Abstract
Trypanosomosis is a major disease of Man and animals. This study investigated the effect of Moringa oleifera leaf extract on the survivability rate, weight gain and histopathological changes of Wister rats experimentally infected with Trypanosoma brucei. A total of thirty (30) rats randomly divided into six groups (A-F). Rats in group A remain untreated and uninfected while rates in group F were infected and untreated. Rats in groups B and C were treated with Moringa oleifera leave extract orally at 200 mg/kg for 14 days pre-infection and the treatment continued in B but not in C. Rats in groups D and E were treated with the extract orally for ninety days at 200 mg/kg (pre-infection) and the treatment continued in D but not in E. The weight changes in all rats were monitored weekly. Rats in B-F groups were infected with 3 × 106 of Trypanosoma brucei per mL of blood. The results showed that all the infected rats died but the treated group survived extra two days when compared with the untreated group. The percentage weight gain of rats in groups B and C was high (23.9% and 21.1%) respectively as against negative control (17.2%). The groups with chronic administration of the extract (D and E) had a lower percentage weight gains (64.3% and 60.3% respectively) when compared with negative control (71.8%). The histopathology results showed that the extract was a potent ameliorative agent that reduced neuronal degeneration and congestion in the brain and the spleen of the infected rats respectively. In conclusion, Moringa Oleifera leave extract has mitigative effects on the pathogenesis of trypanosomosis.
1 Introduction
African trypanosomosis is a major disease that has ravaged the livestock industry in tropical Africa where disease is endemic [Citation1,Citation2]. Trypanosoma congolense, T. vivax and T. brucei remain the major pathogenic tsetse transmitted trypanosome species responsible for the disease in tropical regions of Africa, where the vector (Glossina spp) is prevalent [Citation3]. The disease is an haemoparasitic disease of domestic animals with manifestations such as; severe anaemia, weight loss, reduced productivity, infertility and abortion, with death occurring in some animals during the acute and chronic phase [Citation4].
Drugs of therapy and prophylaxis (Chemotherapy and chemoprophylaxis) are the main methods of controlling trypanosomosis [Citation5]. These methods of control are still the most reliable in spite of problems of drug resistance and toxicity [Citation6] especially since the development of an effective vaccine against the disease has been unsuccessful despite intensive effort in research [Citation2]. Diminazene aceturate is one of the few drugs available for both treatment and prophylaxis of African trypanosomosis in livestock especially in cattle infected with T. congolense, T. vivax and T. brucei [Citation7]. Conventional chemotherapeutic treatment of trypanosomosis is no longer befitting as a result of side effects associated with the drugs and the development of many resistance trypanosome in many part of the world. Research now focuses on new compounds for the treatment of trypanosome infection [Citation8]. One possible source for such affordable treatment lies in the use of natural products which have served as an important source of drugs since ancient times. About half of useful drugs have been derived from natural source [Citation9]. Antiparasitic plant-derived molecules have been used as lead compounds to develop semi-synthetic drugs with better efficacy and safety [Citation10]. Due to the aforementioned reasons and implications this study was aimed at assessing the effect of Moringa oleifera on the survivability rate, weight gain and histopathological changes observed during the course of trypanosomosis.
2 Materials and methods
2.1 Ethics, experimental animals and study design
Ethical approval was sought from Animal Care, Use and Review Ethical Committee (ACUREC), University of Ibadan, Nigeria with approval code number as thus, UI-ACUREC/APP/2016/011
Thirty adult male and female Wistar rats between the ages of 8–12 weeks, weighing from 90 to 105 g were used for this study. The rats were housed in the animal house of the Department of Veterinary Physiology, Biochemistry and Pharmacology, University of Ibadan, Nigeria. The animals were kept in cages under normal environmental temperature (20–22 °C) and fed with standard pelleted feed (Livestock® Lagos, Nigeria) and water given ad libitum. The rats were allowed to acclimatize to the laboratory environment for two weeks before the experiment commenced. Rats were grouped into six groups of five rats each and the extracts were administered through the oral route using oral cannula.
