Abstract
Sheep is an important livestock in the world providing meat, milk and wool for human beings. With increasing human population, the worldwide needs of production of sheep have elevated. To meet the needs, the assistant reproductive technology including ovine in vitro embryo production (ovine IVP) is urgently required to enhance the effective production of sheep in the world. To learn the status of ovine IVP, we collected some publications related to ovine IVP through PubMed and analyzed the progress in ovine IVP made in the last five years (2012–2017). We made comparisons of these data and found that the recent advances in ovine IVP has been made slowly comparable to that of ovine IVP two decades ago. Therefore, we suggested two strategies or approaches to tackle the main problems in ovine IVP and expect that the efficiency of ovine IVP could be improved significantly when the approaches would be implemented.
Introduction
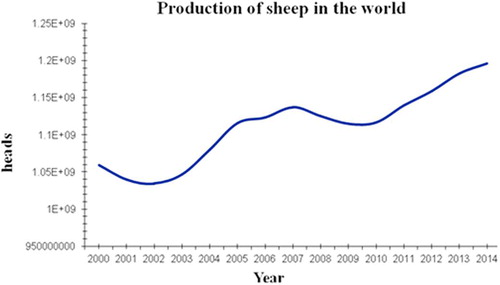
Since the first success of sheep in vitro fertilization (IVF) was reported in Cambridge, the UK in1986 [Citation1], sheep reproductive technology entered a new era, the great efforts had been made in the field by the scientists worldwide. Until the second half of 1980s, the IVF became entirely in vitro systems, called “in vitro embryo production” (IVP) including the three procedures, namely in vitro maturation (IVM), in vitro fertilization (IVF) and in vitro embryo culture (IVC). Up to the early1990s, the basic systems of ovine IVP including the three procedures had been well established and have been utilized until now. IVP is a valuable tool to aid the understanding of early mammalian development with applications ranging from therapeutic treatment of human reproductive failure to the preservation of gametes from animals of high genetic merit [Citation2] and speeding up genetic improvement in livestock. However, the process in sheep is still inefficient: approximately 70–90% of immature oocytes undergo maturation, from prophase I to metaphase II; 50–80% undergo fertilization and cleave to at least the two-cell stage at 24 to 48 h post-insemination; only 20% to 50% of immature oocytes ever reach the blastocyst stage, on day 7 to 8 post fertilization shown in , these results are similar to that reported by Walker et al. [Citation3] in 1996. Additionally, in vivo produced embryos are, in general, of greater quality than in vitro-produced embryos, because of greater implantation rates, high birth rate and high survival rate. The differences imply a great potential in improving ovine IVP. According to the statistical data reported by the United Nation Food and Agricultural Organization; production of sheep in the world has increased from 1060 million in 2000 to 1196 million heads in 2014 (cited from FAOSTAT-DATA 2017 online) (). This tendency indicates that the IVP systems as a new reproductive technology may play an important role for production of sheep in the future to accelerate sheep breeding and to improve the efficiency of production. However, we are currently facing many technical challenges in improving the efficiency of ovine IVP system such as low efficiency and poor quality of embryos, the system remains important, especially in sheep genetic breeding’s compared to natural reproduction and could be used to ensure the sustainable development of sheep production. Therefore, we urgently need to find solutions to overcome the problems so that the system could significantly be improved. Additionally, there are recently many excellent reviews on IVP in sheep [Citation4–Citation9], which not only described the advances in the field, but also pointed out the direction of the technology in the future. Likewise, based on recent publication associated with ovine IVP, in this review, we summarized the recent advances and challenges in sheep IVP including IVM, IVF and IVC procedures and suggested two possible approaches to tackle the problems. At the end, we predict the prospects of applications of sheep IVP systems, particularly in biomedical research.
able 1 Summary of ovine embryo in vitro production in recent years (2012–2017).
1.1 In vitro maturation of ovine oocytes
Immature oocytes to become fertilizable must undergo cytoplasmic and nuclear maturation. Subsequently, oocytes extrude the first polar body and have entered metaphase II [Citation4], waiting to be fertilized. Therefore, in vitro maturation is a key step to provide good quality oocytes for in vitro fertilization and determines the potential developmental competence of the oocytes. In other word, the prerequisite of obtaining a healthy embryo is to produce a good quality oocyte. For example, the inappropriate in vitro maturation of oocytes is the main reason of polyspermy after IVF when compared to developmental competence between in vivo and in vitro produced oocytes [Citation10]. To have a glimpse at what progress in the field has been made over the last 5 years, we collected 25 papers on ovine IVM published by different countries through PubMed and listed them in [Citation11–Citation35]. It should be noticed that in the table, all blastocyst rates have been standardized, which means that all blastocyst rates presented in are calculated based on the number of cleaved embryos. Meanwhile, if many experiments were simultaneously undertaken in a publication, we chose only the highest blastocyst rate in the experiment and the correspondent protocol from individual publications to fill in and made them comparable. From , the basic maturation medium used by most laboratories is still the traditional medium - Tissue Culture Medium 199 (TCM199), supplemented with various serum at 10% including fetal cattle serum (13 of 25 laboratories), sheep serum (5 of 25), BSA (5 of 25), follicular fluid (1 of 25), except, one [Citation36] does not use either serum or BSA. Moreover, hormones such as only FSH (5 of 25 laboratories) or a combination of FSH and LH or 17β - estradiol (20 of 25) are added into maturation medium. In addition to these, conditions for ovine oocyte in vitro maturation are the standard, namely 38.5 °C-39 °C, 5%CO2 for 20–24 h.
.2 Effect of researchers working in ovine IVP
Compared to in vivo maturation oocytes, in vitro maturation of oocytes is designed and performed by researchers. Therefore, the biggest impact to the success of IVP must be the researchers themselves who work with ovine IVP. In , whatever laboratories applied the same or similar protocols or even the same laboratory carried out different experiments with the same protocol, the results showed some differences. Although there could be many reasons to explain for them, it cannot be denied that in the cases the most differences were made by the different people with experiences. What contributions did the experienced researcher made to the successful IVP? There are at least two major contributions: (1) oocytes selection, (2) a short time between aspiration of oocytes from ovarian follicles and culturing the oocytes in maturation medium. In fact, oocytes selected by experienced researchers are more likely to have great developmental potential after in vitro maturation. Furthermore, experienced researchers handle oocytes more quickly than beginners do. Thus, the shorter time of handling oocytes means that mammalian cumulus-oocyte complexes (COCs) would be more synchronized as physical removal of mammalian COCs from ovarian follicles results in spontaneous resumption of meiosis, causing asynchrony between cytoplasmic and nuclear maturation and decreases oocyte developmental competence. Therefore, a proper training to any beginners working in the field is essential and important for the success of ovine IVP meanwhile a team work is also critical in speeding handling oocytes for IVM.
