Abstract
Oocyte cryopreservation is valuable way of preserving the female germ line. Vitrification of immature ovine oocytes decreased the levels of both maturation promoting factor (MPF) and mitogen-activated protein kinase (MAPK) in metaphase II (MII) oocytes after IVM. Our aims were 1) to evaluate the effects of vitrification of ovine GV-oocytes on spindle assembly, MPF/MAP kinases activities, and preimplantation development following IVM and IVF, 2) to elucidate the impact of caffeine supplementation during IVM on the quality and development of vitrified/warmed ovine GV-oocytes. Cumulus-oocyte complexes (COCs) from mature ewes were divided into vitrified, toxicity and control groups. Oocytes from each group were matured in vitro for 18 h in caffeine free IVM medium and denuded oocytes were incubated in maturation medium supplemented with 10 mM (+) or without (−) caffeine for another 6 h. At 24 h.p.m., oocytes were evaluated for spindle configuration, MPF/MAP kinases activities or fertilized and cultured in vitro for 7 days. Caffeine supplementation did not significantly affect the percentages of oocytes with normal spindle assembly in all the groups. Caffeine supplementation during IVM did not increase the activities of both kinases in vitrified groups. Cleavage and blastocyst development were significantly lower in vitrified groups than in control. Caffeine supplementation during the last 6 h of IVM did not significantly improve the cleavage and blastocyst rates in vitrified group. In conclusion, caffeine treatment during in vitro maturation has no positive impact on the quality and development of vitrified/warmed ovine GV-oocytes after IVM/IVF and embryo culture.
Introduction
Cryopreservation of gametes and embryos has become an integral part of assisted reproduction technologies (ART). In particular, sperm cryopreservation has been the most widely and successfully used in humans as well as in variety of mammalian species [Citation1]. Embryo cryopreservation has also become a routine practise in clinical ART and has resulted in delivery of many healthy babies [Citation2]. However, embryo cryopreservation has some drawbacks; it requires the availability of male partner to produce those embryos, in addition, embryo cryopreservation is prohibited due to ethical, legal and religious implications in some countries [Citation3]. Cryopreservation of unfertilized oocytes is an alternative option, giving flexibility in the time of in vitro fertilization (IVF) and a potential to establish oocyte banking with oocyte donation [Citation4–Citation8]. There are two techniques applied to the cryopreservation of gametes and embryos: controlled slow freezing, which was favoured in early procedures, and ultrarapid cooling by vitrification, which is now widely used as it produces less damage to the oocytes and embryos than slow freezing [Citation9].
Although extensive research have been conducted on the cryopreservation of metaphase II (MII) oocytes, vitrification of oocytes at this stage can disrupt the meiotic spindle [Citation10], which could be avoided by freezing of oocytes at the germinal vesicle (GV) stages. Freezing of oocytes at this stage has many clinical advantages in human and animal reproduction. [Citation11–Citation15]. Though, numerous studies have been conducted to cryopreserve GV-oocytes in many mammalian species [Citation12,Citation13,Citation15–Citation27], and reportedly produced live births in humans and animals [Citation17,Citation28,Citation29], the blastocyst development rate remains low [Citation23,Citation24,Citation30]. One major obstacle is the requirement of the frozen GV-oocytes to be matured in vitro (IVM) prior to fertilization [Citation19]. No standard IVM protocol for GV-oocytes has yet been established in humans and in some animal species [Citation31–Citation33].
It is well known that, oocyte meiotic maturation is controlled by the levels of two cytoplasmic protein kinases; maturation promoting factor (MPF) and mitogen-activated protein kinase (MAPK). High activities of both kinases are responsible for the onset of germinal vesicle breakdown and are also essential for the maintenance of the oocytes at MII-stage [Citation34]. Previous studies showed that vitrification of immature ovine oocytes decreased the levels of both kinases after IVM [Citation13].
Caffeine (1,3,7-trimethylxanthine), a phosphodiesterase inhibitor, has been reported to induce dephosphorylation of Y-15 and T14 of p34cdc2, which may have been occurred by inhibition of Myt1/Wee1 kinase resulting in an increase in the activity of MPF in cultured mammalian cells, Xenopus, and porcine oocytes [Citation35]. In sheep, previous studies showed that treatment of in vitro matured oocytes with caffeine increased the activities of both MPF and MAPK kinases, improved frequencies of nuclear envelope breakdown and chromosome condensation of transferred nuclei, increased total cell numbers, and reduced the frequency of apoptotic nuclei in blastocyst embryos produced by somatic cell nuclear transfer (SCNT) [Citation36–Citation38]. Moreover, in aged denuded ovine oocytes, caffeine treatment increased the blastocyst development rates and decreased the frequency of polyspermy following IVF [Citation39]. However, little is known about its role on the development of vitrified/warmed oocytes. Our aims were 1) to evaluate the effects of vitrification of ovine GV-oocytes on spindle assembly, MPF/MAP kinases activities, and preimplantation development following IVM and IVF, 2) to elucidate the impact of caffeine supplementation during IVM on the quality and development of vitrified/warmed ovine GV-oocytes.
Materials and methods
Unless stated otherwise, all chemicals and reagents were purchased from Sigma-Aldrich (Dorset, UK).
