Abstract
Dietary polyunsaturated fatty acids (PUFAs) can influence fertility in farm animals. Some evidence in mice and sheep have suggested that PUFAs may influence offspring sex ratio, which may have significant value for cattle production. To test this hypothesis, three groups of Holstein cows were supplemented with either 0%, 3% or 5% protected fat (PF) in the form of calcium salt of fatty acids (rich in omega-6) from 14–21 days pre-partum until conception. Proven-fertile frozen semen from the same ejaculate was used for insemination. Calf sex recorded at birth was 8/19 (42.1%) male offspring in the control group, increasing to 14/20 (70%, P > 0.05) and 17/20 (85%, P < 0.05) in 3% and 5% PF, respectively. To test if this effect was caused by a direct influence on the oocyte, we supplemented bovine cumulus oocyte complexes during in vitro maturation with either omega-3 alpha-linolenic acid (ALA), omega-6 linoleic acid (LA) or trans-10, cis-12 conjugated linoleic acid (CLA). Sex ratio of the produced transferable embryos was determined using PCR of SRY gene. Similar to the in vivo results, sex ratio was skewed to the male side in the embryos derived from LA- and CLA-treated oocytes (79% and 71%) compared to control and ALA-treated oocytes (44% and 54%, respectively). These results indicate that both dietary and in vitro supplementation of omega-6 PUFAs can skew the sex ratio towards the male side in cattle. Further experiments are required to confirm this effect on a larger scale and to study the mechanisms of action that might be involved.
1 Introduction
Various mechanisms of sex determination are present in amniote vertebrates, including genotypic, environmental (for example temperature in Crocodilian reptiles), or a mixture [Citation1]. There is evidence that mammals also have the ability to skew their sex ratios in response to environmental conditions, a system which is thought to confer evolutionary benefits [Citation2]. This would have the least cost to the mother if the sex ratio was adjusted close to the time of conception. Two main research lines have attempted to explain possible mechanisms involved [Citation3]. The first is based on the finding that more dominant mothers produce more male offspring, possibly mediated via alterations in their testosterone levels [Citation4]. The second has investigated associations between the body condition or diet of the mother and offspring sex. Within the latter category, alterations in sex ratio have been reported in response to pre-conceptual maternal diet [Citation5–Citation8], general dietary supplementation [Citation9], changes in body condition [Citation10] and diabetes [Citation11]. More specifically, the dietary content of unsaturated fats [Citation12], polyunsaturated fatty acids (PUFAs) [Citation13,Citation14], glucose [Citation15] or fructose [Citation16] have also been implicated. Results have not, however, always been consistent. For example, female mice supplemented with an n-6 rich diet gave birth to more female pups than male pups (P < 0.001) compared to control or n-3 fed mothers [Citation13]. Others have shown a skew to the male side when a high fat diet was used in mice [Citation5] or a high n-6 PUFA diet was fed to ewes [Citation14]. In the latter study 69% males were recorded in the n-6 PUFA group fed as a protected soybean meal vs. 38% in the control.
Sex ratio is a key factor in cattle breeding. In the dairy industry a predominance of females is preferred as these are required for milk production. Whereas, beef cattle producers may use sexed semen to produce crossbred female replacements [Citation17]. This has led to the development of semen sexing technologies which can reliably produce a 90% gender bias, but the sorting mechanism remains costly and pregnancy rates are significantly less than those using conventional semen [Citation17,Citation18]. Other potential methods of altering the sex ratio are therefore of significant interest.
Nutritional management can be a natural, effective and less expensive substitute for hormonal and medical intervention that can be a potential health hazard in milk and meat. Dietary PUFAs are already used to increase the energy density of the diet in cattle and also have some specific effects that are energy-independent. These include prostaglandin biosynthesis, steroidogenesis and transcriptional regulation, as well as regulation of genes involved in maternal immune response and tissue remodeling [Citation19]. The predominant PUFA in most seed lipids is linoleic acid (LA; 18:2n-6), while α-linolenic acid (ALA;18:3n-3) predominates in most forage lipids and flaxseed. These two essential fatty acids can be metabolized in animal tissue to other forms of PUFAs. Dietary PUFA supplements in ruminants have to be protected against rumen bio-hydrogenation [Citation20]. This process also results in synthesis of conjugated LA (CLA) from LA [Citation21].