.3 Effects of reproductive and non – Reproductive seasons
Sheep reproduction is seasonal dependent. Because of this, most laboratories experience periodic reductions in embryo yield. Therefore, it is understandable that the season is likely an impact on oocyte quality. A study on this issue shown in [Citation25] was conducted to assess the effect of season on cleavage, blastocyst and lambing rates of in vitro produced ovine embryos during 3 years of data collection. The maturation and embryo culture media were defined, TCM199 + BSA and SOFaa + BSA, respectively. Matured oocytes were fertilized with fresh semen in synthetic oviductal fluid (SOF) with 20% heat inactivated oestrous sheep serum. The results show that there were no significant differences in cleavage rates between seasons in any of the 3 years examined although the blastocyst rate varied significantly between seasons in 2005 and 2007 (P < 0.05), and in 2006 (P < 0.001). Also, there were no differences in pregnancy and lambing rates between embryos during anoestrous versus during the breeding season. Finally, the authors concluded that only the blastocyst rate appeared to have been affected by season [Citation25]. Likewise, an in vivo experiment was carried out to investigate the effects of season on the superovulation in Black Suffolk ewes, particularly the ovulation rate and embryo quality [Citation37]. The ewes were superovulated in reproductive and non-reproductive seasons, respectively, followed by laparoscopic intrauterine artificial insemination. The viable morula and blastocysts were recovered and immediately transferred to recipients. The results showed that the ewe's ovulation rate was higher in non-reproductive season, whereas the viability rate of embryo was higher in reproductive season. Additionally, although no significant difference in the survival rate of the transferred viable embryos and the number of offspring per donor ewe was observed between the two seasons, in contrast, the offspring/ova ratio of the donor ewes superovulated in non- reproductive season was lower (P < 0.01). Indeed, these results directly prove a seasonal effect on oocyte quality in sheep, which could partially explain the reasons for the variation of the efficacy in ovine IVP during seasons.
.4 Effects of age of oocyte donor
In , for ovine IVP the most labs used oocytes collected from local slaughter house, age of oocyte donors is normally unknown. However, four reports used oocytes harvested from younger than 6 months old lambs shown. For example, a report only achieved blastocyst rate of 13.3%, the lowest in regardless of conditions for ovine IVP [Citation29]. The quality from lamb oocytes may partially consider for the poor rate. Like seasonal effect, many publications on the effect of maternal age of oocytes on developmental competence have shown that, age of oocyte donor is a significant factor influencing developmental competence of the oocyte although oocytes collected from lambs are able to develop healthy offspring after IVP embryo transfer. For example, Kochhar, et al. [Citation38] observed that lamb oocytes reached second meiotic metaphase (MII) at lower numbers at 24 h (60.0%) and 26 h (28.6%), whereas, 85.7% of adult-derived oocytes attained MII status by 24 h of maturation. Radiolabeling of oocyte proteins revealed higher incorporation of [(35) S-]-methionine and [(35) S]-cysteine in adult-derived oocytes compared to lamb oocytes. Although the cleavage rate of lamb oocytes was similar to that of ewe oocytes, the proportion reaching blastocyst stage was significantly lower (P < 0.05) in the lamb-derived oocytes. Furthermore, Armstrong [Citation39] pointed out that age-related abnormalities of oocytes of lambs include a) meiotic incompetence or inability to complete meiotic maturation resulting in oocytes incapable of fertilization; b) errors in meiosis that can be compatible with fertilization but lead to genetic abnormalities that compromise embryo viability; and c) cytoplasmic deficiencies that are expressed at several stages of development before or after fertilization. The evidence suggests that in general, oocytes from juvenile donors and the embryos derived therefrom appear less robust and may be less tolerant to suboptimal handling and in vitro culture conditions than are adult oocytes. In addition to less tolerance to inappropriate conditions, it is interesting that a significantly lower rate (P < 0.01) of cleaved prepubertal oocytes was observed at 22 and 26 h after fertilization while it was higher (P < 0.01) at 32 h than in the adult ones. Similarly, this trend was also found in blastocyst formation of in vitro fertilized oocytes from prepubertal sheep [Citation40]. The results may imply that the current protocol of ovine oocyte IVM may not be adaptive for lamb oocytes and suggest that the duration of lamb oocyte IVM may need to be longer compared with adult oocyte IVM.
.5 Effect of serum and bovine serum albumin (BSA)
In , almost all maturation media used for ovine IVM were added with either 10% serum including cattle serum (FCS, FBS), sheep serum (SS, OSS or ESS) and follicular fluid (FF) or BSA (as a defined medium). Despite the undefined and variable nature of serum composition, serum and BSA are among the most common components of the media in mammalian oocyte and embryo culture systems. Shirazi, et al. [Citation41] evaluated the effects of protein source (FBS, and BSA) in maturation medium on developmental competence of ovine oocyte. The results show that FBS supplemented in maturation medium could significantly improve the proportions of cleavage and total blastocysts compared to BSA supplemented. However, the cryotolerance of blastocysts was negatively influenced by the presence of FBS rather than BSA during IVM. Noticeably, not all sera are adaptive for ovine oocyte IVM, serum to be used for IVM should be normally tested among several samples of sera through the IVP process and only the best one is selected for ovine IVP.
.6 Effects of Roscovitine, a-linolenic acid, cerium oxide nanoparticles (CeO2 NPs) and sericin
In , there were several experiments attempting to improve ovine oocyte IVM.
.7 Roscovitine
Roscovitine was added into maturation medium to test the meiotic inhibition strategy. In the experiment, ovine COCs were cultured for 6 h in the presence of (Rosco) or absence (Control) of 75 μm roscovitine and, subsequently, they were subjected to IVM for 18 h in the presence of gonadotropins and followed by IVF and IVC. The results showed that, a high and similar proportion of oocytes from Rosco (93.6%) and Control (88.4%) reached the MII stage after IVM. In both treatments, approximately 70% of oocytes cleaved and 50% of them developed to blastocysts. The authors concluded that roscovitine, was efficient to reversibly inhibit the meiosis of adult sheep oocytes without detrimental effect on development and quality of the in vitro produced embryos [Citation12].
.8 α-linoleic acid
α-linoleic acid is a polyunsaturated fatty acid present in high concentrations in follicular fluid. The compound was supplemented into maturation medium; unfortunately, the compound did not exert any positive effect on maturation and development of ovine oocytes. However, a low concentration of the α-linoleic acid did not harm the developmental competence of the oocytes [Citation14]. In contrast, similar experiment was performed with oocytes collected from ovaries of prepubertal lambs [Citation34]. The different concentrations of α-linoleic acid were added into maturation medium to optimize the concentration. After IVM the oocytes were subjected to IVF and IVC. The conclusion was that the addition of 200 μM of α-linoleic acid to the IVM medium of prepubertal sheep oocytes had negative effects on nuclear maturation and cumulus cell expansion. These negative effects were not the case at 50 and 100 μM concentrations. Although the compound could improve male pronuclear formation and blastocyst quality, no effects were found on cleavage and blastocyst rates. Therefore, a question would be that, if a high concentration of this compound in maturation medium decreased the maturation rate and subsequently developmental competence of ovine oocytes, what is its function at a high concentration in follicular fluid? Perhaps, more work needs to be done to answer this question.
.9 Cerium oxide nanoparticles (CeO2 NPs) [10]
The objective of the experiment was to investigate whether cerium dioxide nanoparticles (CeO2 NPs) during in vitro maturation (IVM) of prepubertal ovine oocytes influenced their embryonic development in vitro. In this experiment, COCs derived from the ovaries of slaughtered prepubertal sheep underwent IVM with CeO2NPs (0, 44, 88 or 220 µg mL−1). Matured oocytes were then fertilized in vitro and zygotes were cultured for 7 days. The results showed that a concentration of 44 µg mL−1 CeO2NPs significantly increased the blastocyst yield and their total, inner cell mass and trophectoderm cell numbers [Citation20]. The authors concluded that a low concentration of CeO2NPs in the maturation medium enhanced in vitro embryo production of prepubertal ovine oocytes. Despite the use of prepubertal sheep oocytes, the blastocyst rate in the experiment was comparable to those of adult ones.
.10 Sericin [25]
Sericin is one of the major components of silks of mulberry as well as non-mulberry silkworms. It has proven that sericin, particularly sericin S, could improve serum-free mammalian cell culture [Citation42]. 0.1% and 0.5% sericin were supplemented into IVM medium. Mature oocytes were fertilized with fresh semen and zygotes were cultured in vitro. The results demonstrated that supplementation of 0.1% and 0.5% sericin during IVM had a significant effect on the nuclear and cytoplasmic maturation and enhanced preimplantation development of in vitro–cultured ovine embryos [Citation35]. This conclusion is also supported by another report [Citation43]. Taken together, this protein may be utilized potentially for ovine oocyte IVM.