The rats were divided into six groups, each comprising five rats and each group was treated as follows: Group A (Negative controls), treated with distil water (uninfected and untreated). Group B: Moringa oleifera was administered orally at 200 mg/kg daily for 14 days before infection and supplement continued after infection for 16 days. Group C: Moringa oleifera was administered orally at 200 mg/kg daily for 14 days before infection and treatment stopped after infection. Group D: Moringa oleifera was administered (daily) orally at 200 mg/kg for 90 days pre-infection and supplement continued after infection for 16 days. Group E: Moringa oleifera was administered orally at 200 mg/kg daily for 90 days pre-infection and treatment stopped post-infection. Group F: Infected and untreated rats (positive control).
2.2 Plant authentication and preparation
The leaves of Moringa oleifera were procured at Ajibode, Akinyele Local Government area of Oyo State, located in the South Western part of Nigeria, which is about 2 Km from University of Ibadan, Ibadan Nigeria. It was taxonomically identified and authenticated in the herbarium of the Department of the Forest Research Institute of Nigeria (FRIN), with voucher number UIH-10847. The leaves of Moringa oleifera were washed thoroughly, dried in shade and pulverized to powder. The methalonic extraction was done at the Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Science, University of Ibadan using the method of Adeyemi et al. [Citation4]. The extract was concentrated to the final concentration of 7.5% stock solution using rotary evaporator and the yield was diluted in soya oil as a vehicle.
2.3 Moringa oleifera phytochemical screening
Phytochemical screening of methanol extract of Moringa oleifera leaves was subjected to qualitative chemical screening for identification of the various classes of the chemical constituents as previously described by Sofowora [Citation11].
2.4 Trypanosoma stock and inoculation
Trypanosoma brucei used in this study was secured from Nigeria institute for Trypanosomiasis and Onchocerciasis research institute, Vom, Plateau state, Nigeria. The dose of inoculum was judged to be 3x106 of Trypanosoma brucei per mL of blood using the “Rapid Matching method” described by Herbert and Lumsden [Citation12]. The parasites were maintained by serial passage in rats. To infect the rat, 1 ml of blood was collected from heavily infected rat and then melded with 2 ml of normal saline. The mixture was viewed under microscope at X40 magnification. The blood containing the parasites was then injected into rats intraperitoneally
2.5 Weight gain determination
The weights of the rats were monitored from first day and later on weekly basis using automated electronic scale (Sensor Disc Technology, London). To weigh a rat, a round plastic container was placed on the scale and tarred to zero following which the rat was dropped inside the container and subsequently weighed.
2.6 Histological procedures
After the death of the rats, the brain and spleen were extracted from the rats and were adequately treated with 10% formaldehyde (fixation) in order to preserve both the structure and molecular composition [Citation13]. The organs were dehydrated by bathing it successively in rated mixture of ethanol and water (70–100%). The ethanol was replaced with a solvent mixable with the embedding medium. The tissues were penetrated with xylene and it became transparent (clearing). The instilled tissue with xylene was placed in melted paraffin in an oven and was maintained at 58–60 °C (embedding). The heat allowed the solvent to evaporate and the spaces within the tissues became filled with paraffin. The tissue together with its impregnating paraffin hardened following removal from the oven. The sections (5 μm) were then floated on water and transferred to a glass slide and stained with haematoxylin and eosin stains. The slides were viewed under light microscope with magnification X40 [Citation13].
2.7 Data analysis
All data generated were expressed as mean ± SD. The differences between the groups were analyzed by one-way analysis of variance (ANOVA) followed by Dunnet’s post hoc multiple comparison test using Graphpad Prism statistical package, San Diego, Califonia, U.S.A (www.Graphpad.Com). Statistical estimates were made at confidence interval of 95%. Probability Values less or equal to 0.05 (P ≤ 0.05) were considered significant.