With respect to reproductive performance in cattle [reviewed by Citation22–Citation25], some PUFA supplements were reported to have positive effects on postpartum uterine involution [Citation26], growth rate and diameter of the ovulatory follicle [Citation27] and in vitro oocyte developmental capacity [Citation28]. Staples et al. [Citation22] reviewed different studies examining the effect of dietary fat supplements on reproductive performance of lactating dairy cows, and reported that eleven out of twenty studies showed an average of 17% improvement in conception or pregnancy rates.
Despite the widespread use of various feeds which alter dietary PUFA concentrations and ratios, the effects of PUFAs on the sex of the offspring in cattle have not to our knowledge been investigated to date. Therefore, the aim of the present study was twofold. Firstly we examined the effect of dietary supplementation of cows with two levels of protected fat (Calcium salt of fatty acids; Magnapac) on the sex of the calves produced. Secondly we tested supplementation with either ALA, LA or CLA during bovine in vitro oocyte maturation media. This was to determine whether any effects on the sex ratio of the embryos produced were directly on the oocyte and to identify differential effects of different PUFAs.
2 Materials and methods
2.1 Experimental animals and feeding
The experimental work of this study was carried out at a private dairy farm in cooperation with the Animal Production Department, Faculty of Agriculture, Mansoura University. Holstein cows in late lactation, 490–540 kg LBW, 1–4 parities were individually fed according to the nutrient requirements recommended by NRC [Citation29]. The control ration consisted of concentrate feed mixture (CFM; 19% Crude Protein), corn silage (ranged from 18–20 kg/day) and berseem hay (2 kg/day). The concentrate feed mixture (CFM) was composed of 46% yellow corn, 10% wheat bran, 10% cottonseed meal, 20% soybean, 10% horse bean, 1% NaCl, 0.1% Toxfree™ (Alfa Chemical, Mansourah, Egypt), 0.4% Premix, 1.3% sodium bicarbonate, 0.2% vitamins mixture, and 1% limestone. Roughage (silage and hay) was offered ad libitum while the CFM was offered individually for each animal twice daily before milking. This resulted in a roughage: concentrate ratio of nearly 60:40%. All animals had free access to clean drinking water and mineralized salt stone.
2.2 Experimental design
Sixty Holstein cows were stratified and randomly divided into three groups according to BW and parity. Experimental cows in group 1 (n = 20) were fed with the control diet without any fat supplementation. Groups 2 and 3 were fed with the CFM of the control diet supplemented with 3% dry matter (DM) or 5% DM respectively of a protected fat (PF) (Magnapac, Norel & Nature Comp., Madrid, Spain, a calcium salt of fatty acids). The fatty acid profile of Magnapac according to the product specifications is myristic acid (C14) 1.5%, palmitic acid (C16) 44.0%, stearic acid (C18) 5.0%, oleic acid (C18:1) 40.0% and linoleic acid (C18:2) 9.5%. The net energy for lactation (NEL) was 1.76, 1.82, and 1.84 Mcal/kg DM in control, 3% PF and 5% PF diets, respectively. The experimental feeding period started at 14–21 days pre-partum and continued up to 120 day-post-partum or conception (1–3 inseminations). The nutrient requirements were adjusted every two weeks according to changes in milk yield. Cows were inseminated by artificial insemination after detection of estrus according to the traditional am–pm rule. The same proven-fertile frozen semen source was used in all treatment groups. Cows that did not conceive at first AI were re-inseminated at the next estrous. Cows that did not return to estrus after insemination were examined transrectally by ultrasonography to confirm pregnancy at 35 days post insemination. A minimum of one and a maximum of three inseminations per conception were required. During the post-partum period, live body weight (LBW) was measured and the body condition score was estimated on a scale of 1–5 (1 = emaciated, 5 = extremely fat) by the same trained technician at 15, 30, 45 and 60 days in milk (DIM). One cow in the control group was excluded from the study due to repeat breeding. The sex of the offspring was then recorded at birth.
