Abstract
Rabies is a vaccine-preventable fatal disease in man and most mammals. Although rabies is recorded in 150 territories and is responsible for at least 60,000 human deaths every year worldwide, it is a neglected tropical problem. Most of the rabies free countries are considered to be fragile free as the disease may re-emerge easily through wild mammals. For the performance of effective rabies eradication programs, a complex set of strategies and activities is required. At the time, a joint project of WHO–OIE–FAO which was announced in 2015, plans to control animal–human–ecosystems rabies interface. For effective rabies control, prophylactic policies must be applied. These include various educational outreaches for farmers and people living in endemic areas, enforced legislation for responsible dog ownership, control programs for the free-ranging stray dog and cat populations, field large-scale vaccination campaigns, and the development of new vaccine delivery strategies for both humans and animals. The present work presents the advances in the development of new safe, effective and economic vaccines for domestic dogs, and oral vaccines for the control of the disease in wild animals. It presents also some therapeutic protocols used for the treatment of patients.
1 Introduction
Rabies is a zoonotic viral disease infecting all mammals, resulting in the highest fatality rate among all known infectious diseases. The disease is caused by a negative-stranded RNA “bullet” shaped member of the genus Lyssavirus, which is a member of Rhabdoviridae family. Rabies is a vaccine-preventable fatal disease which has almost a case fatality score of 100% in none vaccinated cases [Citation1,Citation2]. The current formal classification of the genus Lyssavirus no longer considers genotypes. The genotypes were upgraded to species to be more in agreement with the taxonomical nomenclature used for higher organisms namely, Rabies lyssavirus, Duvenhage lyssavirus, European bat 1 lyssavirus, European bat 2 lyssavirus, Australian bat lyssavirus, Aravan lyssavirus, Khujand lyssavirus, Irkut lyssavirus, Bokeloh bat lyssavirus, Gannoruwa bat lyssavirus, Taiwan bat lyssavirus, Lagos bat lyssavirus, Mokola lyssavirus, Shimoni bat lyssavirus, West Caucasian bat lyssavirus, Ikoma lyssavirus and Lleida bat lyssavirus. Although, phylogroups are not recognized by ICTV as taxonomical units of classification, rather as an evolutionary and functional sub-classification scheme. Based on such criteria members of the genus are divided into three Phylogroups namely; Phylogroup 1 contains the rabies virus (RABV), Aravan virus (ARAV), Khujand virus (KHUV), European bat lyssavirus type 1 and 2 (EBLV-1, -2), Bokeloh bat lyssavirus (BBLV), Australian bat lyssavirus (ABLV), Irkut virus (IRKV), Duvenhage virus (DUVV) and the two newly recognized Gannoruwa bat lyssavirus (GBLV) and Taiwan bat lyssavirus (TBLV) [Citation3,Citation4]. The phylogroup 1 members seem to have a common ancestor. They are 100% neutralized when rabies-virus-based biologics are used. The second phylogroup contains the African Lyssaviruses. In opposite to the first group, they are not neutralize by rabies virus –based biologics. This group contains the Lagos bat virus (LBV), Shimoni bat virus (SHIBV), and Mokola virus (MOKV). Among phylogroup 2 members, only MOKV was shown to have zoonotic impact. The third phylogroup is represented by the most genetically distant lyssaviruses. It is represented by three members, which have no zoonotic importance, namely, the West Caucasian bat virus (WCBV), Lleida bat virus (LLEBV) and the Ikoma lyssavirus (IKOV) [Citation5,Citation6]. Based on their evolutionary history, members contained within the Rabies lyssavirus species could be divided into two phylogroups bat-related RABVs and dog-related RABVs [Citation7,Citation8].