.1 Oocyte collection
Ovine ovaries were collected from a local slaughterhouse (Nottingham, UK) and kept in a thermos flask filled with pre-warmed phosphate-buffered saline (PBS) at 25 °C during transportation to the laboratory. Cumulus–oocyte complexes (COCs) were aspirated using a 21-gauge needle attached to a 10-mL syringe from 2 to 3-mm follicles. The follicular fluid and COCs were placed into 50-mL conical tubes and kept in a warming box at 39 °C for 15 min so that COCs sank to the bottom of the tubes. The upper follicular fluid was removed and 5 mL of follicular fluid containing COCs was poured into a 90-mm Petri dish containing oocyte washing medium consisting of HEPES-buffered TCM 199 (H-TCM 199; Gibco BRL BRL/Life Technologies, Paisley, Renfrewshire, UK) supplemented with 10% v/v fetal bovine serum (FBS; Gibco). The COCs, with at least two to three compact layers of cumulus cells and a homogeneous cytoplasm, were selected under a light microscope (Leica, Wetzlar, Germany). The selected oocytes were randomly divided into three groups; vitrified, toxicity (exposed only to vitrification and warming solutions without freezing), and control (directly subjected to IVM).
.2 Vitrification and warming of COCs
COCs were vitrified according to the method described by Moawad et al. [Citation13]. Briefly, COCs were rinsed three times in base medium (BM; H-TCM 199 supplemented with 10% FBS), and then transferred into 500 μL equilibration solution (10% v/v ethylene glycol (EG) plus 0.25 M trehalose in BM) for 3 min on a warm stage at 39 °C. 3–5 oocytes were then transferred into a small droplet of 20 μL vitrification solution (20% v/v EG and 20% v/v dimethylsulfoxide (DMSO)) before being immediately treated in warming solution as toxicity or vitrified. For vitrification, the cryoloop (Hampton Research, Aliso Viejo, CA, USA) was dipped in the vitrification solution so that a thin film was created by surface tension. Three to five oocytes were gently placed on the film using a glass-mouth pipette and the cryoloop device containing the oocytes was plunged directly into a cryovial that had been submerged and filled with liquid nitrogen (LN2). The cap of the cryovial was then tightened and the vial was returned to LN2. The whole process was performed in 1 min. For thawing, the cap of the cryovial submerged in LN2 was carefully unscrewed and opened and the cryoloop containing the vitrified oocytes was transferred immediately into warming solution, which consisted of 500 mL BM plus 10% v/v EG and 1 M trehalose. The oocytes came off the cryoloop into the solution and were kept in this solution for 3 min before being transferred into 500 μL BM plus 0.5 M trehalose and then to BM (3 min in each solution). All solutions were kept at 39 °C. Finally, the COCs were examined morphologically to assess their viability under a stereomicroscope (MZ 12.5; Leica Microsystems, Wetzlar, Germany). Oocytes with a spherical and symmetrical shape and evenly granulated cytoplasm were regarded as viable, whereas oocytes exhibiting membrane damage, a swollen or ruptured zona pellucida and/or degenerated cytoplasm were considered as non-viable. Only morphologically viable COCs were selected for further experiments.
.3 In vitro maturation
IVM was performed as described previously [Citation15]. Briefly, COCs from vitrified, toxicity and control groups were washed twice in maturation medium (bicarbonate-buffered TCM 199 with Earle salts; Gibco) supplemented with 10% FBS, 5 μg/mL FSH (Vetropharm, Belleville, ON, Canada), 5 µg/mL LH (Vetropharm), 1 µg/mL 17β-oestradiol, 0.3 mM sodium pyruvate, 100 µM cysteamine and 50 μg/mL gentamicin. Groups of 40–45 COCs were then transferred into four-well dishes (Nunc, Roskilde, Denmark) containing 500 μL maturation medium covered with mineral oil. All dishes were pre-warmed in an incubator at 39 °C under 5% CO2 in air before use. All COCs were cultured in the medium for 18 h under the same conditions.
.4 Caffeine treatment
At 18 h post IVM, cumulus cells were removed from all the groups by repeated pipetting of COCs in H-TCM 199 supplemented with 4 mg/mL polyvinylpyrrolidone (PVP) and 300 IU/mL hyaluronidase. After washing in maturation medium, oocytes from each group were incubated in maturation medium supplemented either with 10 mM (+) or without (−) caffeine for another 6 h. IVM was then continued till 24 h.
.5 Spindle and chromosome configuration
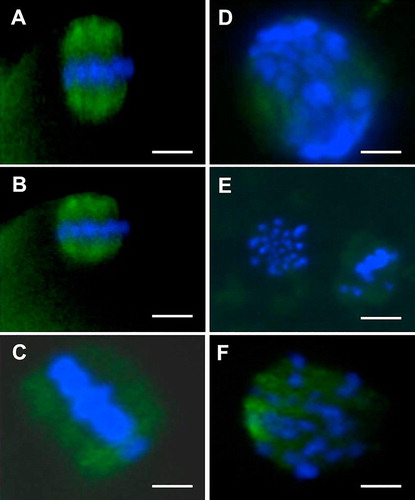
IVM oocytes were immunostained for tubulin and counterstained to assess chromosomes as previously described [Citation13]. Briefly, oocytes (at least 44 oocytes/group) were fixed in 4% (w/v) paraformaldehyde (PFA) for 30 min at room temperature and then washed three times (10 min each) in PBS supplemented with 20% FBS (PBS-FBS). All oocytes were then transferred to PBS-FBS containing 0.5% Triton X-100 (permeabilization medium) and kept in this medium for 30 min at room temperature. Subsequently, the primary antibody (mouse monoclonal anti-α-tubulin antibody; 1:200 dilution) was added and oocytes were further cultured at 4 °C overnight. Then, oocytes were rinsed three times in PBS-FBS as described above before being incubated with a fluorescein isothiocyanate (FITC)-labelled goat secondary antibody (1:200 dilution) at room temperature for 60 min. Finally, oocytes were stained with 10 μg/mL Hoechst 33342 and then rinsed three times in PBS-FBS. Oocytes were then transferred to a small drop of Vectashield mounting medium containing 4′,6′-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA) on a diagnostic microscope slide (Erie Scientific, Portsmouth, UK) and then covered with a coverslip. The slides were examined by epifluorescence (Leica DMR, Wetzlar, Germany), and the images captured using a digital camera (Hamamatsu, Shizuoka, Japan) and analyzed by Simple PCI software (Compix, Cranberry Township, PA, USA). Based on the morphology of the meiotic spindles and chromosomes [Citation13], oocytes with a classical symmetric barrel shaped spindle were considered normal, whereas oocytes with disorganized, clumped, dispersed or missing (completely or partially) spindles were considered abnormal. Similarly, normal chromosomes were defined as those in which two sets of chromosomes were regularly aligned along the equatorial plate of the meiotic spindle, whereas abnormal chromosomes appear clumped or dispersed ().