Additionally, an attempt listed in was to in improve ovine oocyte maturation through addition of insulin–transferrin–selenium and ascorbic acid into maturation medium. Unfortunately, the attempt [Citation29] was not successful. Obviously, the progress in ovine oocyte IVM has been made over the last 5 years. However, compared to the efficiency of ovine oocyte IVM achieved by Walker [Citation3] two decades ago, the current efficiency, in general, is almost the same if it is measured by blastocyst rate. Therefore, we will discuss the problems and possible solutions in ovine IVM in the late section of this review.
1.11 In vitro fertilization of ovine oocytes
Unlike IVM, oocytes and spermatozoa both are involved in IVF process. Whereas oocyte and spermatozoa are also timing dependent, in other words, both have the ability of being fertilized or fertilizing oocytes with the limited time. This limited time of oocytes or sperm is called “fertile span”. A long or short fertile span greatly depends on many factors such as breeds, season, donor's age, donor nutrition statue, gamete quality, culture or preservation conditions including pH value, osmolarity, compounds, gases and so on. The fertile span of gametes is variable, even in the same individuals. Therefore, for a successful IVF, timing is very critical. Oocytes and spermatozoa must be co-incubated together within their fertile spans to ensure that the maximum number of mature oocytes can be fertilized by capacitated and appropriate spermatozoa. In , in general, IVF takes place after 20–24 h of IVM when the majority oocytes in maturation medium are considered to have already reached at the metaphase of the second meiotic division (MII). The procedures of ovine IVF shown in are similar among these laboratories. Also, frozen/thawed spermatozoa are utilized in majority of laboratories, nonetheless, some laboratories still prefer to use fresh semen if available. The most common fertilization medium for ovine oocytes is SOF medium supplemented with 1–2 μg/mL heparin+ 2–20% either fetal bovine serum or sheep serum, respectively. Regardless of other conditions, the blastocyst rate of oocytes IVF with 2% serum ranges from 20% to 59.2%, whereas with 20% serum it ranges from 24.1% to 42% (see ). It is relevant that ovine oocytes fertilized in SOF medium + 2% serum develop to higher blastocyst rates than those develop in SOF medium supplemented with 20% serum. In goat IVF, the similar results showed that the presence of 2% estrous sheep serum (ESS) achieves better results than the use of no serum or the standard 20% concentration as the percentage of zygotes with 2PN was higher in the SOF+2% serum than in the SOF+20% serum treatment group (27.7% versus 2.9% P < 0.05) [Citation44]. Therefore, a high concentration of serum seems to contribute to more polyspermy in fertilized oocytes and reduces the number of normal embryo development. It should be noted again that like serum used in IVM, not all sera would be suitable for ovine oocyte IVF and that the appropriate serum should be tested and selected for successful ovine IVF.
1.12 In vitro culture of ovine embryos
Compared to IVM and IVF, duration of embryo culture is much longer, needs 6–8 days. Therefore, culture medium is extremely important for embryo development in vitro. Like ovine oocyte IVM and IVF, the procedures of ovine embryo in vitro culture have not been significantly altered in majority of laboratories in the world for more than two decades. Ovine zygotes are cultured in synthetic oviduct fluid (SOF) supplemented with amino-acids and BSA. This combination is the standard or conventional basic culture medium for sheep embryos. To reduce incidence of oxidation, oxygen concentration is kept at 5% (5% O2, 5% CO2, 90% N2) (). The blastocyst formation occurs on days 6–8 of culture. During embryo culture, the culture medium is usually changed every 48 h, or applied with two- step culture, namely, on the first three days embryos are cultured in SOF+ AA+ BSA and on day 4, they were transferred in SOF+ AA+ glucose+ bovine/ovine serum or BSA and cultured in the medium up to day 8. Apparently, due to embryos kept in culture for a long period of time, inappropriate culture conditions for some mammalian embryos may cause epigenetic changes in genomic imprinting, thus leading to developmental anomalies. For example, Schwarzer et al. [Citation45] found that 13 different embryo culture protocols resulted in distinct cellular and molecular phenotypes in mouse embryos, suggesting that certain culture medium components can interfere with the epigenetic regulation of genes. Studies on the embryonic genome indicate that currently used in vitro embryo development conditions cannot fully mimic in vivo conditions about mRNA expression [Citation46]. Additionally, the most impressive example is “large offspring syndrome” observed in cattle and sheep caused by aberrant effects on fetal growth since serum complemented the pre-implantation culture medium [Citation47]. Furthermore, Fernández-Gonzalez et al. [Citation48] observed that the mRNA expression of some imprinting genes was significantly affected in blastocysts cultured in the presence of FCS. The collective evidence postulates that certain culture medium components can interfere with the epigenetic regulation of genes. Although addition of serum into ovine embryo culture medium could result in “large offspring syndrome” at a high risk if the embryos are transferred into recipients, many laboratories still prefer to use it as the cell numbers/blastocyst would be increased. However, an early experiment performed by Gardner, et al. [Citation49] showed that sheep blastocysts after culture in the presence of amino acids based on serum free -SOF medium was significantly greater and that the cell number per blastocyst significantly increased when the medium was renewed every 48 h to alleviate ammonium toxicity. Yet, non-essential amino acids and glutamine also significantly decreased the number of arrested embryos (P < 0.05). Interestingly, they also observed that culturing embryos singly or in groups in SOF medium with all Eagle's amino acids that was renewed every 48 h resulted in significant increases in blastocyst hatching and mean cell number. They also found that after culture in groups of blastocysts, cell numbers were equivalent to in vivo-developed controls and significantly greater than those developed in serum (103 cells; P < 0.01). Until now this protocol of ovine IVC has still been utilized successfully by many laboratories (). However, studies on embryo developmental potential and the embryonic genome in both ovine in vitro and in vivo embryos, indicate that either developmental competence or the expression pattern of genes in the former is largely different from that in the latter. Therefore, the ovine IVP embryos cannot fully mimic in vivo those in many ways [Citation50]. Taken together, it is relevant that inappropriate in vitro culture conditions currently used affect not only cleavage rate, blastocyst rate and mean cell numbers per blastocyst during the culture of embryos, but also pregnancy rate, fetal development and birth weight, even healthy state of offspring after embryo transfer. Thus, optimizing culture media for ovine embryos should be continuing so that the quality of ovine IVP embryos would eventually be closer to that of in vivo ones.
Possible solutions to improve ovine embryo IVP
After in vitro maturation, the population of oocytes can be divided into three subpopulations, namely degenerated oocytes, immature oocytes including nuclear mature but cytoplasmic immature oocytes, mature oocytes including fertilizable oocytes and aged oocytes. Degenerated oocytes can be easily identified and removed whereas the others are difficult to be separated from each other before IVF takes place. Therefore, to maximize the population of fertilizable oocytes and to minimize the others will be the key for improving the efficiency of ovine IVP. In other words, the success of IVP may largely depend on the proportions of the subpopulations of oocytes except degenerated oocytes. It is very likely that the proportions of the three subpopulations of oocytes may have been decided in the first place in term of developmental potential when in vitro maturation begins as asynchrony among oocytes exist at that time. If oocytes could be synchronized at the beginning of in vitro maturation, it means that the population of fertilizable oocytes would be significantly increased so that the efficiency of IVP should be improved. Noticeably, physical removal of mammalian COCs from ovarian follicles results in spontaneous resumption of meiosis, largely because of a decrease in cyclic adenosine monophosphate (cAMP) concentrations, causing asynchrony between cytoplasmic and nuclear maturation and a reduction in oocyte developmental competence [Citation36]. Therefore, controlling the delay of meiotic progress by means of adenylate cyclase activators, cAMP analogues, or phosphodiesterase inhibitors is very critical during IVM [Citation51]. Obviously, two asynchronies could highly affect the efficiency of ovine IVP, (1) asynchrony between cytoplasmic and nuclear maturation in individual oocytes, (2) asynchrony among oocytes at developmental stages. The first asynchrony can be improved by optimizing the system of ovine in vitro maturation. For example, the most experiments of IVM in were carried out for this purpose whereas the second is likely ignored. Based on these considerations we discuss the first strategy or approach to tackle the asynchrony among oocytes.