2 History, distribution and transmission of the disease
The first case of rabies was recorded in 2300 BCE, where Aristotle described the saliva of rabid dog as a venomous. The origin of the word rabies comes from the Latin word ‘rabere: to be mad’ or from the word Rabbahs in old Indian language (Sanskrit) which means (violent) [Citation9–Citation11]. Rabies virus has a wide mammalian reservoir host species from the orders Carnivora and Chiroptera including dog, cat, wild animals as fox, wolves, raccoons, jackal, skunk, coyote, and bats [Citation8]. Infections result mainly from bites of rabied dogs. Rabies virus cannot penetrate intact skin. However, the contact between infected saliva and wounds, mucous membranes or skin abrasions may lead to infections. Aerosol and transplantation transmission were also reported [Citation12–Citation15]. The rabies virus was isolated from the brain and salivary glands of slaughtered dogs to be eaten in South East Asia and some parts of Africa. So that butchers in these countries are at risk [Citation16,Citation17].
Although the humans are considered to be (dead end host), person to person transmission was reported in Ethiopia where the disease was transmitted through direct contact with infected saliva in two patients [Citation5,Citation13]. Additional 15 cases were infected via recipient of transplanted organs or corneas. Transplacental transmission of rabies was also recorded in Turkey. The child was infected inside the uterus before being normally born via the vagina. Vertical transmission is more common in animals than in humans. This may be attributed to the anatomical differences of the placenta between man and animals [Citation18,Citation19].
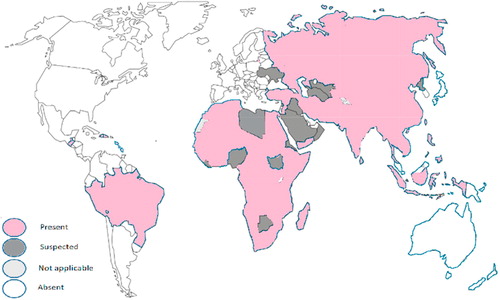
Rabies occurs in more than 150 countries and territories worldwide (). The disease is a neglected tropical problem because it is distributed mainly in the slums of Africa and Asia. Every year at least 60,000 people die from rabies worldwide. Every 15 min, one person dies and 300 people get in risk mostly children under 15 years. The WHO plans to eliminate human rabies mortalities worldwide by the year 2030. To achieve this goal, a joint project of WHO–OIE–FAO was announced in 2015 to control animal–human–ecosystems rabies interface [Citation5,Citation20–Citation22]. It was previously suggested that different forms of rabies infections exist in nature according to the invading virus [Citation23]. While infection with paralytic rabies virus (PRV) results in dog paralysis within 6 days, the convulsive rabies viruses (CRV) induces convulsions in dogs after a longer incubation period [Citation24]. Rabies caused by some abortive rabies strains – in opposite to encephalic strains- may run unnoticed in man and animals without leaving any health abnormalities. The patients even survive without getting intensive medical care. However, in 60% of the cases, they may suffer later from neurological disorders such as limb paralysis [Citation15,Citation25–Citation27]. It was also noticed, that people bitten by a bat have a better chance to survive than those bitten by a rabied dog [Citation28].
The disease is transmitted mainly through dog bites. Blood and blood products do not play any role in disease transmission as the disease is not accompanied with viremia. In about 54% of the cases the incubation period ranges from 1 to 3 months. In 30% of the cases the incubation period may be less than 30 days, and in 15% may last over 3 months. In the rest cases (1%), the incubation period may extend beyond 1 year. However, in exceptional cases it would extend up to 25 years. The length of the incubation period depends upon many factors including; the species of the biting animal, the severity and site of the bite; the virulence of virus strain and the dose of virus inoculated. In addition to other factors related to the bitten animal including previous vaccination and the general immune status [Citation29–Citation34].
In Europe, rabies was almost eradicated from dogs in the 20 century. However, the virus was kept maintained in wild animals there. The rabies virus became adapted to the red fox (Vulpes vulpes) in Russia and east Europe in the 1940 s and spreads west and south words with about 20–60 km per year. Although Europe is almost rabies free continent, new human cases were reported in Europe between 2008 and 2013 The recurrence of rabies in rabies free countries indicates how fragile is the (rabies free Status) as long as the virus is maintained in wild animals [Citation35]. To overcome this problem oral vaccination control programs were applied to eliminate rabies in wild animals. The recent discovery of new rabies virus variants complicates this mission [Citation8,Citation35,Citation36].
