.JorssenE.BosmansP.E.J.BolsUnravelling the needs of singly in vitro-produced bovine embryos: from cumulus cell co-culture to semi-defined, oil-free culture conditionsReprod Fertil Dev24201210841092
- R.L.KelleyD.K.GardnerIn vitro culture of individual mouse preimplantation embryos: the role of embryo density, microwells, oxygen, timing and conditioned mediaReprod Biomed Online342017441454
- I.G.F.GoovaertsJ.L.M.R.LeroyA.Van SoomJ.B.P.De ClercqS.AndriesP.E.J.BolsEffect of cumulus cell coculture and oxygen tension on the in vitro developmental competence of bovine zygotes cultured singlyTheriogenology712009729738
- M.MoriS.KasaM-aHattoriS.UedaDevelopment of a single bovine embryo improved by co-culture with trophoblastic vesicles in vitamin-supplemented mediumJ Reprod Dev582012717721
- I.M.SaadeldinB.KimB.LeeG.JangEffect of different culture media on the temporal gene expression in the bovine developing embryosTheriogenology7520119951004
- I.M.SaadeldinW.ChoiB.Roibas Da TorreB.KimB.LeeG.JangEmbryonic development and implantation related gene expression of oocyte reconstructed with bovine trophoblast cellsJ Reprod Dev582012425431
- K.T.LimG.JangK.H.KoW.W.LeeH.J.ParkJ.J.KimImproved in vitro bovine embryo development and increased efficiency in producing viable calves using defined mediaTheriogenology672007293302
- S.-Y.YumS.-J.LeeH.-M.KimW.-J.ChoiJ.-H.ParkW.-W.LeeEfficient generation of transgenic cattle using the DNA transposon and their analysis by next-generation sequencingSci Rep6201627185
- W.ChoiE.KimS.-Y.YumC.LeeJ.LeeJ.MoonEfficientPRNPdeletion in bovine genome using gene-editing technologies in bovine cellsPrion92015278291
- G.M.MachadoJ.O.CarvalhoE.S.FilhoE.S.CaixetaM.M.FrancoR.RumpfEffect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryosTheriogenology71200912891297
- A.ChamberlandV.FournierS.TardifM.A.SirardR.SullivanJ.L.BaileyThe effect of heparin on motility parameters and protein phosphorylation during bovine sperm capacitationTheriogenology552001823835
- S.KimI.M.SaadeldinW.J.ChoiS.J.LeeW.W.LeeB.H.KimProduction of transgenic bovine cloned embryos using piggybac transpositionJ Vet Med Sci73201114531457
- E.M.O'DohertyM.G.WadeJ.L.HillM.P.BolandEffects of culturing bovine oocytes either singly or in groups on development to blastocystsTheriogenology481997161169
- L.M.PegoraroJ.M.ThuardN.DelalleauB.GuerinJ.C.DeschampsB.Marquant Le GuienneComparison of sex ratio and cell number of IVM-IVF bovine blastocysts co-cultured with bovine oviduct epithelial cells or with Vero cellsTheriogenology49199815791590
- U.MittwochBlastocysts prepare for the race to be maleHum Reprod8199315501555
- H.HirayamaS.KageyamaS.MoriyasuK.SawaiA.MinamihashiEmbryo sexing and sex chromosomal chimerism analysis by loop-mediated isothermal amplification in cattle and water buffaloesJ Reprod Dev592013321326
- H.HirayamaS.KageyamaS.MoriyasuK.SawaiS.OnoeY.TakahashiRapid sexing of bovine preimplantation embryos using loop-mediated isothermal amplificationTheriogenology622004887896