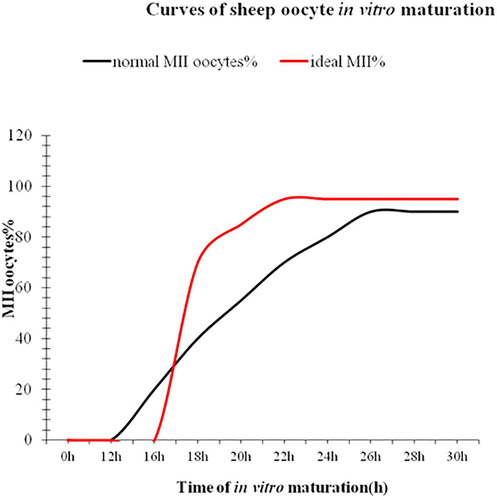
.1 1.“Synchronization” of cumulus-oocyte complexes
The asynchrony among oocytes at the beginning of oocytes affects not only maturation rate, but also subsequently developmental competence of oocytes. shows a curve of ovine oocyte in vitro maturation observed in our laboratory. The curve in black reveals that the first MII oocytes appear at 16 h of maturation and that the maturation curve gradually increases from 16 to 24 h of in vitro maturation and reaches the maximal maturation rates (over 80% of oocytes at MII stage) at approximately 24–26 h of maturation. However, not all MII oocytes have the same developmental competence. For example, those MII oocytes are present at the early stage, presumably from 16 to 20 h post maturation may have been aged whereas their fertile span is normally estimated to be approximate 2–4 h. Sometimes, fertile span may vary depending on the quality of oocytes, poor quality or aged oocytes may have shorter fertile span. In other words, some MII oocytes may have already lose their capability of being fertilized at IVF. Perhaps this phenomenon appears to be more significant when oocytes are collected from sheep at non-breeding season or from lambs. This suggests that “synchronization” of oocytes at the beginning of in vitro maturation might be the key to improve the efficiency of ovine IVP. The strategy or approach will be to “synchronize” oocytes at the germinal vesicle (GV stage) to stop or control “spontaneous resumption of meiosis” so that all oocytes could stand at the same developmental stage or called “same start line” for maturation. Thus, all oocytes would be synchronized at the metaphase of the second meiotic division by the end of in vitro maturation; the presumably ideal curve in red is shown in . Obviously, we expect that this synchronization could bring two significant changes (see the ideal curve in red) compared to the normal one in black: (1) the first presence of MII oocytes should delay; (2) meanwhile the whole population of oocytes should develop to the MII at the highest rate earlier, maybe at 22 h of maturation. This means that more MII oocytes at the best time, namely within their fertile span - the period of appropriate time for IVF or parthenogenetic activation. However, how we can turn the strategy to the reality? In fact, this approach has been suggested by several reviews [Citation52–Citation54]. There are currently many inhibitors available for the purpose, such as cycloheximide (CHX) [Citation55], a protein synthesis inhibitor, roscovitine, a potent inhibitor of M-phase Promoting Factor (MPF) kinase activity [Citation56], 6-dimethylaminopurine (6-DMAP) a phosphorylation inhibitor [Citation57–Citation60] vanadate (NaV03), an inhibitor of protein tyrosine phosphatases [Citation57], an invasive extracellular adenylate cyclase (iAC) [Citation53], phosphodiesterase (PDE) inhibitor (IBMX) [Citation61,Citation62] butyrolactone I, a nuclear maturation inhibitor [Citation53,Citation63]. All these inhibitors can be reversible. There have been a number of publications [Citation53,Citation62,Citation64,Citation65] attempting to “synchronize” oocytes with these inhibitors in different species since 1990 s. However, the results revealed controversy effects of these inhibitors on meiotic cell cycle arrest, maturation rate and subsequently developmental potential. Nonetheless, agents that modulate oocyte cAMP during IVM showed greater potential, possibly as these compounds could extend oocyte-cumulus cells gap- junctional communication [Citation36,Citation66]. Additionally, some inhibitors such as CHX seem to have a positive effect on meiotic cell cycle arrest within 24 h and on developmental potential in some species including porcine and bovine [Citation67,Citation68]. Therefore, use of the inhibitors might pave not only the way for improving ovine IVP, but also would benefit researchers with a flexible alternative in working practice without compromising developmental competence of oocytes, such as oocyte transportation for a long distance and a flexible starting time for oocyte IVM in 24 h. Nevertheless, the similar reports on ovine IVM are few compared to those in bovine. For example, Rose et al. [Citation36] reported that slaughterhouse-derived sheep COCs were cultured for 2 h (pre-IVM) in 100 mM forskolin (FSK) plus 500 mM 3-isobutyl-1-methylxanthine (IBMX). Pre-IVM (100 mM FSK and 500 mM IBMX) culture increased COCs cAMP concentrations by 10- fold compared with controls (P < 0.05) and concluded that regulation of ovine oocyte cAMP concentrations during IVM improved embryo quality compared with embryos produced by standard IVM methods. Moreover, milrinone, a specific inhibitor of type 3 phosphodiesterases (expressed in the oocyte only) and rolipram, a specific inhibitor of type 4 phosphodiesterases (expressed in cumulus cells only) were supplemented into maturation medium together, increase embryo production post-IVM [Citation36]. Therefore, with such treatments, we expect that the ideal curve of ovine IVM in could be achieved, which should significantly contribute to the successful ovine IVP. Additionally, in our laboratory CHX was used to test this idea with ovine oocyte IVM and the preliminary data were encouraging (unpublished data), implying that the idea could potentially benefit ovine IVP. Perhaps, the pre-treatment of an inhibitor to stop spontaneous resumption of meiosis in ovine oocyte IVM might become a routine procedure of the ovine IVM protocol in near future. As the expected, adding the cAMP-modulating or other agents to IVM media often improves oocyte maturation [Citation62,Citation69–Citation71] or has at least no detrimental effect [Citation53,Citation72,Citation73] on subsequent oocyte developmental potential. Nevertheless, to screen effective inhibitors with less toxicity and to optimize concentrations of the selected inhibitors for ovine oocyte IVM are certainly necessary.