3 Advances in rabies prevention and treatment
Prophylactic immunization and treatment of clinical cases are critical components of disease control. However, disease control entails more comprehensive activities and strategies targeting the disease major reservoir hosts and most vulnerable affected populations. Prophylaxis is possible since Pasteur developed his first anti-rabies vaccine in 1885. However, due to the poor immune response of the CNS, no prophylaxis could be achieved once the virus already entered the CNS. The prognosis in such cases is very poor [Citation14]. The mechanism how the rabies causes death is not completely clear. The actual cause of death following rabies infection has not been conclusively established. It is speculated that the massive viral multiplication in the CNS leads to serious complications due to the upregulation of the interferons, cytokines, and chemokines production in the CNS. This leads to the increase chemokine production and intensive immune response in CNS particularly T cells [Citation5]. It was also hypothesized that rabies infection leads to mitochondrial disorders in the nerve cells. Cell death occurs due to the resulting mitochondrial dysfunction and oxidative stress [Citation37].
3.1 Immunization
The virus avoids the stimulation of the immune system before reaching the CNS through its low replication rate in the periphery and by avoiding the induction of viremia [Citation5]. In addition, the virus developed many strategies to kill migratory T cells, to escape to the CNS without causing apoptosis of infected neurons and to maintain the BBB impermeability as will be discussed later [Citation38]. There are pre- and post- viral exposure immunization protocols. Pre-exposure immunization (active immunization, vaccines) are usually given to veterinarians and forest workers while the post-exposure ones are mainly injectable anti-rabies human (HRIG) or equine (ERIG) origin immunoglobulin (passive immunization) usually described in combination with active vaccines (active immunization). Regular booster doses are required to maintain the immunity [Citation14].
3.1.1 Immunoglobulins
Due to the high price and unavailability of large amounts of natural antibodies, possibly blood born infections, and the immunogenicity of the plasma itself, trials were carried out to develop new approaches including the use of monoclonal antibodies or bacterial produced virus-neutralizing nanobodies (VHH) against G protein. The new approaches showed promising results. Due to the narrow scope of the monoclonal antibodies, it is recommended to use a cocktail of them to cover all possible variants. Additional disadvantage of the monoclonal antibodies is their poor tissue penetration capacity and high cost of production [Citation39,Citation40].
3.1.2 Vaccination
Rabies virus is a highly neurotrophic single stranded RNA virus which encodes 5 proteins; namely the nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G) and finally the RNA polymerase (L) [Citation14]. The development of rabies vaccines passed through 4 historical stages (1) the production of neural tissue vaccines, (2) followed by non-neural vaccines, (3) cell culture vaccines and recently (4) rabies recombinant DNA vaccines. During these developmental stages, various living attenuated or inactivated vaccines were tested. In general, the killed vaccines were less efficient as they are poor immunogenic and do not induce strong inflammatory response required for effective T- and B- cell responses [Citation41].
The first known rabies study was done by Georg Gottfried Zinke in 1804. He infected a rabbit by the saliva of a rabied dog. Later on, he immunized a sheep by intravenous injection of the treated saliva, which protected the sheep from later challenge. In 1885, Pasteur developed the first live attenuated rabies vaccine. The vaccine was attenuated in a rabbit where it was harvest from the spinal cord. The vaccine could protect dogs against challenge. It was later applied on a boy whom was bitten by a rabied dog. By the year 1886, Pasteur could treat 350 patients all over Europe [Citation10,Citation42]. However, the vaccine developed by Pasteur contained high concentration of myelin which caused neurological disorders in recipients and lead some times to their death. Other concerns accompanied the vaccine developed by Pasteur, such as resumption of the virulence status of the attenuated viruses and the inability of rabbit based vaccine to meet the large demand from the vaccine [Citation14]. To overcome the resumption of virus virulence, the vaccine was inactivated by phenol. The phenol helped not only to inactivate the virus but also to preserve the vaccine for a longer time and prevents its contamination. In the same time, for mass production of the vaccine, the sheep and goat replaced the rabbits as vaccine producers. Their brains were used in vaccine preparation [Citation43,Citation44]. However, the newly modified Pasteur vaccine after its treatment with phenol and preparation in sheep and goat CNS still had serious side effects such as paralysis and allergic encephalomyelitis. These side effects occurred mainly due to the use of adult mammalian nervous tissue in vaccine preparation or the incomplete inactivation of the virus. To overcome this problem, Fuenzalida and colleagues developed myelin-free killed rabies vaccines prepared in neonatal mouse brains [Citation10]. The members of the first generation of rabies vaccines (vaccines prepared in neural tissues) included: Pasteur vaccine (1885, living attenuated), Hogyes Vaccine (1887, Live attenuated) and Puscariu Vaccine (1895, inactivated vaccine). Later on, brains of baby animals were used instead of those of adult animals as they contain less myelin and therefore have less side effects, such as Suckling Mouse Brain Vaccine (1955, Inactivated), Suckling rat Brain Vaccine (1955, Inactivated) and Suckling Rabbit Brain Vaccine (1955, Inactivated). All these vaccines advocated autoimmune reactions, caused radiculitis and acute inflammatory demyelinating polyradiculoneuropathy in the patients [Citation45–Citation47].