2.3 Chemicals and reagents used for the in vitro experiment
All chemicals and reagents were purchased from Sigma Chemical Company (Poole, Dorset, UK) unless otherwise stated.
2.4 Collection of oocytes
Bovine ovaries were collected immediately after slaughter from a local abattoir and transported within 2 h to the laboratory in phosphate buffered saline (PBS) at 37 °C. Ovaries were washed in PBS and 70% ethanol. COCs were aspirated from antral follicles (3–8 mm in diameter) and only grade-I COCs were used for the experiment [Citation30]. Serum-free TCM-199 medium supplemented with 20 mM HEPES and 0.4% (w/v) BSA was used during COC selection and washing.
2.5 In vitro maturation and PUFA supplementation
Selected COCs were cultured in four-well dishes (NUNC, Thermo Fisher Scientific, Loughborough, Leicestershire, UK) in serum-free maturation medium (TCM-199) supplemented with 10 µg/mL LH (Leutropin; Bioniche Animal Health, Belleville ON), 10 µg/mL FSH (Follitropin; Bioniche), 1 µg/mL oestradiol, 0.6% (w/v) fatty acid-free BSA, and 50 µg/mL gentamycin. Stock solutions of alpha-linolenic acid (ALA; n-3 18:3), linoleic acid (n-6; 18:2) and trans-10, cis-12 conjugated linoleic acid (CLA; n-6 18:2) were prepared in DMSO (100 mM). They were added to maturation media at a final concentration of 50 µM. Media was incubated without COCs for 2 h at 38.5 °C to allow binding of PUFAs to BSA which acts as a carrier. DMSO was added to the control group at the same concentration used with PUFAs (0.05%). We have previously shown that DMSO at this concentration has no effect on maturation and embryo development compared to DMSO-free controls [Citation31]. COCs were incubated in maturation media containing DMSO or PUFAs for 24 h at 38.5 °C under 5% CO2 in humidified air. A total of 773 COCs were used in this experiment.
2.6 In vitro fertilization and embryo culture
In vitro matured oocytes (in the presence or absence of 50 µM ALA, LA or CLA) were fertilized with frozen semen from a single bull as previously described by Fouladi-Nashta and Campbell [Citation32]. Briefly, swim up technique was used to select motile spermatozoa using calcium-free medium (for 45 min). Supernatant was then centrifuged at 300 × g at room temperature and spermatozoa were re-suspended (at a concentration of 1 × 106 sperm/mL) in fertilization medium (Fert-TALP) supplemented with 0.6% (w/v) fatty acid-free BSA, 1 µg/mL heparin, 50 ng/mL hypotaurine, and 50 ng/mL epinephrine. COCs were fertilized in 4-well dishes containing 400 µl of fertilization medium with 1 × 106 sperm/mL for 18 h at 38.5 °C, in a humidified incubator of 5% CO2 in air. Afterwards, presumptive zygotes were denuded from cumulus cells by gentle pipetting and transferred to 4-well plates containing 500 µL/well of SOFaaci (synthetic oviductal fluid medium containing amino acids, myoinositol, and sodium citrate [Citation33]) supplemented with 0.4% (w/v) fatty acid-free BSA, then incubated at 38.5 °C in a humidified incubator with 5% O2, 5% CO2, and 90% N2. The culture was continued up to Day 7. Fetal calf serum (FCS) was added at 5% to the embryo culture media starting from day 3. Blastocysts on day 7 were washed in PBS and individually snap frozen in 1.5 mL microcentrifuge tubes using liquid nitrogen. Samples were kept at −20 °C until genomic DNA extraction.