Extension of fertile span of ovine oocytes matured in vitro
The “synchronization” of ovine oocytes at IVM maturation could improve oocyte quality and increases the number of MII oocytes capable of being fertilized or activated at a similar period of IVM. However, the fertile span for individual MII oocytes may vary, likely depending on many different factors such breeds, ages, seasons, locations, nutrition, body weight, management, reproductive performance, climate (light, temperature, humidity) as well as the protocol used for IVM and conditions such as medium, technical skills, gases, temperature and humidity and so on. In addition to these, fertilization must take place at the right time when most MII oocytes have reached at the beginning of their fertile span so that spermatozoa could penetrate and fertilize oocytes before they become aged. In other words, the timing of fertilization to the MII oocytes is extremely important to ensure the subsequent embryo development However, individual oocytes may have different fertile spans and fertile span could be variable from lot to lot of oocytes, likely depending on the oocyte quality. Understandably, compared to in vivo oocytes, in vitro matured oocytes may have a shorter fertile span. Apparently, the different and shorter fertile spans in IVM oocytes would reduce the efficiency of subsequent IVF because IVM oocytes only have “a variable and shorter window” to be fertilized. This may at least partially explain why IVF oocytes could have a high cleavage rate but suffering a low blastocyst rate. Based on these considerations we raise the second strategy or a question, whether fertile span in IVM oocytes could be extended to ensure that spermatozoa would have enough time to penetrate and fertilize quality oocytes?
Spontaneous activation in rat oocytes is a big obstacle for somatic cell nuclear transfer (SCNT). Rat oocytes cultured in a culture medium for about 1.5 h will rapidly enter a cell cycle stage of the second meiotic division called MIII. Shortly after in vitro culture, the oocyte extrudes the second polar body without pronuclear formation. Such MIII oocyte will completely lose the developmental competence. Obviously, rat oocytes cultured in vitro have a short fertile span. However, if rat oocytes harvested from in vivo are treated with demecolcine for 6 h, the developmental competence of the oocytes will not be compromised [Citation74]. The result clearly shows that it is possible to extend fertile span in rat oocytes treated with demecolcine. Also, similar experiments were conducted in ovine oocytes. For example, Choi, et al. [Citation75] treated ovine oocytes with 10 mM caffeine for 6 h and subsequently used oocytes as enucleated recipient cytoplasm for somatic cell nuclear transfer, the results elucidate that the enucleated cytoplasts treated with caffeine for 6 h could improve reprogramming of a somatic cell after SCNT, resulting in a birth of SCNT healthy lamb. In addition, in vitro aged then fertilized oocytes exhibit an increased frequency of polyspermy and fragmentation, a decrease in the frequency of cleavage and a decrease in frequency of development to the blastocyst stage. Interestingly, treatment of ovine oocytes with 10 mM caffeine from 18 to 24 h post maturation can increase the activities of both MPF and MAPK in MII oocytes, whereas treatment of MII oocytes from 24 to 30 h post maturation with 10 mM caffeine can prevent the decline in MPF and MAPK activities associated with aging and prevent the acquisition of activation competence [Citation76]. In other words, MII ovine oocytes treated with caffeine can have a prolonged fertile span. Moreover, Maalouf et al. [Citation10] found that ovine denuded oocytes treated with 10 mM caffeine had an improved rate of development to blastocyst, but there were no effects on the quality of blastocysts produced in terms of mean cell number or (inner cell mass) ICM: trophectoderm (TE) ratios. Notably, caffeine treatment of aged COCs had no significant effect on the frequency of development; however, in aged and denuded oocytes (DO’s) caffeine treatment significantly increased development to blastocyst and decreased the frequency of polyspermy. Additionally, the level of MPF activity at MII is lower in oocytes obtained from prepubertal sheep than mature ewes [Citation77], indicating that treatment of caffeine to IVM oocytes collected from prepubertal sheep may benefit the extension of their fertile span more effectively compared to mature ewes as caffeine treatment could not only maintain, but also increase the MPF level [Citation75]. Maturation promoting factor (MPF) is responsible for governing meiotic cell cycle arrest of MII oocytes. Therefore, maintaining MPF in ovine MII oocytes at a high level is essential for this purpose. For example, caffeine induced dephosphorylation of the catalytic subunit of MPF, p34cdc2, to elevate the activity of MPF [Citation78]. There are several inhibitors available such as caffeine, demecolcine, nocodazon and MG-132, all these inhibitors have been proven to effectively block resumption of meiotic oocytes in different species [Citation75]. In addition to this they all are reversible and ideal for this propose. In , we presume that after implementing the first approach, the more ovine oocytes at 22–26 h of in vitro maturation reach at the MII stage ideally for IVF. IVF must take place within this “2–4 h window” to obtain the maximal fertilization rate as penetration of spermatozoon into an oocyte would normally take approximate 1.5–3 h. If missed “the window”, the fertilization rate would dramatically drop shown in black line in as aged oocytes will lose the capability of being fertilized rapidly or even could be fertilized but would have the poor developmental competence. If the window could be widened doubled from 20 to 28 h or even wider like the ideal curve shown in red in , it means that such the “strong” oocytes treated with an inhibitor would have more time to meet suitable spermatozoa to be fertilized compared to untreated oocytes. The fertilization and subsequent development should be improved.
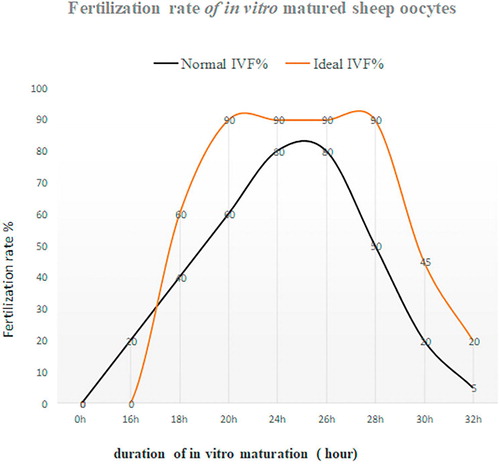
All the results elucidate that the “window” for optimal fertilization can be extended. Meanwhile, it should be noted that caffeine treatment to mouse oocytes could cause zona pellucida hardened which reduced blastocyst rate [Citation79], the result clearly indicates that caffeine treatment may cause some side-effects on the developmental potential in certain species. Therefore, further investigation to the effects of the inhibitors on ovine oocytes is required. Indeed, we currently face many challenges in ovine IVP meanwhile less progress in ovine IVP has been made in recent years. Therefore, we need to consider new ideas or strategies to tackle the most important issues in ovine IVP so that the efficiency of ovine IVP could be improved significantly in near future.
Prospect of ovine IVP in biomedical research
In last decade, it seems to be that sheep is not only as a valuable livestock producing meats, wool and skin for human beings, but also becomes an important large animal model for use in biomedical research to mimic human diseases as many mouse or rat models do not always show the same syndromes as which in humans sometimes. We are also difficult to observe small organs or tissues in a mouse or rat model. However, compared to cattle, sheep are more suitable as an animal model with a reasonable size, handling easiness, a short reproduction cycle, and cost-efficiency. Moreover, sheep have a similar pattern of diseases to humans, including allergic rhinitis, sinusitis, and nasal polyposis. Other advantages include their tolerance to long surgical procedures and their large nasal cavity that renders them suitable or repeated endoscopic sinus surgery (ESS) [Citation80]. In fact, until now sheep have been used as animal models in biomedical research for osteoarthritis [Citation81,Citation82], Chondral Defects [Citation83], a far-lateral disc herniation [Citation84], microdiscectomy [Citation85], antibiotic-eluting orthopedic device to prevent early implant associated infections [Citation86], meniscus tissue engineering [Citation87], transvaginal mesh insertion [Citation88], altered hemostasis [Citation89]. Therefore, the tendency to be an animal model may become a new force to drive the development of ovine IVP as such sheep models need ovine IVP as a technical platform. We expect that the development of ovine IVP could catch up the needs of both production of sheep and biomedical research. In summary, the improvement of ovine IVP system has been slow over the last 5 years, the efficiency remains low and no any significant progress has been made yet. On the other hand, the needs of production of sheep in the world and sheep models for use in biomedical research both have been increased rapidly, which could create an opportunity for improvement of ovine IVP. Also, the two strategies or approaches raised in the review may provide the possible solutions or considerations to the main problems in ovine IVP system.