.6 Analysis of MPF and MAPK activity
Analysis of MPF and MAPK activities was performed according to the method described by Ye et al. [Citation40] with some modifications. Briefly, groups of 10 oocytes (3 replicates were repeated in each experimental group) were thoroughly washed in DPBS containing 0.1% polyvinyl alcohol (PVA) at 39 °C and then placed into 5 μL of ice-cold lysis buffer containing 45 mM β-glycerophosphate (pH 7.3), 12 mM ρ-nitrophenylphosphate, 2 mM 3-(N-morpholino)-propanesulfonic acid (MOPS), 12 mM MgCl2, 12 mM ethyleneglycol bis (2-aminoethyl-ether) tetraacetic acid (EGTA), 0.1 mM EDTA, 20 mM Na3VO4, 10 mM NaF, 2 mM dithiothreitol (DTT), 2 mM phenylmethylsulphonyl fluoride, 2 mM benzamidine, 20 μg/mL leupeptin, 20 μg/mL pepstatin A and 19.5 μg/mL aprotinin. The samples were frozen in LN2 and stored at −80 °C until analyzed. The activities of MPF and MAPK kinases were measured simultaneously using histone H1 and bovine myelin basic protein (MBP) as their in vitro substrates, respectively. The oocyte lysate was thawed and then refrozen in liquid nitrogen (−196 °C) once. The kinase reaction was started by adding the oocyte lysate to 5 μL kinase assay buffer containing 45 mM β-glycerophosphate (pH 7.3), 12 mM ρ-nitrophenylphosphate, 20 mM MOPS, 12 mM MgCl2, 12 mM EGTA, 0.1 mM EDTA, 2 mM Na3VO4, 10 mM NaF, 4 μg/mL H1, 6 mg/mL MBP, 40 μM protein kinase A (PKA) inhibiting peptide (Santa Cruz Biotechnology; Autogen Bioclear, Clane), 43 μM protein kinase C (PKC) inhibiting peptide (Promega, Southampton) and 10 Ci/mmol [γ-32P] ATP (PerkinElmer). The mixtures were incubated at 37 °C for 30 min with gentle shaking. The reaction was stopped by adding 10 μL ice-cold 2 × SDS sample buffer which was made from [125 mMTris-Cl(pH6.8)(FisherScientific), 200 mMDTT, 4%(w/v)SDS(FisherScientific), 0.01%(w/v)bromophenolblueand20%(w/v)glycerol]. After boiling for 5 min, the substrates were separated by standard polyacrylamide gel electrophoresis (SDS-PAGE, 15% gels) using a Mini-Protean II dual slab cell (Bio-Rad, Hercules, CA) at 140 V for 1.5 h. Gels were dried on 3 mm filters and exposed to phosphor-screens (Fuji film). The phosphor images of gels (screens) were captured and the kinase activities were quantified using an FX phosphor image analysis system (Bio-Rad).
.7 In vitro fertilization and embryo culture
IVF was performed using frozen-thawed semen pellets obtained from a Texel ram (Britbreed, Tranent, UK) as described previously [Citation13]. Briefly, matured oocytes (24 h.p.m) were washed once in pre-warmed ovine fertilization medium (m-SOF supplemented with 2% sheep serum) at 39 °C. Groups of 40–50 oocytes were then transferred into four-well dishes (Nunc) containing 500 μL fertilization medium and 2.0 × 106 spermatozoa/mL. After co-incubation of oocytes with spermatozoa for 18 h at 39 °C in a humidified atmosphere of 5% CO2 in air, presumptive zygotes were washed three times in 500 μL H-SOF containing non-essential amino acids and 4 mg/ml BSA at 39 °C to remove adhered spermatozoa. After that, they were washed twice in embryo culture medium (C.SOF; m-SOFaaci containing BME-essential amino acid and MEM-non essential amino acids and FAF-BSA 4 mg/mL). Following washing, the embryos (15–20) were transferred into 50 μL drops of pre-warmed and equilibrated C.SOF-BSA culture medium covered with mineral oil and incubated at 39 °C in a humidified atmosphere of 5% O2, 5% CO2 and 90% N2 until day 7 (Day 0 = Day of insemination). Cleavage was assessed at 24 and 48 h post insemination (h.p.i.). Development to morula and blastocyst was evaluated on day 5 and 7 p.i., respectively. Blastocysts were morphologically evaluated under a stereomicroscope and divided into early, expanded or hatched blastocysts. Mean cell numbers per blastocyst was evaluated by staining of blastocysts with 10 μg/mL Hoechst 33342 [Citation13]. The samples were examined under fluorescence microscope (Leica DMR, Germany) fitted with a digital camera (Hamamatsu, Japan) and image analysis software (Simple PCI, Compix Inc., USA).
.8 Statistical analysis
At least three replicates were repeated for each experimental group. Data were analyzed using Chi-squared test. MPF and MAPK activities were analyzed by un-paired student's t-test. All results are considered to be statistically significant at P ≤ 0.05. The statistical analysis was performed using Graph Pad Software (http://www.graphpad.com).