2.7 Genomic DNA extraction and conventional PCR
Genomic DNA was extracted from individual blastocyst using QIAamp DNA Micro Kit (QIAGEN Ltd, West Sussex, UK) with carrier RNA following manufacturer guidelines.
Conventional PCR amplification was performed using 5 µL purified DNA and 2 µm primers () specific to SRY using a Multiplex PCR kit (Qiagen). All samples were also analyzed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as a control. Male and female DNA samples from bull liver and uterus were used as positive and negative controls. PCR products were visualized using ethidium bromide staining on agarose gel electrophoresis.
Table 1 Oligonucleotide primer sequence and amplicon size used for PCR.
2.8 Statistical analysis
Mean body condition score and live body weight (LBW, as log transformed data) were compared using ANOVA followed by Bonferroni correction for multiple comparison. Sex ratio was analyzed using binomial analyses in SPSS against the expected proportion of 0.5 (1:1). Differences of P values ≤0.05 were considered as significant.
3 Results
3.1 Effect of protected fat dietary supplementation on the live body weight (LBW) and body condition score of the dam
In the control group, a gradual loss of live body weight resulted in an overall reduction of 28 ± 16.3 kg within 45 days postpartum. Weight loss was less marked in cows receiving 3% PF (P > 0.05 compared to control) while, 5% PF supplementation prevented weight loss and resulted in an average body weight gain of 8 ± 7.6 kg during the same period (). This was also observed in body condition score.
Table 2 Effect of dietary protected fat on live body weight (LBW) and body condition score (BCS) of dairy cows during early postpartum period.
3.2 Effect of protected fat dietary supplementation on sex ratio of the offspring
The sex ratio of calves produced by cows in the different dietary treatment groups (0%, 3% and 5% PF) during treatment period is presented in . Cows in both fat-supplemented treatment groups produced significantly more male than female calves (P < 0.05), while the number of males in the 5% PF group was relatively higher than that in the 3% PF group.
Table 3 Sex ratio of offspring produced from cows fed different experimental diets during the postpartum period.
3.3 Effect of PUFA supplementation during IVM on the sex of embryos produced
Morulae and blastocysts produced in vitro following exposure to free fatty acids were individually analyzed by PCR to determine their sex (). In COCs treated with n-6 LA or CLA fatty acids during maturation, the sex ratio was skewed toward males (P < 0.05), whereas in control and ALA groups the sex ratio was not significantly different from the expected proportion of 50% ().
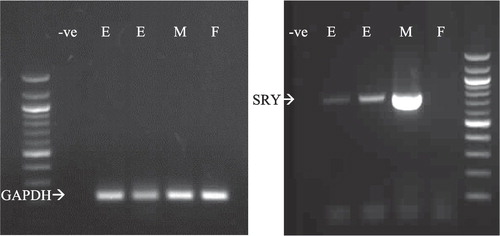
Table 4 Proportion of male and female embryos produced from COCs matured in the presence or absence of 50 µM of ALA, LA or CLA. The control group was supplemented with 0.05% DMSO equivalent to that used to dissolve fatty acids. P values are generated by comparing treatment group proportions to the expected sex ratio of 0.5.
4 Discussion
Our results revealed a significant shift to a more male sex ratio in offspring of cows supplemented with a Ca salt of fatty acids that is rich in LA. Furthermore, oocytes matured in vitro in the presence of the omega-6 PUFAs, LA or CLA, were more likely to produce a male embryo compared to those supplemented with the omega-3 ALA or to FA-free controls. This may have very important implications in cattle production systems as such feeds are currently used for their potential to improve fertility. Positive impact of PF on some fertility outcome parameters of the cows used in this study (n = 5 per group) namely: duration of placental drop, uterine involution, and conception rate have been previously reported [Citation34]. Similarly, dairy cows (n = 511) fed with Ca-soap of long chain FAs enriched with LA had higher pregnancy rates at 27 days (P = 0.07) and 41 days (P = 0.05) after insemination, compared to cows fed Ca-Palm oil [Citation35]. This FAs supplementation also reduced incidence of metritis during the early postpartum period.