In the second stage, non-neural based rabies vaccines were developed. These included different embryonated (duck or chick) egg inactivated vaccines. These vaccines are simple and economic to produce. They are safer than the first generation vaccines. Several attenuated rabies strains are adopted for use in vaccine production including Pasteur rabies strain, Evelyn Rokitniki Abelseth and Street-Alabama-Dufferin. Additional viruses are also used such as 3aG, Fuenzalida S-51 and S-91, Ni-Ce, SRV9, PM, Nishigahara, RC-HL, Kelev, Flury, “Shelkovo-51”, “O-73 Uz-VGNKI”, “RV-71”, “Krasnopresnenskii-85”, and finally RV-97 isolate. The major disadvantage of these vaccines is the need for large number of doses to induce sufficient protection beside their serious side effects [Citation3]. A new era started with the introduction of tissue culture based vaccines. The first TC rabies vaccine was grown on hamster kidney cells, followed by human diploid cell line (HDCV, inactivated vaccine) and purified chick embryo cells (PCECV, inactivated) in the 1970s. Other commonly used tissue cultures involved in the production of inactivated vaccines include: Rhesus diploid foetal lung T.C., Purified vero cells T.C., Primary hamster kidney T.C., Foetal bovine kidney T.C., and Primary dog kidney T.C. Living attenuated vaccines were also produced using tissue culture cell lines inoculated with certain rabies virus strains usually primarily attenuated through low egg passage (LEP) such as Hamster kidney – LEP flury strain, Vnukovo 32, and porcine kidney – ERA LEP. The tissue culture based vaccines are more economic than previous vaccines, much saver, highly immunogenic and are protective in small doses. However, they are still expensive as the given doses must be repeated for at least 3–4 weeks, and must be stored in refrigerators which is not practical everywhere in Africa and Asia where it is needed. Moreover, they deliver poor immune response in malnourished animals or humans [Citation20,Citation48–Citation52]. The fourth stage in the development of rabies vaccines started with the evolution of DNA recombinant technology. The concept of DNA vaccines is based on the insertion of a viral gene, which encodes an immunogenic protein, in a plasmid. For this purpose, rabies virus glycoproteins (G) are usually used as target of immunization as they are surface-exposed protein [Citation53]. The expression of the plasmid in a eukaryotic expression vector leads to the production of high amounts of viral proteins in an economic way. The genetic manipulation of the inserted gene enables the increase of protein immunogenicity. In addition, the vaccine production in this way protects the laboratory workers, veterinarians and the wild animals from getting in contact with the living pathogen. DNA vaccines, in opposite traditional vaccine, are easy and cheap to produce, requires only few doses and are stable if stored at room temp. The plasmids coated in gold beads can be introduced to the host either intradermally or intramuscularly by the mean of gene-gun. Field trials could not detect anti-DNA antibodies [Citation20]. In addition, the used plasmids can also be constructed to deliver protection against more than one disease, e.g. the plasmid vector (pIRES) encodes antigens of both rabies virus glycoprotein and VP2 protein of canine parvo virus [Citation54]. The resulting immune response can be improved if the vaccine is given with a suitable DNA adjuvant such as plasmids encoding cytokines Tumor Necrosis Factor (TNF) [Citation55].