Results
.1 Effect of caffeine supplementation during IVM on spindle assembly and chromatin configuration of ovine oocytes vitrified at the GV-stage
As shown in , irrespective to caffeine supplementation, vitrification of ovine GV-oocytes reduced the proportions of oocytes with normal spindle and chromosome configuration (43.8–47.8%) as compared with those in the control groups (67.6–77.3%). However, the difference between vitrified and control – oocytes (matured in caffeine free media) was not significant. Furthermore, the percentages of oocytes with missing spindle significantly increased in both toxicity and vitrified groups (22.7–28.3%) than in the control (6.8–9.1%). Caffeine supplementation during the last 6 h of IVM did not significantly improve (P > 0.05) the percentages of oocytes with normal spindle assembly and chromosome configuration in vitrified, toxicity and control groups.
able 1 Effect of caffeine treatment during IVM on spindle and chromatin configuration of ovine oocytes vitrified at GV-stage.
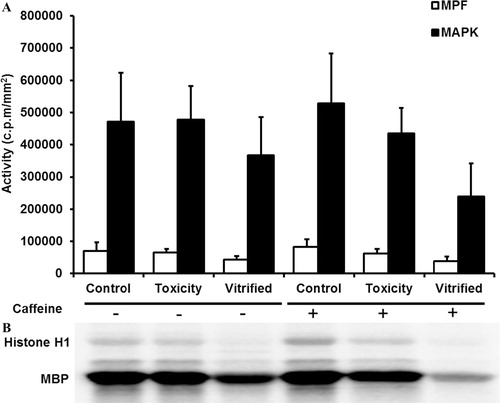
.2 Effect of caffeine supplementation during IVM on MPF and MAP kinases activities of ovine oocytes vitrified at the GV-stage
As presented in , the activities of both MPF and MAP kinases were reduced in vitrified groups as compared to the controls; however, the differences were not statistically significant. Exposure of oocytes to vitrification and warming solutions (toxicity group) did not affect the activities of both kinases in comparison with the control group. The levels of both MPF and MAP kinases were lower in vitrified oocytes matured in vitro in caffeine supplemented medium (vitrified+) than those matured in non-supplemented medium (vitrified−).
.3 Effect of caffeine supplementation during IVM on cleavage rates and preimplantation development of ovine oocytes vitrified at the GV-stage following IVF and embryo culture
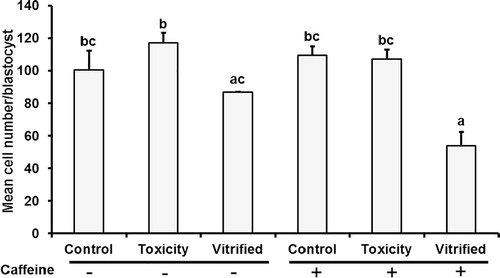
As summarized in , cleavage at 24 and 48 h.p.i. was significantly lower (P ≤ 0.05) in vitrified groups either treated with (2.8% and 30.3%) or without (4.3% and 32.2%) caffeine than those in the other groups. Irrespective to caffeine supplementation, no significant differences were observed in the percentages of cleaved embryos at 48 h.p.i. between toxicity and control groups (values ranged from 58.1% to 78.6%). Development to morula (day 5 p.i.) was significantly lower (P ≤ 0.05) in both vitrified groups (values ranged from 24.0% to 25.4%) than those in toxicity (45.1%) and control (41.6%) groups in vitro matured in caffeine supplemented media. However, no significant differences were observed in morula development between vitrified, toxicity, and control oocytes matured in caffeine free IVM media. Irrespective to caffeine supplementation, blastocyst rates were significantly lower (P ≤ 0.05) in vitrified groups (1.7–4.2%) than those in toxicity and control ones (30.2–32.2%). The same trend was observed when blastocyst rates were calculated on the basis of the number of the cleaved embryos. Caffeine supplementation during the last 6 h of IVM did not improve blastocyst development in vitrified groups. Morphological evaluation of the blastocysts revealed no hatched blastocysts produced in both vitrified groups; however, from 8.3% to 16.1% were recorded in toxicity and control groups. Mean cell numbers per blastocysts were significantly lower (P ≤ 0.05) in vitrified (+) blastocyst (54.0) as compared to other treatments (107.2, 117.3, 109.6, and 100.6, in toxicity and control + and −, respectively); however, this value did not differ significantly from those reported in the vitrified (caffeine −, 87.0) group ().
able 2 Effect of caffeine treatment during IVM on developmental potential of ovine oocytes vitrified at the germinal vesicle stage.
Discussion
In the present studies we hypothesized that incubation of vitrified/warmed ovine GV-oocytes with caffeine during IVM could restore spindle and chromosome integrities, the activities of MPF and MAP kinases, and embryo development following IVM/IVF. We demonstrated that vitrification of ovine oocytes at the GV-stage reduced the frequencies of oocytes with normal spindle configuration and MPF/MAP kinases activities and preimplantation development after IVM/IVF and embryo culture and caffeine supplementation during IVM failed to restore the quality and development potential of vitrified/thawed oocytes. In the present study the regimen of caffeine treatment (10 mM for the last 6 h of IVM) was chosen based on the previous studies that have reported the positive impact of caffeine on in vitro matured ovine oocytes on the activities of MPF and MAP kinases and on the development of SCNT and IVF embryos [Citation36–Citation39,Citation41]. In these studies the authors have reported the encouraging results based on exposure of denuded oocytes to caffeine; therefore, in our study we also used denuded oocytes to study the effects of caffeine treatment. In mice, recent studies showed that supplementation of vitrification and warming solutions with caffeine maintained MPF levels and improved embryonic development after IVF of vitrified/warmed MII denuded oocytes [Citation42]. The mechanism of action of caffeine on the levels of both kinases in oocytes is not well recognized and may differ between cumulus oocyte complexes and denuded oocytes [Citation39].