3 Results
3.1 Findings of phytochemical analysis
Phytochemical analysis of methanolic extract of Moringa oleifera revealed the presence of flavonoid, tannin, saponin, steroid, phenol carbohydrate and glycoside as stated in .
Table 1 The phytochemical constituents of M. oleifera leaves.
3.2 Survivability rate
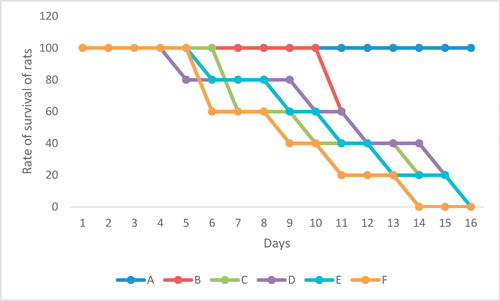
All the rats infected with T. brucei died but the group treated with moringa oleifera leaves died at day 17 when compared with the untreated group (positive control) that died at day 15 as shown in .
3.3 Weight gain
3.3.1 Effect of administration of M.O leaf extract for 14 days before infection
The starting weights are the initial mean weight of the rats in the groups after 2 weeks of acclimatization in the animal house. The mean weights were measured on weekly basis and compared with the control. The weight of rats in group C were found to be statistically significantly higher (P < 0.05) in both first and second week. The percentage weight gain of rats in group B was lower (15.9%) when compared with other groups as stated in .
Table 2 Weight changes (%) in rats dosed with methanolic extract of moringa oleifera leaves for 14 days.
3.3.2 Effect of administration of M.O. leaf extract for 90 days before infection
On the 90th day, the final weight was compared with the controls (groups A and F). The weight of rats in group D were found to be significantly lower (P < 0.05) at day 21, 42, 76 and 83 while the weight of rats in group E were found to be significantly lower (P < 0.05) at day 56, 76 and 83. The rats in groups (D and E) treated with Moringa oleifera leaves had percentage weight gain of 64.3% and 60.3% respectively when compared with the controls (A and F) that had 71.8 and 70.6 respectively ().
Table 3 Weight changes (%) in rats dosed with methanolic extract of moringa oleifera leaves for 90 days.
3.4 Histopathological findings
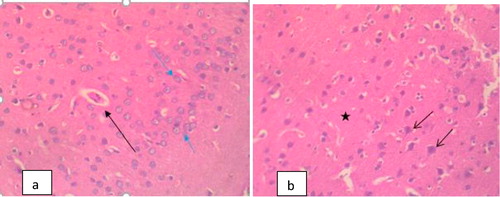
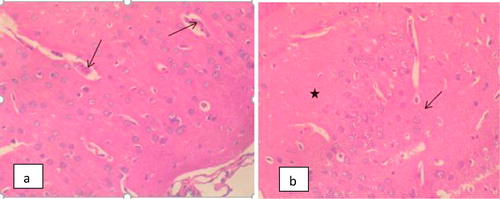
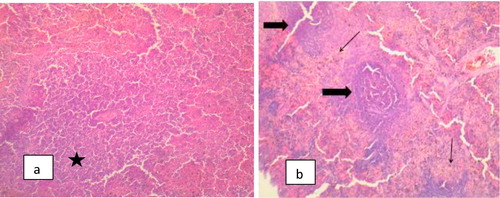
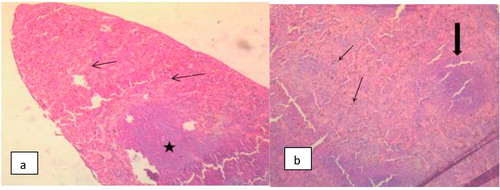
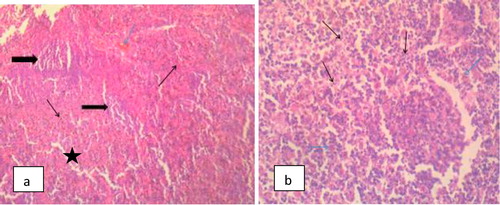
The Photomicrograph of brain in rats (Group F) that are infected and untreated showed multiple foci of neuronal degeneration, marked proliferation of oligodendroglia and neuropil vacuolation, moderate congestion of cerebral blood vessels () while that of rats uninfected and untreated (Group A) appeared normal with no visible lesion (). Photomicrograph of brain in rats treated with M.O for 14 days before trypanosomal infection also appeared normal although, there is moderate congestion of cerebral blood vessels () while that of rats treated with M.O 14 days pre-infection and continued post-infection had no visible lesion (). However, the photomicrograph of brain in rats treated with M.O 90 days pre-infection had few foci of neuronal degeneration and necrosis, moderate swelling of endothelial cells of the cerebral blood vessels as well as congestion of the blood vessels () while that of group treated with M.O for 90 days pre-infection and continued post-infection had multiple foci of neuronal degeneration and marked proliferation of glia cells as well as vacuolation of the neuropil ().