Several factors have been proposed to modulate the sex ratio of mammalian offspring. Most relevant to the present study are the effects of maternal diet, type and quantity as reviewed by Rosenfeld [Citation36]. Among all studies that investigated the effect of maternal nutrition on sex ratio of offspring, the underlying mechanism favouring the dominance of one sex over the other remains unknown. The sex allocation hypothesis proposed by Trivers and Willard [Citation37] predicted that females with higher body condition score (BCS) would be better in producing male offspring, allowing the genes of these fitter females to be propagated in the gene pool of following generations. In the present study the BCS of the females supplemented with protected fat was higher than in the controls. This is also in line with the effect of maternal caloric intake in mice on the sex ratio of the offspring. Female mice supplemented with very high dietary fat (55%) resulted in a high male to female ratio of up to 71% [Citation5,Citation6]. On the contrary, calorie restriction resulted in 1:3 male to female ratio in mice compared to 1:1 ratio in controls [Citation38,Citation39]. A similar positive correlation between the socioeconomic patterns of food availability and male births have been reported in humans [Citation40].
It was suggested that the dietary effects on sex ratio are mainly maternal since exposing the breeder male mice to high fat diets failed to alter sex ratio and resulted in a normal percentage of X: Y spermatozoa [Citation12]. We therefore hypothesized that the effect of protected fat supplementation mentioned above is mediated by a direct effect on the oocyte. Many studies have clearly shown that the metabolic changes in blood serum are reflected in the follicular fluid composition and may thus directly influence cumulus cells and oocyte composition and quality [Citation41–Citation43]. PUFAs constitute the major portion of the fatty acid content of bovine follicular fluid, with linoleic acid (18:2) contributing about one third of the total fatty acid composition [Citation44]. Fatty acid composition of lipids in the oocytes and their surrounding cumulus cells was found to vary according to many factors including quality of the oocyte [Citation45], the season [Citation46], as well as dietary fatty acid composition [Citation47] and may have direct effects on oocyte cellular functions and developmental potential. For example, feeding encapsulated sunflower (E-SUN) or flaxseed (E-FLAX) oils to Holstein dairy cows markedly increased the corresponding fatty acid content in the COCs [Citation47]. The percentage of LA in the COCs was 54% higher in E-SUN vs. E-FLAX and was 2.4-fold higher than the controls fed no fat supplement [Citation47]. This clearly shows that the FA composition of the oocyte can be manipulated by diet. In sheep, n-3 (salmon oil) and n-6 (sunflower oil) enriched diets also resulted in significant increases in the n-3 and n-6 content of the granulosa cells and oocytes, respectively [Citation43].
We also tested the individual effects of ALA, LA and CLA, the most predominant PUFAs in dietary supplements, during oocyte maturation on the sex ratio of in vitro produced embryos. We found that the omega-6 LA and CLA, but not the omega-3 ALA, skewed the sex ratio towards male embryos. We have previously shown that supplementation of PUFAs (LA and ALA) during oocyte maturation significantly increase prostaglandin production as detected in the spent media [Citation31,Citation48]. This proves that the treated COCs could incorporate and use the supplemented fatty acids. Fatty acid incorporation into the cellular structure may affect various signaling molecules as well as cell membrane properties [Citation24].