It is well known that normal spindle configuration and chromosome alignment in MII-oocytes is crucial for successful fertilization and embryo development after IVF [Citation43]. The effects of cryopreservation on an oocyte’s spindle assembly and chromosome arrangement have been reported in different species, including the mouse [Citation10,Citation25], human [Citation44,Citation45], bovine [Citation46,Citation47], porcine [Citation48], equine [Citation26], and ovine [Citation13,Citation49]. In sheep, previous studies have shown that spindle configuration in vitrified-warmed MII oocytes is influenced by the cryodevice used, with higher percentages of oocytes with abnormal spindle morphology noted following the use of open pulled straw (OPS) and cryoloop compared with cryotop devices [Citation49]. In the present study we found that vitrification of ovine GV-oocytes negatively impacted spindle assembly and chromosome arrangement after IVM. We also noticed that caffeine supplementation during the last 6 h of IVM did not improve spindle alignment in vitrified group (). In contrast to our observations, previous studies showed that caffeine treatment at 20 mM during the last three hours of IVM before vitrification was able to keep the normality of chromatin organization in vitrified/warmed ovine MII-oocytes in similar values as in the control group. Discrepancies between the results could be due to the stage at which oocytes were vitrified, oocyte quality, method of vitrification, and the regimen of caffeine treatment. In mice, previous studies showed that caffeine treatment during IVM impacts the spindle morphology in dose dependant manner; a high percentage of oocytes with normal spindle morphology were noticed in 5 or 10 mM caffeine treated groups as compared to those treated with 0 or 1 mM [Citation50]. Perturbations in the meiotic spindle configuration following IVM of vitrified GV-oocytes could be associated with cryodamage to key regulatory proteins, such as MPF and MAPK, which are involved in spindle formation and microtubule organization [Citation13]. Herein, there was a tendency for the activity of both kinases to be lower in vitrified oocytes compared with control oocytes, although the differences failed to reach statistical significance. However, the activities of both kinases were comparable in the toxicity group and the control one. These observations indicate that cryopreservation may induce the degradation of molecules involved in the signalling pathways that are involved in the regulation of kinase activity. Previous studies have reported that the activity of MPF decreased significantly in OPS, cryoloop, and cryotop vitrified-warmed MII ovine oocytes compared with fresh controls; however, this activity was restored after 2 h in vitro culture in OPS- and cryoloop-vitrified oocytes, but not in cryotop-vitrified oocytes [Citation49]. Our results also showed that caffeine supplementation during IVM did not improve the levels of MPF and MAPK in vitrified oocytes suggesting that the damaging effects on both kinases during vitrification could not be restored by caffeine treatment. Further studies are needed to elucidate the effects of other caffeine concentrations or methods of treatment on the quality of vitrified oocytes. In sheep, it has been shown that the treatment of COCs with 20 mM caffeine for the last 3 h of IVM and before vitrification stabilizes the activity of MPF at similar levels to that of control oocytes [Citation51]. In mice, recent studies have illustrated that caffeine supplementation at 10 mM during vitrification and warming of MII-oocytes maintained MPF activity after cryopreservation [Citation42].
The deleterious consequences arising from cryodamage may also appear during cleavage or pre-implantation development [Citation52]. In fact, it has been documented that, vitrified-warmed oocytes cleave and reach blastocyst stage in significantly lower proportions as compared to control [Citation12,Citation15,Citation53,Citation24]. The reduction in developmental rates obtained after IVM and IVF of vitrified/warmed immature oocytes can be ascribed to structural, biochemical, and molecular changes that could occur as a consequence of vitrification process [Citation12–Citation15,Citation25,Citation24] or as a result of exposure to cryoprotectants [Citation54]. Our results showed that frequencies of cleaved embryos (24 and 48 h.p.i.) and blastocyst rates were significantly lower in vitrified oocytes than in toxicity and control groups. We also demonstrated that caffeine supplementation during the last 6 h of IVM did not improve these percentages in vitrified oocytes (). Again, under our experimental conditions, these results confirm inability of caffeine to restore the quality and development of vitrified/warmed oocytes. The positive impact of caffeine on developmental potential of verified/warmed oocytes has been reported in different species. For example, in sheep, previous studies showed that treatment of oocytes before vitrification with 20 mM caffeine for the last three hours of IVM reduced spontaneous activation than non-treated groups [Citation51]. The authors attributed their findings to the prolonged period of meiotic arrest of vitrified MII oocytes in caffeine treated group [Citation51]. It has been documented that caffeine could maintain the quality of aged oocytes safely in mouse as aged oocytes treated by caffeine has a potential to be activated [Citation55]. In mice, supplementation of vitrification and warming media with 10 mM caffeine significantly improved blastocyst rates after IVF of MII-oocytes [Citation42]. In cattle, pre-treatment of oocytes with caffeine before IVM has shown to delay meiotic resumption and improved blastocyst quality and their tolerance to cryopreservation [Citation56]. Regarding the quality of in vitro produced blastocysts, our results revealed that vitrification and warming of oocytes reduced the mean cell numbers/blastocyst as compared with the control; however, the difference was not statistically significant. We also demonstrated that caffeine treatment failed to recover or even it dramatically reduced the quality of blastocysts (). In mice, it has been shown that vitrification of MII-oocytes in the presence of 10 mM caffeine did not significantly impact the total cell numbers per blastocyst [Citation42].