Competing interests
The authors declare no competing interests.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- W.T.K.ChengR.M.MoorC.E.PolgeIn vitro fertilization of pig and sheep oocytes matured in vivo and in vitroTheriogenology25198614
- Rizos D, Clemente M, Bermejo-Alvarez P, Fuente J de La, Lonergan P, Gutie’rrez-Ada’n A. Consequences of In Vitro Culture Conditions on Embryo Development and Quality. Reprod Dom Anim 2008;43(Suppl. 4):44–50.
- S.K.WalkerJ.L.HillD.O.KleemannC.D.NancarrowDevelopment of Ovine Embryos in Synthetic Oviductal Fluid Containing Amino Acids at Oviductal Fluid ConcentrationsBiol Reprod551996703708
- Cognié Y, Baril G, Poulin N, Mermillod P. Current status of embryo technologies in sheep and goat. Theriogenology 2003;59(1):171–88. (a review).
- Cox JF, Alfaro V. In vitro fertilization and development of OPU derived goat and sheep oocytes. Reprod Domest Anim 2007;42(1):83–7. (a review).
- Joanna Maria Gonçalvesde Souza-Fabjan BarbaraPanneau NicolasDuffard YannLocatelli José Ricardode Figueiredo Vicente Joséde Figueirêdo Freitas Pascal Mermillod. In vitro production of small ruminant embryos: late improvements and further research (a review). Theriogenology 2014;81(9):1149–1162.
- Maria-Teresa Paramio Dolor Izquierdo. Recent advances in in vitro embryo production in small ruminants. Theriogenology 2016;86(1):152–159.
- Botigelli RC, Razza EM, Pioltine EM, Nogueira MF. New approach regarding the in vitro maturation of oocytes: manipulating cyclic nucleotides and their partners in crime JBRA Assisted Reprod 2017;21(1):35–44 517.
- Amiridisa GS, Cseh S. Assisted reproductive technologies in the reproductive management of small ruminants. Anim Reprod Sci 2012;130:152–16(A review).
- W.E.MaaloufJ.-H.LeeK.H.S.CampbellEffects of caffeine, cumulus cell removal and aging on polyspermy and embryo development on in vitro matured and fertilized ovine oocytesTheriogenology71200910831092
- S.LeddaA.IddaJ.KellyF.AriuL.BoglioloD.BebbereA novel technique for in vitro maturation of sheep oocytes in a liquid marble microbioreactorJ Assist Reprod Genet3342016513518
- L.F.CrocomoF.AriuL.BoglioloD.BebbereS.LeddaS.D.BicudoIn vitro Developmental Competence of Adult Sheep Oocytes Treated with RoscovitineReprod Domest Anim5122016276281 10.1111/rda.12677
- P.C.Dos Santos NetoM.VilariñoN.BarreraF.CuadroM.CrispoA.MenchacaCryotolerance of Day 2 or Day 6 in vitro produced ovine embryos after vitrification by Cryotop or Spatula methodsCryobiology70120151722
- E.AminiR.AsadpourL.RoshangarR.Jafari-joozaniEffect of α-linoleic acid supplementation on in vitro maturation, embryo development and apoptotic related gene expression in ovineInt J Reprod Biomed (Yazd)1442016255262
- Z.WangP.LinS.YuEffects of ghrelin on developmental competence and gene expression of in vitro fertilized ovine embryosTheriogenology7942013695701
- O.García-ÁlvarezA.Maroto-MoralesM.RamónE.Del OlmoP.Jiménez-RabadánM.R.Fernández-SantosDynamics of sperm subpopulations based on motility and plasma membrane status in thawed ram spermatozoa incubated under conditions that support in vitro capacitation and fertilisationReprod Fertil Dev2652014725732 10.1071/RD13034
- A.R.MoawadJ.ZhuI.ChoiD.AmarnathW.ChenK.H.S.CampbellProduction of good-quality blastocyst embryos following IVF of ovine oocytes vitrified at the germinal vesicle stage using a cryoloopReprod Fertil Dev258201312041215 10.1071/RD12215
- A.Mohammadi-SangcheshmehM.SoleimaniH.DeldarM.SalehiS.SoudiS.M.HashemiPrediction of oocyte developmental competence in ovine using glucose-6-phosphate dehydrogenase (G6PDH) activity determined at retrieval timeJ Assist Reprod Genet2922012153158 10.1007/s10815-011-9625-6
- A.R.MoawadP.FisherJ.ZhuI.ChoiZ.PolgarA.DinnyesIn vitro fertilization of ovine oocytes vitrified by solid surface vitrification at germinal vesicle stageCryobiology6522012139144 10.1016/j.cryobiol.2012.04.008
- Ariu F, Bogliolo L, Pinna A, Malfatti L, Innocenzi P, Falchi L, Bebbere D, Ledda S. Cerium oxide nanoparticles (CeO2 NPs) improve the developmental competence of in vitro-matured prepubertal ovine oocytes. Reprod Fertil Dev. 2017;29(5):1046–1056. doi: https://doi.org/10.1071/RD15521.
- A.ShiraziE.MotaghiThe in vitro fertilization of ovine oocytes in the presence of oviductal cells and its effect on the expression of zygote arrest 1 (zar1) and subsequent embryonic developmentJ Reprod Infertil142013816
- M.NaderiS.BoroujeniA.SarvariB.HeidariM.AkhondiA.ZarnaniThe effect of media supplementation with angiotensin on developmental competence of ovine embryos derived from vitrified-warmed oocytesAvicenna J Med Biotechnol832016139144
- M.G.CatalD.IzquierdoM.Rodriguez-PradoS.HammamiM.T.ParamioEffect of oocyte quality on blastocyst development after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in a sheep modelFertil Steril974201210041008
- Abolfazl.ShiraziMohammad Ansari.ArdaliEbrahim.AhmadiHassan.NazariMorteza.MamueeBanafsheh.HeidariThe Effect of Macromolecule Source and Type of Media During In vitro Maturation of Sheep Oocytes on Subsequent Embryo DevelopmentJ Reprod Infertil13120121319
- Mara1L, Sanna D, Casu S, Dattena M, Mayorga Muñoz IM, 2013. Blastocyst rate of in vitro embryo production in sheep is affected by season. Reprod Dom Anim 49 (Suppl. 4), 37–48 (2014).
- Catalá MG1, Izquierdo D, Rodríguez-Prado M, Hammami S, Paramio MT. Effect of oocyte quality on blastocyst development after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI) in a sheep model. Fertil Steril 2012 Apr; 97(4):1004–8. doi: https://doi.org/10.1016/j.fertnstert.2011.12.043. Epub 2012 Jan 21.
- B.LahozJ.L.AlabartJ.FolchP.SánchezE.EchegoyenM.J.CoceroInfluence of the FecX(R) allele in heterozygous ewes on follicular population and outcomes of IVP and ET using LOPU-derived oocytesReprod Domest Anim4852013 Oct717723 10.1111/rda.12150 Epub 2013 Feb 26
- Ni H, Sheng X, Cui X, Gu M, Liu Y, Qi X, Xing S, Guo Y. Epidermal growth factor-mediated mitogen-activated protein kinase3/1 pathway is conducive to in vitro maturation of sheep oocytes. PLoS One 2015;10(3):e0120418. doi: https://doi.org/10.1371/journal.pone.0120418. eCollection.