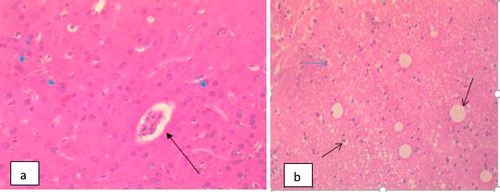
The different histopathologies in the spleen as a result of Trypanosomal infection either with acute or chronic treatment with methanolic extract of M.O pre-infection only or pre and post-infection are shown in –.
4 Discussion
The qualitative phytochemical screening of the methanolic extract of moringa oleifera leaves used in this study indicated the presence of tannins, alkaloid, flavonoid, saponin and glycosides. This is in line with previous work described by other workers that analyze the phytochemical component of moringa oleifera as a medicinal plant [Citation14]
The study revealed that Moringa oleifera leaves increase the survival rate of infected rats for extra two days when compared with the positive control that died at day 15. This showed that methanolic extract of M. oleifera leaves does not possess trypanocidal activities against rats infected with T. brucei as earlier concluded in the work of Atawodi et al. [Citation15] which reported that different parts of M. oleifera possess anti-trypanocidal effect against different species of trypanosome such as T. brucei and T. congolensis.
The outcome of percentage weight gain in this study showed that Moringa oleifera leaves improve the percentage weight gain on acute dosing (14 days) as observed in 23% weight gain when compare with 17% observed in the negative control group. On the other hand, in chronic dosing (90 days) there was a decrease in percentage weight gain (63%) as against 73% seen in the negative control group. This decrease in percentage weight gain may be as a result of presence of tannins which produce anti-nutritive effects thereby reducing feed consumption. This is in accordance with the work of Awotunde et al. [Citation16] that reported that Moringa oleifera reduces feed consumption when used for long term. The Trypanosome brucei DNA is mainly present in the brain of animal at day 6 post-infection and increase more than 15-fold on reaching day 21. Parasites and T-cells are observed in the parenchyma from day 9 onwards. Parasites traversing blood vessel walls may also be seen in the hypothalamus and other brain regions and the body weight gain will significantly reduce from day 7 onwards [Citation17,Citation18].
Trypanosome parasites penetrate the brain leading to vascular degeneration [Citation19]. The histopathological lesions seen in the brain of infected and untreated group are in agreement with Weber [Citation19]. The brain histopathology of the groups with acute administration of Moringa oleifera extracts (groups B and C) had lower neuronal degeneration and the neurons appear normal with no visible lesion especially the group B. The result of this study further emphasized that methanolic extract of Moringa oleifera leaves is a potent ameliorative agent that reduces neuronal degeneration in the brain of rats infected with T. brucei [Citation20,Citation21]. However, the groups with chronic administration of Moringa oleifera extracts (D and E) showed histopathological lesions compared with positive control (Group F). This may be as a result of excessive antioxidant effect of Moringa oleifera extracts that in turned lead to prooxidant effects [Citation22].