The results presented herein indicate that exposure of oocytes to vitrification solutions (toxicity group) has no detrimental impact on the development of oocytes. This improvement in the toxicity trials may be related to the strict short exposure time to the cryoprotectants during the vitrification and warming procedures. This regimen of exposure is beneficial to surpass the toxic effects of high concentrations of chemicals.
Conclusions
In conclusion, our results suggest that vitrification of ovine GV- oocytes negatively impact oocyte quality and development after IVM/IVF and embryo culture. Supplementation of in vitro maturation media with 10 mM caffeine during the last 6 h of IVM failed to restore the spindle integrities, MPF/MAPK activities and subsequent development of vitrified/warmed oocytes at the germinal vesicle stage. Further studies are needed to test different regimen (i.e. different concentrations, various time and methods of treatment) of caffeine on developmental potential of cryopreserved oocytes.
Acknowledgments
This work was supported by Scholarship from Ministry of Higher Education, Egypt to A.R.M. This work was performed in the Division of Animal Sciences, University of Nottingham, UK.
Competing interests
All authors declare no competing interests.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- J.KopeikaA.ThornhillY.KhalafThe effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidenceHum Reprod Update2122015209227 10.1093/humupd/dmu063 PubMed PMID: 25519143
- K.E.LoutradiE.M.KolibianakisC.A.VenetisE.G.PapanikolaouG.PadosI.BontisCryopreservation of human embryos by vitrification or slow freezing: a systematic review and meta-analysisFertil Steril9012008186193 10.1016/j.fertnstert.2007.06.010 PubMed PMID: 17980870
- R.C.ChianL.GilbertJ.Y.HuangE.DemirtasH.HolzerA.BenjaminLive birth after vitrification of in vitro matured human oocytesFertil Steril9122009372376 10.1016/j.fertnstert.2007.11.088 PubMed PMID: 18514195
- Z.P.NagyC.C.ChangD.B.ShapiroD.P.BernalH.I.KortG.VajtaThe efficacy and safety of human oocyte vitrificationSemin Reprod Med2762009450455 10.1055/s-0029-1241054 PubMed PMID: 19806513
- N.NoyesJ.KnopmanP.LabellaC.McCaffreyM.Clark-WilliamsJ.GrifoOocyte cryopreservation outcomes including pre-cryopreservation and post-thaw meiotic spindle evaluation following slow cooling and vitrification of human oocytesFertil Steril946201020782082 10.1016/j.fertnstert.2010.01.019 PubMed PMID: 20188356
- N.NoyesJ.M.KnopmanK.MelzerM.E.FinoB.FriedmanL.M.WestphalOocyte cryopreservation as a fertility preservation measure for cancer patientsReprod Biomed Online2332011323333 10.1016/j.rbmo.2010.11.011 PubMed PMID: 21570353
- N.NoyesE.PorcuA.BoriniOver 900 oocyte cryopreservation babies born with no apparent increase in congenital anomaliesReprod Biomed Online1862009769776 PubMed PMID: 19490780
- A.CoboA.CoelloJ.RemohiJ.SerranoJ.M.de Los SantosM.MeseguerEffect of oocyte vitrification on embryo quality: time-lapse analysis and morphokinetic evaluationFertil Steril10832017491497e3 10.1016/j.fertnstert.2017.06.024
- L.L.KuleshovaA.LopataVitrification can be more favorable than slow coolingFertil Steril.7832002449454 PubMed PMID: 12215314
- C.M.GomesC.A.SilvaN.AcevedoE.BaracatP.SerafiniG.D.SmithInfluence of vitrification on mouse metaphase II oocyte spindle dynamics and chromatin alignmentFertil Steril904 Suppl200813961404 10.1016/j.fertnstert.2007.08.025 PubMed PMID: 18359482
- F.BrambillascaM.C.GuglielmoG.CoticchioM.Mignini RenziniM.Dal CantoR.FadiniThe current challenges to efficient immature oocyte cryopreservationJ Assist Reprod Genet3012201315311539 https://doi.org/10.1007/s10815-013-0112-0. PubMed PMID: 24114631; PubMed Central PMCID: PMCPMC3843180
- A.R.MoawadJ.ZhuI.ChoiD.AmarnathK.H.CampbellEffect of Cytochalasin B pretreatment on developmental potential of ovine oocytes vitrified at the germinal vesicle stageCryo Lett3462013634644 PubMed PMID: 24441374
- A.R.MoawadJ.ZhuI.ChoiD.AmarnathW.ChenK.H.CampbellProduction of good-quality blastocyst embryos following IVF of ovine oocytes vitrified at the germinal vesicle stage using a cryoloopReprod Fertil Dev258201312041215 10.1071/RD12215 PubMed PMID: 23336581
- A.R.MoawadI.ChoiJ.ZhuK.H.CampbellOvine oocytes vitrified at germinal vesicle stage as cytoplast recipients for somatic cell nuclear transfer (SCNT)Cell Reprogram1342011289296 10.1089/cell.2010.0089 PubMed PMID: 21718110
- A.R.MoawadP.FisherJ.ZhuI.ChoiZ.PolgarA.DinnyesIn vitro fertilization of ovine oocytes vitrified by solid surface vitrification at germinal vesicle stageCryobiology6522012139144 10.1016/j.cryobiol.2012.04.008 PubMed PMID: 22579520