- Catalá.María-GraciaRoura.MontserratIzquierdo.DolorsMorato.RoserHammami.SondesParamio.María-TeresaBlastocyst development, MPF activity and ATP content of lamb oocytes supplemented with insulin–transferrin–selenium (ITS) and ascorbic acid at IVMSmall Ruminant Res1121–32013103107
- Romaoa R, Marquesb CC, Baptistab MC, Vasquesb MI, Barbasb JP, Hortab AEM, Carolinob N, Bettencourta dE, Planchac C, Rodriguesc P, Pereira RM. Evaluation of two methods of in vitro production of ovine embryos using fresh or cryopreserved semen. Small Ruminant Res 2013;(110):36–41.
- Forouzanfar M, Fekri Ershad S, Hosseini SM, Hajian M, Ostad-Hosseini S, Abid A, ... Nasr Esfahani MH. Can permeable super oxide dismutase mimetic agents improve the quality of frozen-thawed ram semen? Cryobiology 2013;66(2), 126–130.
- Wei X, Xiaoling Z, Kai M, Rui W, Jing X, Min G, Zhonghong W, Jianhui T, Xinyu Z, Lei A. Characterization and comparative analyses of transcriptomes for in vivo and in vitro produced peri-implantation conceptuses and endometria from sheep. J Reprod Dev 2016; 62(3):279–87. doi: https://doi.org/10.1262/jrd.2015-064.
- S.M.HosseiniV.AsgariS.OstadhosseiniM.HajianH.R.GhanaeiM.H.Nasr-EsfahaniDevelopmental competence of ovine oocytes after vitrification: Differential effects of vitrification steps, embryo production methods, and parental origin of pronucleiTheriogenology8332015366376
- V.GhaffarilalehA.Fouladi-NashtaM.T.ParamioEffect of α-linolenic acid on oocyte maturation and embryo development of prepubertal sheep oocytesTheriogenology8252014686696
- F.AghazH.HajarianH.K.ShabankarehA.AbdolmohammadiEffect of sericin supplementation in maturation medium on cumulus cell expansion, oocyte nuclear maturation, and subsequent embryo development in Sanjabi ewes during the breeding seasonTheriogenology849201516311635
- Rose RD, Gilchrist RB, Kelly JM. Thompson JG, Sutton-McDowall ML, Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology 2013;564(79):142–148.
- Shi JM, Yi JY, Tian XZ, Wang F, Lian ZX, Han HB, Fu JC, Lv WF, Liu GS. Effects of seasonal changes on the ovulation rate and embryo quality in superovulated Black Suffolk ewes. Neuro Endocrinol Lett 2015;36(4):330–6.
- Kochhar HP1, Wu B, Morris LH, Buckrell BC, Pollard JW, Basrur PK, King WA. Maturation status, protein synthesis and developmental competence of oocytes derived from lambs and ewes. Reprod Domest Anim 2002;37(1):19–25.
- D.T.ArmstrongEffects of maternal age on oocyte developmental competenceTheriogenology5562001 Apr 113031322
- Leoni GG1, Succu S, Berlinguer F, Rosati I, Bebbere D, Bogliolo L, Ledda S, Naitana S. Delay on the in vitro kinetic development of prepubertal ovine embryos. Formation Anim Reprod Sci 2006; 92(3–4):373–83.
- A.ShiraziM.A.ArdaliE.AhmadiH.NazariM.MamueeB.HeidariThe Effect of Macromolecule Source and Type of Media During In vitro Maturation of Sheep Oocytes on Subsequent Embryo DevelopmentJ Reprod Infertil1312012 Jan1319
- Sasaki M, Kato Y, Yamada H, Terada S. Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnol Appl Biochem 2005;42:183–188 e.
- C.YasminT.OtoiM.A.SetiadiN.W.K.KarjaMaturation and fertilization of sheep oocytes cultured in serum-free medium containing silk protein sericinActa Vet Hung632015110117
- López-Saucedo J, Santiago-Moreno J, Fierro R, IzquierdoD, Coloma MA, Catalá MG, Jiménez I, Paramio MT. Fertilization capacity of cryopreserved Iberian ibex epididymal sperm in a heterologous in vitro fertilization assay. Zygote 23:136–144. c Cambridge University Press 2013 doi:https://doi.org/10.1017/S0967199413000518).
- C.SchwarzerT.C.EstevesM.J.Arau-BravoS.Le GacV.NordhoffS.SchlattART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responsesHum Reprod272012 2627–536
- E.M.SadeeshN.L.SelokarA.K.BalharaP.S.YadavDifferences in developmental competence and gene expression profiles between buffalo (Bubalus bubalis) preimplantation embryos cultured in three different embryo culture mediaCytotechnology685201619731986
- S.KhoslaW.DeanW.ReikR.FeilCulture of pre-implantation embryos and it long-term effects on gene expression and phenotypeHum Reprod Update742001419427
- Fernández-Gonzalez R, Moreira P, Bilbao A, Jiménez A, Pérez-Crespo M, Ramírez MA, Rodríguez De Fonseca F, Pintado B, Gutiérrez-Adán A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behaviour. Proc Natl Acad Sci USA 2004;101(16):5880–5.
- D.K.GardnerM.LaneA.SpitzerP.A.BattEnhanced rates of cleavage and development for sheep zygotes cultured to the blastocyst stage in vitro in the absence of serum and somatic cells: amino acids, vitamins, and culturing embryos in groups stimulate developmentBiol Reprod5021994 Feb390400
- Xia Wei, Zhang Xiao ling, Miao Kai, Wang Rui, Xu Jing, Guo Min, Wu Zhong Hong, Tian Jian Hui, Zhang Xin Yu, An Lei. Characterization and comparative analyses of transcriptomes for in vivo and in vitro produced peri-implantation conceptuses and endometria from sheep. J Reprod Dev. 2016;62:279–287.
- V.A.Absalón-MedinaW.R.ButlerR.O.GilbertPre-implantation embryo metabolism and culture systems: experience from domestic animals and clinical implicationsJ Assist Reprod Genet3142014 Apr393409
- S.HashimotoN.MinamiR.TakakuraH.ImaiBovine immature oocytes acquire developmental competence during meiotic arrest in vitroBiol Reprod66200216961701
- Aktas H, Wheeler MB, First NL, Leibfried Rutledge ML. Maintenance of meiotic arrest by increasing [cAMP]i may have physiological relevance in bovine oocytes. J Reprod Fertil 1995;105:237–45.
- G.CoticchioM.Dal CantoM.Mignini RenziniM.C.GuglielmoF.BrambillascaD.TurchiOocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganizationHum Reprod Update2142015427454
- S.D.GermanJ.H.LeeK.H.CampbellD.SweetmanR.AlberioActin depolymerization is associated with meiotic acceleration in cycloheximide-treated ovine oocytesBiol Reprod9242015 Apr103
- Letícia Ferrari Crocomo, Carla Maria Vela Ulian, Naiana dava Silva Branchini, Denise Theodoro da Silva, Wolff Camargo Marques Filho, Fernanda da Cruz Landim Alvarenga, Sony Dimas Bicudo. Meiotic arrest of sheep oocytes using roscovitine under different medium compositions Small Ruminant Res. 2015;126:52–58.