In a recent review examining sex ratio after assisted reproduction in humans, four suggestions were made to account for skewed sex ratios in 4–8 cell embryos produced by IVF [Citation49]. These were: (i) male embryos have a developmental advantage after fertilization; (ii) the sperm preparation technique may favour Y-bearing spermatozoa; (iii) the composition of the zona pellucida may increase the chances of Y spermatozoa fertilizing the oocyte or (iv) Y bearing spermatozoa may have higher fertilizing ability. Of these, the effects on the zona pellucida were considered most likely although Zuccotti et al. [Citation50] reported that mammalian oocytes are not selective towards X- or Y-bearing spermatozoa. Another suggestion was that culture conditions could influence the process of X-inactivation, which normally occurs in female embryos via interference with DNA methylation, chromatin modification or histone deacetylation [Citation49]. Interestingly, a recent study found that knockout of Annexin A1, a glucocorticoid-regulated anti-inflammatory protein, in mice resulted in a significantly higher percentage of female pups, without affecting the proportion of Y and X chromosomes in the males [Citation51]. Annexin A1 inhibits phospholipase A2 inhibitory activity, needed for the biosynthesis of inflammatory mediators such as prostaglandins [Citation52]. Omega 3 PUFAs (EPA and DHA) can differentially affect the expression patterns of cell-associated Annexin A2 in cultured human umbilical vein cells [Citation53], however effects on Annexin A1 on sex ratio have not been reported.
5 Conclusions
In conclusion, this study is the first to report effects of omega 6 PUFAs on the sex ratio of the offspring in cattle. Dietary protected fat supplementation increased the male: female ratio. Our data also suggest that this might be mediated, at least in part, via a direct effect of PUFAs on the oocyte. Further research is required to determine the associated structural and molecular mechanisms involved.
Competing interests
The authors have no conflict of interest to declare.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
Reference
- M.Johnson PokornaL.KratochvilWhat was the ancestral sex-determining mechanism in amniote vertebrates?Biol Rev Camb Philos Soc912016112
- S.KrackowPotential mechanisms for sex ratio adjustment in mammals and birdsBiol Rev Camb Philos Soc701995225241
- V.J.GrantL.W.ChamleyCan mammalian mothers influence the sex of their offspring peri-conceptually?Reproduction1402010425433
- T.B.KimJ.K.OhK.T.KimS.J.YoonS.W.KimDoes the mother or father determine the offspring sex ratio? Investigating the relationship between maternal digit ratio and offspring sex ratioPLoS One102015e0143054
- C.S.RosenfeldK.M.GrimmK.A.LivingstonA.M.BrokmanW.E.LambersonR.M.RobertsStriking variation in the sex ratio of pups born to mice according to whether maternal diet is high in fat or carbohydrateProc Natl Acad Sci U S A100200346284632
- C.S.RosenfeldR.M.RobertsMaternal diet and other factors affecting offspring sex ratio: a reviewBiol Reprod71200410631070
- E.Z.CameronP.R.LemonsP.W.BatemanN.C.BennettExperimental alteration of litter sex ratios in a mammalProc Biol Sci2752008323327
- F.MathewsP.J.JohnsonA.NeilYou are what your mother eats: evidence for maternal preconception diet influencing foetal sex in humansProc Biol Sci275200816611668
- S.N.AustadM.E.SunquistSex-ratio manipulation in the common opossumNature324198658