- T.SomfaiM.OzawaJ.NoguchiH.KanekoN.W.Kuriani KarjaM.FarhudinDevelopmental competence of in vitro-fertilized porcine oocytes after in vitro maturation and solid surface vitrification: effect of cryopreservation on oocyte antioxidative system and cell cycle stageCryobiology5522007115126 10.1016/j.cryobiol.2007.06.008 PubMed PMID: 17681290
- A.D.VieiraF.ForellC.FeltrinJ.L.RodriguesCalves born after direct transfer of vitrified bovine in vitro-produced blastocysts derived from vitrified immature oocytesReprod Domest Anim4332008314318 10.1111/j.1439-0531.2007.00899.x PubMed PMID: 18086255
- A.D.VieiraA.MezzaliraD.P.BarbieriR.C.LehmkuhlM.I.RubinG.VajtaCalves born after open pulled straw vitrification of immature bovine oocytesCryobiology45120029194 PubMed PMID: 12445553
- C.J.Ruppert-LinghamS.J.PaynterJ.GodfreyB.J.FullerR.W.ShawDevelopmental potential of murine germinal vesicle stage cumulus-oocyte complexes following exposure to dimethylsulphoxide or cryopreservation: loss of membrane integrity of cumulus cells after thawingHum Reprod1822003392398 PubMed PMID: 12571179
- S.S.KimR.OlsenD.D.KimD.F.AlbertiniThe impact of vitrification on immature oocyte cell cycle and cytoskeletal integrity in a rat modelJ Assist Reprod Genet3162014739747 https://doi.org/10.1007/s10815-014-0216-1. PubMed PMID: 24668208; PubMed Central PMCID: PMCPMC4048389
- V.IsachenkoM.MontagE.IsachenkoS.DessoleF.NawrothH.van der VenAseptic vitrification of human germinal vesicle oocytes using dimethyl sulfoxide as a cryoprotectantFertil Steril8532006741747 10.1016/j.fertnstert.2005.08.047 PubMed PMID: 16500347
- A.R.MoawadA.DasariI.ChoiP.FisherJ.ZhuCampbell KH2008Effect on in vitro fertilization rates. CryobiologyVitrification of immature ovine oocytes with Cryoloop57
- A.R.MoawadS.L.TanT.TaketoBeneficial effects of glutathione supplementation during vitrification of mouse oocytes at the germinal vesicle stage on their preimplantation development following maturation and fertilization in vitroCryobiology76201798103 10.1016/j.cryobiol.2017.04.002 PubMed PMID: 28412286
- A.R.MoawadS.L.TanB.XuH.Y.ChenT.TaketoL-carnitine supplementation during vitrification of mouse oocytes at the germinal vesicle stage improves preimplantation development following maturation and fertilization in vitroBiol Reprod8842013104 10.1095/biolreprod.112.107433 PubMed PMID: 23446455
- A.R.MoawadB.XuS.L.TanT.TaketoL-carnitine supplementation during vitrification of mouse germinal vesicle stage-oocytes and their subsequent in vitro maturation improves meiotic spindle configuration and mitochondrial distribution in metaphase II oocytesHum Reprod2910201422562268 10.1093/humrep/deu201 PubMed PMID: 25113843
- T.TharasanitB.ColenbranderT.A.StoutEffect of maturation stage at cryopreservation on post-thaw cytoskeleton quality and fertilizability of equine oocytesMol Reprod Dev7352006627637 10.1002/mrd.20432 PubMed PMID: 16477611
- T.TharasanitS.ColleoniG.LazzariB.ColenbranderC.GalliT.A.StoutEffect of cumulus morphology and maturation stage on the cryopreservability of equine oocytesReproduction13252006759769 10.1530/rep.1.01156 PubMed PMID: 17071777
- M.J.TuckerG.WrightP.C.MortonJ.B.MasseyBirth after cryopreservation of immature oocytes with subsequent in vitro maturationFertil Steril7031998578579 PubMed PMID: 9757897
- N.AonoY.AbeK.HaraH.SasadaE.SatoH.YoshidaProduction of live offspring from mouse germinal vesicle-stage oocytes vitrified by a modified stepwise method, SWEIDFertil Steril84Suppl 2200510781082 10.1016/j.fertnstert.2005.03.077 PubMed PMID: 16209996
- C.E.ArgyleJ.C.HarperM.C.DaviesOocyte cryopreservation: where are we now?Hum Reprod Update2242016440449 10.1093/humupd/dmw007 PubMed PMID: 27006004
- A.ShahediA.HosseiniM.A.KhaliliM.NorouzianM.SalehiA.PiriaeiThe effect of vitrification on ultrastructure of human in vitro matured germinal vesicle oocytesEur J Obstet Gynecol Reprod Biol167120136975 10.1016/j.ejogrb.2012.11.006 PubMed PMID: 23260597
- A.S.El-ShalofyA.R.MoawadG.M.DarwishS.T.IsmailA.B.BadawyM.R.BadrEffect of different vitrification solutions and cryodevices on viability and subsequent development of buffalo oocytes vitrified at the germinal vesicle (GV) stageCryobiology7420178692 10.1016/j.cryobiol.2016.11.010 PubMed PMID: 27908686
- M.FathiA.A.SeidaR.R.SobhyG.M.DarwishM.R.BadrA.R.MoawadCaffeine supplementation during IVM improves frequencies of nuclear maturation and preimplantation development of dromedary camel oocytes following IVFTheriogenology819201412861292 10.1016/j.theriogenology.2014.02.010 PubMed PMID: 24630529
- P.NurseUniversal control mechanism regulating onset of M-phaseNature34462661990503508 10.1038/344503a0 PubMed PMID: 2138713
- C.SmytheJ.W.NewportCoupling of mitosis to the completion of S phase in Xenopus occurs via modulation of the tyrosine kinase that phosphorylates p34cdc2Cell6841992787797 PubMed PMID: 1531451