Splenomegaly was a prominent feature of infection with both T. brucei and T. congolensis [Citation23]. This is associated with acute or parasitemic phase of trypanosome infection and attributed its cause to red blood cell sequestration and expanded macrophage population [Citation23,Citation24]. The hyperplasia of germinal center and proliferation of macrophages and plasma cells observed in this study are in agreement with Clyton et al. [Citation22] who reported increase in splenic macrophages and the profound effects of trypanosomes on mature splenic lymphocytes. The presence of trypanosomes has been demonstrated to stimulate and cause proliferation of splenic B-lymphocytes [Citation25]. Some of the proliferating B-cells differentiate and secretes large amount of immunoglobulins of diverse specificity [Citation26]. The proliferation of immune competent splenic cells may be responsible for prominent increase size following infection with T. brucei. Treatment using Moringa oleifera gave variable results regarding response of the spleen to treatment. It is however difficult to compare this observation with those of other workers as a result of dearth of data on Moringa oleifera supplementation following trypanocide treatment in trypanosomosis albeit Aremu et al., [Citation23] reported that methanolic extract of M. oleifera potentiates diamenazine aceturate in the treatment of trypanosomosis. The photomicrographs of spleen in this study showed marked congestion of the splenic sinuses and sinusoids suggesting marked splenic depletion. This is as a result of erythrocytes destruction by the trypanosome organisms which is in line with the report of a previous work [Citation26] that showed the pathogenesis of anaemia in T. congolensis infected mice. The congestion in the spleen in M. oleifera treated group was considerably reduced when compared with the positive control group. This result is in line with report of the findings of Shetty and Naryana [Citation27] that compared the histopathology in rats infected with T. brucei.
5 Conclusions
The study revealed that Moringa oleifera supplement for short period of time (1–14 days) improves the weight gain but when administered for long time (90–100 days), the weight will be reduced. The study also deduced that Moringa oleifera supplement reduces tissue and organ damage caused during trypanosomal infection and this is as a result of the potent antioxidant effect of the crude extract. It can also be concluded that M. oleifera improves survivability of rats infected with Trypanosoma spp.
Competing interest
None
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- l.D.B.KinaboL.A.BoganThe pharmacology of isometamidiumJ Vet Pharmacol Ther111988233245
- A.R.GriffinB.J.AllanbyEpidemiology of bovine trypanosomiasis in the Ghibe valley, southwest EthiopiaJ Trop Med531999151163
- V.M.NantulyaTrypanosomosis in domestic animals: problems of diagnosisRev Sci Tech Off Intl Epiz91990357367
- O.S.AdeyemiM.A.AkanjiJ.T.EkanemEthanolic extract of Psidium guajava influences protein and bilirubin level in Trypanosoma brucei infected ratsJ Biol Sci122012111116
- A.C.DolanD.DamperC.L.PattonPentamidine transport in Trypanosoma brucei pharmacokinetics and pharmacodynamic sequenceBlackwill publishers, London.81990400420
- Ross AD, Taylor HA. Standardization of routine observation of blood trypanosomes E.A.T.R.O review of report in East Africa community 1990; pp. 15–16.
- K.I.EghianruwaP.C.G.OdiakaJ.OgunpoluA preliminary comparative efficacy study of isometamedium following oral and intramuscular administration in mice experimentally infected with T. congolenseSah J Vet Sci320043337
- Kibona J, Camara M, Cooper A, Tait A, Jamonneau V, et al. Population genomics reveals the origin and asexual evolution of human infective trypanosomes. eLife 2016;5:14–73.