Later on, the use of plasmids as a carrier for the expression of the rabies virus G protein was replaced by certain viruses such as Vaccinia-recombinant DNA vaccine. Later on, this vaccine was shown to be inefficient in the immunization of skunks which are rabies reservoir animals. In addition to other safety concerns as it is a live attenuated virus. Therefore another canary-pox virus recombinant vaccine was developed. The vaccine is safe and efficient in foxes but less efficient in other species. Other recombinant vaccines were developed later including the human adenovirus type 2 and 5 (AdHu2 and 5) recombinant vaccine, and the Parainfluenza Virus 5. [Citation4,Citation56–Citation58]. In the fact, the pox virus family members are commonly used in the manufacturing of recombinant vaccines as they are easy to be manipulated in the lab. In addition, they have (1) large DNA genome which allows external insertions of up to 30,000 bp of foreign DNA. (2) potent antigenicity so that they can induce both arms of the immune response, (3) members like vaccinia virus can infect a wide range of animal species and finally (4) high thermal stability under normal conditions [Citation59,Citation60].
Another evolutionary step began with the development of orally administrated rabies vaccines. Oral vaccines were developed mainly to enable vaccination of wild animals. Most rabies strains used in the preparation of oral vaccines are derived from the parent strain SAD (Street-Alabama-Dufferin) which was isolated from a rabied stray dog in 1935 in USA. The virus strain was attenuated through passage in chick embryos, baby mice, and many other cell lines. The resulting attenuated strain (recalled ERA; Evelyn Rokitnicki Abelseth) is now used for oral vaccine preparation in USA. Other variants from SAD were used in different countries such as SAD Bern strain used in Switzerland. The SAD Bern was further adapted to baby hamster kidney cell line (BHK21) to produce SAD B19 and SAD 5/88 strains. However, the use of all these variants in the field failed as they could not completely eliminate their pathogenicity [Citation61–Citation63].
Later on, a new modified SAD Bern strain was selected for vaccine production. The selected strain possesses a mutation in position 333 of the viral glycoprotein. The new strain was called SAG1 (SAD Avirulent Gif) which was seen to be avirulent strain but remained immunogenic like the parent strain. As the frequency of mutations in RNA viruses is relatively high, great concerns about the resumption of the viral pathogenicity of SAG1 strains was made. Therefore, another strain with double mutations was selected (SAG2), the strain was seen to be double avirulent mutant and is now in use for oral vaccine preparation in Europe in the form of baits. The baits are made of paraffin cover (to resist water and to give the shape) in which fats (vehicle) and fish aroma (for palatability) are mixed. The commonly used biomarker in the baits is the tetracycline hydrochloride which can be deposit in the teeth and bones of vaccinated animals. The baits are also labeled to warn humans from touching it. If humans come in contact with them, they must receive the standard rabies post exposure prophylaxis vaccines and immunoglobulins. The baits are designed to be water and heat resistant as they are mostly dropped by helicopters [Citation64,Citation65].