- P.LonerganH.KhatirC.CarolanP.MermillodBovine blastocyst production in vitro after inhibition of oocyte meiotic resumption for 24hJ Reprod Fertil1091997355365
- Pascal.MermillodMilan.TomanekRé Jane.MarchalLaurent.MeijerHigh Developmental Competence of Cattle Oocytes Maintained at the Germinal Vesicle Stage for 24 Hours in Culture by Specific Inhibition of MPF Kinase ActivityMol Reprod Dev5520008995
- B.AveryA.Hay-SchmidtP.HyttelT.GreveEmbryo development, oocyte morphology, and kinetics of meiotic maturation in bovine oocytes exposed to 6-dimethylaminopurine prior to in vitro maturationMol Reprod Dev501998334344
- M.A.DodeP.R.AdonaDevelopmental capacity of Bos indicus oocytes after 607 inhibitions of meiotic resuption by 6-dimethylaminopurineAnim Reprod Sci652001171180
- M.BuellJ.L.ChitwoodP.J.RosscAMP modulation during sheep in vitro oocyte maturation delays progression of meiosis without affecting oocyte parthenogenetic developmental competenceAnim Reprod Sci1542015 Mar1624
- R.E.ThomasJ.G.ThompsonD.T.ArmstrongR.B.GilchristEffect of specific phosphodiesterase isoenzyme inhibitors during in vitro maturation of bovine oocytes on meiotic and developmental capacityBiol Reprod71200411421149
- Elisa Melo Ferreira, Alessandra Aparecida Vireque, Paulo Roberto Adona, Rui Alberto Ferriani, Paula Andrea Navarro. Prematuration of bovine oocytes with butyrolactone I reversibly arrests meiosis without increasing meiotic abnormalities after in vitro maturation. Eur J Obst Gynecol Reprod Biol 2009(145):76–80.
- P.R.AdonaC.Lima Verde LealMeiotic inhibition with different cyclin-dependent kinase inhibitors in bovine oocytes and its effects on maturation and embryo developmentZygote122004197204
- P.MermillodM.TomanekR.MarchalL.MeijerHigh developmental competence of cattle oocytes maintained at the germinal vesicle stage for 24 h in culture by specific inhibition of MPF kinase activityMol Reprod Dev5520008995
- Gilchrist RB, Thompson JG. Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007;67(1):6–15. Review.
- M.G.MarquesA.B.NascimentoR.P.GergerJ.S.GonçalvesA.R.CoutinhoR.SimõesEffect of culture media on porcine embryos produced by in vitro fertilization or parthenogenetic activation after oocyte maturation with cycloheximideZygote1942011331337
- K.SaekiY.NagaoM.KishiM.NagaiDevelopmental capacity of bovine oocytes following inhibition of meiotic resumption by cycloheximide or 6 dimethylaminopurineTheriogenology487199711611172
- H.FunahashiT.C.CantleyB.N.DaySynchronization of meiosis in porcine oocytes by exposure to dibutyryl cyclic adenosine monophosphate improves developmental competence following in vitro fertilizationBiol Reprod5719974953
- M.A.BaggM.B.NottleC.G.GrupenD.T.ArmstrongEffect of dibutyryl cAMP on the cAMP content, meiotic progression, and developmental potential of in vitro matured pre-pubertal and adult pig oocytesMol Reprod Dev73200613261332
- D.NogueiraR.CortvrindtD.G.De MatosL.VanhoutteJ.SmitzEffect of 650 phosphodiesterase type 3 inhibitor on developmental competence of immature mouse oocytes in vitroBiol Reprod696200320452052
- A.M.LucianoP.PocarE.MilanesiS.ModinaD.RiegerA.LauriaEffect of different levels of intracellular cAMP on the in vitro maturation of cattle oocytes and their subsequent development following in vitro fertilizationMol Reprod Dev5419998691
- D.NogueiraR.Ron-ElS.FriedlerM.SchachterA.RazielR.CortvrindtMeiotic arrest in vitro by phosphodiesterase 3-inhibitor enhances maturation capacityof human oocytes and allows subsequent embryonic developmentBiol Reprod742006177184
- Zhu.J, Amarnath D, Choi I, Tsunoda Y, Campbell K. Demecolcine can overcome rat oocyte spontaneous activation. J J Vet Sci Res 2015;2(1):1–12.
- I.ChoiJ.H.LeeP.FisherK.H.CampbellCaffeine treatment of ovine cytoplasts 661 regulates gene expression and foetal development of embryos produced by somatic cell nuclear transferMol Reprod Dev77102010876887
- J.H.LeeK.H.CampbellEffects of enucleation and caffeine on maturation- promoting factor (MPF) and mitogen-activated protein kinase (MAPK) activities in ovine oocytes used as recipient cytoplasts for nuclear transferBiol Reprod7442006691698
- S.LeddaL.BoglioloG.LeoniS.NaitanaCell coupling and maturation-promoting factor activity in in vitro-matured prepubertal and adult sheep oocytesBiol Reprod652001247252
- G.J.JiangK.WangD.Q.MiaoL.GuoY.HouH.SchattenProtein profile changes during porcine oocyte aging and effects of caffeine on protein expression patternsPLoS One6122011e28996
- Xia.ZhangXiaoyan.LiuLi.ChenWu.Dan-YaZhe.-Wen.NieYi.-Ying.GaoYi-LiangMiaoCaffeine delays oocyte aging and maintains the quality of aged oocytes safely in mouseOncotarget8132017 Mar 282060220611
- Shin HW Animal Models in CRS and Pathophysiologic Insights Gained: A Systematic Review Laryngoscope Investig Otolaryngol. 2016 Aug 2;1(5):116–123.
- Hill RJ, Mason HM, Yeip G, Merchant SS, Olsen AL, Stott RD, Van Wettere AJ, Bressel E, Mason JB. The Influence of Oblique Angle Forced Exercise in Surgically Destabilized Stifle Joints Is Synergistic with Bone, but Antagonistic with Cartilage in an Ovine Model of Osteoarthritis Arthritis 2017:7481619. doi: https://doi.org/10.1155/2017/7481619. P p1-10.
- Mastbergen SC1, Pollmeier M, Fischer L, Vianen ME, Lafeber FP. The groove model of osteoarthritis applied to the ovine fetlock joint Osteoarthritis. Cartilage 2008;16(8):919–28.
- Zedde P, Cudoni S, Manunta L, Passino ES, Masala G, Brunetti A, Uboldi FM, Manunta AF. Second Generation Needling Techniques for the Treatment of Chondral Defects in Animal Model Joints 2017;5(1):27–33.
- S.SchwanC.LudtkaI.WiesnerA.BaerthelA.FriedmannF.GöhrePercutaneous posterolateral approach for the simulation of a far-lateral disc herniation in an ovine modelEur Spine J2017 Oct 27 10.1007/s00586-017-5362-6
- Daly CD, Ghosh P, Zannettino ACW, Badal T, Shimmon R, Jenkin G, Oehme D, Jain K, Sher I, Vais A, Cohen C, Chandra RV, Goldschlager T. Mesenchymal progenitor cells primed with pentosan polysulfate promote lumbar intervertebral disc regeneration in an ovine model of microdiscectomy Spine J 2017;18. pii: S1529–9430(17)31059–8.
- M.GimenoP.PinczowskiG.MendozaJ.AsínF.J.VázquezE.VispeAntibiotic-eluting orthopedic device to prevent early implant associated infections: efficacy, biocompatibility and biodistribution studies in an ovine modelJ Biomed Mater Res B Appl Biomater2017 Oct 4 10.1002/jbm.b.34009
- A.BrzezinskiS.A.GhodbaneJ.M.PatelB.A.PerryC.J.GattM.G.DunnThe Ovine Model for Meniscus Tissue Engineering: Considerations of Anatomy, Function, Implantation, and EvaluationTissue Eng Part C Methods2017 10.1089/ten.TEC.2017.0192
- Urbankova I, Callewaert G, Sindhwani N, Turri A, Hympanova L, Feola A, Deprest J. Transvaginal Mesh Insertion in the Ovine Model. J Vis Exp 2017(125). doi: https://doi.org/10.3791/55706.
- M.R.PassmoreY.L.FungG.SimonovaS.R.FoleyS.D.DiabK.R.DunsterEvidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation supportCrit Care2112017 Jul 29191 10.1186/s13054-017-1788-9