- E.Z.CameronW.L.LinklaterExtreme sex ratio variation in relation to change in condition around conceptionBiol Lett32007395397
- S.F.EhrlichB.EskenaziM.M.HeddersonA.FerraraSex ratio variations among the offspring of women with diabetes in pregnancyDiabet Med292012e273e278
- A.P.AlexenkoJ.MaoM.R.EllersieckA.M.DavisJ.J.WhyteC.S.RosenfeldThe contrasting effects of ad libitum and restricted feeding of a diet very high in saturated fats on sex ratio and metabolic hormones in miceBiol Reprod772007599604
- E.D.FountainJ.MaoJ.J.WhyteK.E.MuellerM.R.EllersieckM.J.WillEffects of diets enriched in omega-3 and omega-6 polyunsaturated fatty acids on offspring sex-ratio and maternal behavior in miceBiol Reprod782008211217
- M.P.GreenL.D.SpateT.E.ParksK.KimuraC.N.MurphyJ.E.WilliamsNutritional skewing of conceptus sex in sheep: effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA)Reprod Biol Endocrinol6200821
- K.KimuraL.D.SpateM.P.GreenR.M.RobertsEffects of D-glucose concentration, D-fructose, and inhibitors of enzymes of the pentose phosphate pathway on the development and sex ratio of bovine blastocystsMol Reprod Dev722005201207
- C.GrayS.LongC.GreenS.M.GardinerJ.CraigonD.S.GardnerMaternal fructose and/or salt intake and reproductive outcome in the rat: effects on growth, fertility, sex ratio, and birth orderBiol Reprod89201351
- G.E.SeidelJr.Update on sexed semen technology in cattleAnimal8Suppl 12014160164
- S.T.ButlerI.A.HutchinsonA.R.CromieL.ShallooApplications and cost benefits of sexed semen in pasture-based dairy production systemsAnimal8Suppl 12014165172
- S.M.WatersG.S.CoyneD.A.KennyD.E.MacHughD.G.MorrisDietary n-3 polyunsaturated fatty acid supplementation alters the expression of genes involved in the control of fertility in the bovine uterine endometriumPhysiol Genomics442012878888
- R.MattosC.R.StaplesW.W.ThatcherEffects of dietary fatty acids on reproduction in ruminantsRev Reprod520003845
- K.W.WahleS.D.HeysD.RotondoConjugated linoleic acids: are they beneficial or detrimental to health?Prog Lipid Res432004553587
- C.R.StaplesJ.M.BurkeW.W.ThatcherInfluence of supplemental fats on reproductive tissues and performance of lactating cowsJ Dairy Sci811998856871
- R.S.RobinsonP.G.PushpakumaraZ.ChengA.R.PetersD.R.AbayasekaraD.C.WathesEffects of dietary polyunsaturated fatty acids on ovarian and uterine function in lactating dairy cowsReproduction1242002119131
- D.C.WathesD.R.AbayasekaraR.J.AitkenPolyunsaturated fatty acids in male and female reproductionBiol Reprod772007190201
- J.E.SantosT.R.BilbyW.W.ThatcherC.R.StaplesF.T.SilvestreLong chain fatty acids of diet as factors influencing reproduction in cattleReprod Domest Anim43Suppl 220082330
- J.O.LindellH.KindahlExogenous prostaglandin F2 alpha promotes uterine involution in the cowActa Vet Scand241983269274
- M.CarriquiryC.R.DahlenW.J.WeberG.C.LambB.A.CrookerPostpartum ovarian activity in multiparous Holstein cows treated with bovine somatotropin and fed n-3 fatty acids in early lactationJ Dairy Sci92200948764888
- W.F.MareiM.A.AlvarezV.Van HoeckA.Gutierrez-AdanP.E.BolsJ.L.LeroyEffect of nutritionally induced hyperlipidaemia on in vitro bovine embryo quality depends on the type of major fatty acid in the dietReprod Fertil Dev2016
- National Research Council (U.S.). Subcommittee on Dairy Cattle Nutrition. Nutrient requirements of dairy cattle. Washington, D.C.: National Academy Press; 2001.