- I.ChoiJ.H.LeeP.FisherK.H.CampbellCaffeine treatment of ovine cytoplasts regulates gene expression and foetal development of embryos produced by somatic cell nuclear transferMol Reprod Dev77102010876887 10.1002/mrd.21230 PubMed PMID: 20740651
- I.ChoiK.H.CampbellTreatment of ovine oocytes with caffeine increases the accessibility of DNase I to the donor chromatin and reduces apoptosis in somatic cell nuclear transfer embryosReprod Fertil Dev226201010001014 10.1071/RD09144 PubMed PMID: 20591334
- J.H.LeeK.H.CampbellEffects of enucleation and caffeine on maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) activities in ovine oocytes used as recipient cytoplasts for nuclear transferBiol Reprod7442006691698 10.1095/biolreprod.105.043885 PubMed PMID: 16371593
- W.E.MaaloufJ.H.LeeK.H.CampbellEffects of caffeine, cumulus cell removal and aging on polyspermy and embryo development on in vitro matured and fertilized ovine oocytesTheriogenology717200910831092 10.1016/j.theriogenology.2008.12.001 PubMed PMID: 19185338
- J.YeA.P.FlintM.R.LuckK.H.CampbellIndependent activation of MAP kinase and MPF during the initiation of meiotic maturation in pig oocytesReproduction12552003645656 PubMed PMID: 12713427
- J.H.LeeK.H.CampbellCaffeine treatment prevents age-related changes in ovine oocytes and increases cell numbers in blastocysts produced by somatic cell nuclear transferCloning Stem Cells1032008381390 10.1089/clo.2007.0091 PubMed PMID: 18673075
- J.I.BaekD.W.SeolA.R.LeeW.S.LeeS.Y.YoonD.R.LeeMaintained MPF Level after Oocyte Vitrification Improves Embryonic Development after IVF, but not after Somatic Cell Nuclear TransferMol Cells40112017871879 https://doi.org/10.14348/molcells.2017.0184. PubMed PMID: 29145719; PubMed Central PMCID: PMCPMC5712517
- D.KeefeL.LiuW.WangC.SilvaImaging meiotic spindles by polarization light microscopy: principles and applications to IVFReprod Biomed Online7120032429 PubMed PMID: 12930570
- W.H.WangL.MengR.J.HackettR.OdenbourgD.L.KeefeLimited recovery of meiotic spindles in living human oocytes after cooling-rewarming observed using polarized light microscopyHum Reprod1611200123742378 PubMed PMID: 11679523
- W.H.WangL.MengR.J.HackettD.L.KeefeDevelopmental ability of human oocytes with or without birefringent spindles imaged by Polscope before inseminationHum Reprod167200114641468 PubMed PMID: 11425830
- R.MoratoD.IzquierdoM.T.ParamioT.MogasCryotops versus open-pulled straws (OPS) as carriers for the cryopreservation of bovine oocytes: effects on spindle and chromosome configuration and embryo developmentCryobiology5722008137141 10.1016/j.cryobiol.2008.07.003 PubMed PMID: 18680737
- R.MoratoD.IzquierdoJ.L.AlbarracinB.AnguitaM.J.PalomoA.R.Jimenez-MacedoEffects of pre-treating in vitro-matured bovine oocytes with the cytoskeleton stabilizing agent taxol prior to vitrificationMol Reprod Dev7512008191201 10.1002/mrd.20725 PubMed PMID: 17474095
- C.RojasM.J.PalomoJ.L.AlbarracinT.MogasVitrification of immature and in vitro matured pig oocytes: study of distribution of chromosomes, microtubules, and actin microfilamentsCryobiology4932004211220 10.1016/j.cryobiol.2004.07.002 PubMed PMID: 15615607
- S.SuccuG.G.LeoniD.BebbereF.BerlinguerF.MossaL.BoglioloVitrification devices affect structural and molecular status of in vitro matured ovine oocytesMol Reprod Dev7410200713371344 10.1002/mrd.20693 PubMed PMID: 17290423
- Y.L.MiaoL.H.ShiZ.L.LeiJ.C.HuangJ.W.YangY.C.OuyangEffects of caffeine on in vivo and in vitro oocyte maturation in miceTheriogenology6842007640645 10.1016/j.theriogenology.2007.04.061 PubMed PMID: 17576000
- F.AriuL.BoglioloG.LeoniL.FalchiD.BebbereS.M.NiedduEffect of caffeine treatment before vitrification on MPF and MAPK activity and spontaneous parthenogenetic activation of in vitro matured ovine oocytesCryo Lett3562014530536 PubMed PMID: 25583014
- G.CoticchioM.A.BonuA.BoriniC.FlamigniOocyte cryopreservation: a biological perspectiveEur J Obstet Gynecol Reprod Biol115Suppl 12004S27 10.1016/j.ejogrb.2004.01.006 PubMed PMID: 15196707
- D.H.KimH.S.ParkS.W.KimI.S.HwangB.C.YangG.S.ImVitrification of immature bovine oocytes by the microdrop methodJ Reprod Dev5342007843851 PubMed PMID: 17460392
- L.BoglioloF.AriuS.FoisI.RosatiM.T.ZeddaG.LeoniMorphological and biochemical analysis of immature ovine oocytes vitrified with or without cumulus cellsTheriogenology688200711381149 10.1016/j.theriogenology.2007.08.013 PubMed PMID: 17868798
- X.ZhangX.LiuL.ChenD.Y.WuZ.W.NieY.Y.GaoCaffeine delays oocyte aging and maintains the quality of aged oocytes safely in mouseOncotarget81320172060220611 https://doi.org/10.18632/oncotarget.15292. PubMed PMID: 28206974; PubMed Central PMCID: PMCPMC5400529
- S.M.Bernal-UlloaA.Lucas-HahnD.HerrmannK.G.HadelerP.AldagU.BaulainOocyte pre-IVM with caffeine improves bovine embryo survival after vitrificationTheriogenology865201612221230 10.1016/j.theriogenology.2016.04.013 PubMed PMID: 27180328