- G.J.BawnA.CaceresA.SaraviaS.RizzoPharmacologic properties of Moringa oleifera. Screening for antispasmodic, antiinflammatory and diuretic activityJ Ethnopharmacol3632011233237
- A.B.TowsonP.TahilianiA.KarRole of Moringa oleifera leaf extract in the regulation of thyroid hormone status in adult male and female ratsPharmacol Res412001319323
- E.A.SofoworaMedicinal plant and traditional use in African2006Spectrum books LtdIbadan Nigeria134137
- W.J.HerbertW.H.R.LumsdenTrypanosoma brucei: A Rapid, “Matching” method for Estimating the host parasitemiaExp Parasitol401976427431
- Bing QH, Edward CY. Chemical and Physical Fixation of Cells and Tissues: An Overview. In: Yeung ECT, et al. (eds.), Plant Microtechniques and Protocols, Springer International Publishing Switzerland, 2015.
- M.P.BannettiR.J.BurchmoreA.StichThe trypanosomiases in Africa, human African trypanosomiasisNeuropathol Appl Neurobiol1220038194
- S.E.AtawodiT.BulusS.IbrahimD.A.AmehA.J.NokM.MammanIn vitro trypanocidal effect of methanolic extract of some Nigeria savannah plantsAfr J Biotechnol22003317321
- A.J.AwotundeS.IbrahimD.A.AmehIn vitro trypanocidal effects of methanolic extract of some Nigeria savanna plantsAfr J Biotechnol22002217221
- L.ClaudiaP.MariaF.S.PaulR.JeanB.BarbaraM.PaulTrypanosoma brucei Invasion and T-Cell Infiltration of the Brain Parenchyma in Experimental Sleeping Sickness: Timing and Correlation with Functional ChangesPLoS ONE22016317321
- A.R.DiwaoB.D.RoyStudies on binding of Berenil to microsomal protein and its significance with respect to microsomal metabolism of trypanocidal drugIndian J Exp Biol262000464466
- B.C.WeberExtra vascular foci of Trypanosoma vivaxin goats: the central nervous system and aqueous humor of the eye as potential sources of relapseJ. Proteom132009234239
- Zilva AR, Pannall BC. In vivo and in vitro sensitivity of Trvpanosoma evansi and T. equiperdum to diminazene, suramin,: The treatment of T. simiae in pigs with diminazene aceturate in combination with a 5-substituted 2-nitroimidazole compound. Trop Med Par 1990;38:175–76.
- Kaikabo A, Salako E. Effect of dietary vitamin C and E on oral administration to establish free radical roles in the pathogenesis of spleenomegally and hepatomegaly in rats experimentally infected with trypanosome brucei and T. congolensis. Diag Microbiol Inf Dis 2006;61(4):428–33.
- V.ClytonW.MulatuP.A.O.MajiwaS.G.A.LeakG.J.RowlandsE.AuthiEpidemiology of bovine trypanosomiasis in the Ghibe valley, southwest Ethiopia. 3. Occurrence of populations of Trypanosoma congolense resistant to diminazene, isometamidium and homidiumActa Trop531980151163
- A.AremuK.I.EghianruwaK.T.BiobakuA.O.AhmedA.BasiruCrude methanolic extract of Moringa oleifera leaves improves the efficacy of Diminazene Aceturate in the treatment of Trypanosome infected ratsCeylon J Sci46420174353
- C.A.HunterJ.W.GowP.G.E.KennedyF.W.JenningsM.MurrayImmunopathology of experimental African sleeping sickness: detection of cytokine mRNA in the brains of Trypanosoma brucei brucei-infected miceInf Immun59200446364640
- T.M.MartinfH.M.KaluluY.MitsuruO.MayumiK.TarohD.MichaelKola acuminate proanthocyanidins: a class of anti-trypanosomal compounds effective against Trypanosoma bruceiInt J Parasitol35200591103
- A.CosiriniA.SaraviaS.RizzoPharmacologic properties of Moringa oleifera. Screening for antispasmodic, antiinflammatory and diuretic activityJ Ethnopharmacol3631997233237
- B.C.ShettyA.R.NaryanaMolecular analysis of archived blood slides reveals an atypical human Trypanosoma infectionDiag Microb Inf Dis6142007428433