- W.F.MareiD.R.AbayasekaraD.C.WathesA.A.Fouladi-NashtaRole of PTGS2-generated PGE2 during gonadotrophin-induced bovine oocyte maturation and cumulus cell expansionReprod Biomed Online282014388400
- W.F.MareiD.C.WathesA.A.Fouladi-NashtaThe effect of linolenic Acid on bovine oocyte maturation and developmentBiol Reprod81200910641072
- A.A.Fouladi-NashtaK.H.CampbellDissociation of oocyte nuclear and cytoplasmic maturation by the addition of insulin in cultured bovine antral folliclesReproduction1312006449460
- P.HolmP.J.BoothM.H.SchmidtT.GreveH.CallesenHigh bovine blastocyst development in a static in vitro production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum-proteinsTheriogenology521999683700
- W.A.KhalilM.A.El-HarairyA.A.Abul-AttaImpact of dietary protected fat (magnapac) on productive and reproductive performances of lactating holstein cowsJ Animal Poultry Prod, Mansoura University32012437450
- S.JuchemR.CerriM.VillasenorK.GalvaoR.BrunoH.RutiglianoSupplementation with Calcium Salts of Linoleic and trans-Octadecenoic Acids Improves Fertility of Lactating Dairy CowsReprod Domest Anim2008
- C.S.RosenfeldPericonceptional influences on offspring sex ratio and placental responsesReprod Fertil Dev2420114558
- R.L.TriversD.E.WillardNatural selection of parental ability to vary the sex ratio of offspringScience17919739092
- J.P.RiversM.A.CrawfordMaternal nutrition and the sex ratio at birthNature2521974297298
- D.B.MeikleL.C.DrickamerFood availability and secondary sex ratio variation in wild and laboratory house mice (Mus musculus)J Reprod Fertil781986587591
- D.P.WilliamsS.B.GoingT.G.LohmanD.W.HarshaS.R.SrinivasanL.S.WebberBody fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescentsAm J Public Health821992358363
- J.L.LeroyT.VanholderJ.R.DelangheG.OpsomerA.Van SoomP.E.BolsMetabolic changes in follicular fluid of the dominant follicle in high-yielding dairy cows early post partumTheriogenology62200411311143
- A.A.Fouladi-NashtaK.E.WonnacottC.G.GutierrezJ.G.GongK.D.SinclairP.C.GarnsworthyOocyte quality in lactating dairy cows fed on high levels of n-3 and n-6 fatty acidsReproduction2009
- K.E.WonnacottW.Y.KwongJ.HughesA.M.SalterR.G.LeaP.C.GarnsworthyDietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryosReproduction13920105769
- S.T.HomaC.A.BrownChanges in linoleic acid during follicular development and inhibition of spontaneous breakdown of germinal vesicles in cumulus-free bovine oocytesJ Reprod Fertil941992153160
- J.Y.KimM.KinoshitaM.OhnishiY.FukuiLipid and fatty acid analysis of fresh and frozen-thawed immature and in vitro matured bovine oocytesReproduction1222001131138
- Y.ZeronA.OcheretnyO.KedarA.BorochovD.SklanA.AravSeasonal changes in bovine fertility: relation to developmental competence of oocytes, membrane properties and fatty acid composition of folliclesReproduction1212001447454
- M.ZachutI.DekelH.LehrerA.ArieliA.AravL.LivshitzEffects of dietary fats differing in n-6:n-3 ratio fed to high-yielding dairy cows on fatty acid composition of ovarian compartments, follicular status, and oocyte qualityJ Dairy Sci932010529545
- W.F.MareiD.C.WathesA.A.Fouladi-NashtaImpact of linoleic acid on bovine oocyte maturation and embryo developmentReproduction1392010979988
- J.J.TarinM.A.Garcia-PerezC.HermenegildoA.CanoChanges in sex ratio from fertilization to birth in assisted-reproductive-treatment cyclesReprod Biol Endocrinol12201456
- M.ZuccottiV.SebastianoS.GaragnaC.A.RediExperimental demonstration that mammalian oocytes are not selective towards X- or Y-bearing spermMol Reprod Dev712005245246
- C.B.HebedaI.D.MachadoI.Reif-SilvaJ.B.MoreliS.M.OlianiS.NadkarniEndogenous annexin A1 (AnxA1) modulates early phase gestation and offspring sex-ratio skewingJ Cell Physiol2017
- N.K.LiuY.P.ZhangS.HanJ.PeiL.Y.XuP.H.LuAnnexin A1 reduces inflammatory reaction and tissue damage through inhibition of phospholipase A2 activation in adult rats following spinal cord injuryJ Neuropathol Exp Neurol662007932943
- J.ParkT.YamauraJ.KawamotoT.KuriharaS.B.SatoReciprocal modulation of surface expression of annexin A2 in a human umbilical vein endothelial cell-derived cell line by eicosapentaenoic acid and docosahexaenoic acidPLoS One92014e85045