Another concept was developed by Cenna 2009, in which two variants of replication-deficient rabies virus were developed. The first variant lacks the matrix (M) gene completely while the second one lacks the phosphoprotein (P) gene. The virus lacking the M gene was shown to offer rapid and better protection than the second variant [Citation66]. Replication deficient variants have the advantage in being safe, unable to multiply inside the cells without affecting its capacity to induce the inflammatory response [Citation41]. The Phosphoprotein (P) gene acts as a non-enzymatic cofactor which regulates the viral polymerase gene (L) responsible for viral replication. The P protein is also an antagonist of IFN-a/β antagonist. It interferes with the phosphorylation of the host IFN regulatory factor 3 (IRF-3) and to consequently inhibit type I IFN induction and prevents IFN-a/β stimulated JAK– STAT signaling usually occurs in rabies virus infected cells. In vaccine variants lacking the P gene, the virus can infect the cell, where the genes will be transcripted, without being able to replicate. As a result, the viruses do not spread from the periphery to the CNS. Although the virus was able to express limited amounts of the G protein, it produced 10-fold more immune response than the killed vaccines. The nature of the produced antibodies (balanced IgG2a/IgG1) was similar to that following natural infection but not that resulting from injection of killed vaccine (Dominance of IgG1) [Citation41,Citation67]. Another variant of the recombinant vaccines is the V-RG vaccine. It is a recombinant vaccinia virus vector live attenuated vaccine in which the rabies virus glycoprotein gene was inserted to replace the non‑essential vaccinia thymidine kinase (TK) gene. The vaccine is genetically stable, safe for more than 50 susceptible species, and disappears from the saliva within 48 h after ingestion. It is not shed from the animal in the environment and is thermally stable. No reversion to virulence could be observed in field trials. It was great safety concerns wither the V-RG vaccine can recombine with other orthopoxviruses in the environment such cow pox virus is endemic in European forests and infects wild rodents. If recombination occurs, the new virus may infect non target species. This assumption was excluded through field studies [Citation60]. In the former Soviet Union, another rabies virus (strain RV-97) was adapted for use in oral vaccination programs. The strain has close relationship to the Japanese vaccine strains. Both G and P genes show several unique mutations which attenuated the isolate without affecting its immunogenicity [Citation4].
Another alternative concept for rabies control in stray dogs through hormone-based vaccine-induced contraception was tested in Latin American countries. Field trials showed promising results. However, the use of immune-contraceptive vaccines needs more investigations about their efficacy, safety and practicality in the field. For the effective mass vaccination programs, the vaccination campaigns must be accompanied with legislation and strong law enforcement of responsible dog ownership [Citation68].
3.1.3 Safety and efficiency of modern vaccines
Some concerns started to occur after massive application of oral vaccine to vaccinate wild animals. Hypersensitivity reactions following the ingestion of the baits were recorded in dogs due to the presence of tetracycline markers. Other adverse effects include gastrointestinal disturbances and behavioural changes as restlessness and listlessness [Citation11].
Typing of the rabies virus using monoclonal antibodies could differentiate among 5 different field types, two of them reacted in a similar pattern as the vaccinal SAD virus group. This could be related to reverted vaccine strains [Citation4]. Therefore, beside their ability to induce protective immune response, the vaccine candidate strains must also be safe, i.e. unable to resume their virulence with time, have minimal side effects, not oncogenic, stable and do not recombine with other viruses in the environment, not shed outside the host and if shed must be none hazardous, be safe for public health, and finally they must have genetic markers to be traced [Citation69]. The safety and efficiency of SAG2 vaccines were evaluated in dogs, cats and other non-target species. Monitoring of 3160 orally vaccinated dogs did not reveal any adverse effects following vaccination. The neutralizing antibodies were released after 14 days of vaccine ingestion. Challenge test of the dogs indicated that all dogs which had neutralizing antibodies in any concentration survived the exposure. No virus or viral antigens could be detected in the saliva or brain of the euthanized dogs. Six hours following oral vaccination of 15 cats, shedding of the vaccinal virus could be detected in the saliva of 3 cats, which disappeared later within 3 days after vaccination. Challenge test with a street canine rabies virus killed 2 out of 7 vaccinated dogs, compared with 5 out of 6 of the negative control group. Safety investigation of SAD B19 could not detect living virus particles in the saliva or organs of 5 out of 6 orally vaccinated dogs, while the virus could be recovered from the intestine of the sixth dog one days after vaccination. In the same time, the virus could be isolated from the tonsils of 7 out of 8 foxes, and from the oral mucosa of 3 out of 8 foxes up to the 4th day. In parallel, 82 wild rodents were also involved in the investigation. During the investigation period, 8 rodents died. Rabies could be diagnosed in 5 of them. None of the non-vaccinated rodents in contact attracted the virus [Citation70]. Both DNA rabies vaccine (DRV) and the combination rabies vaccine (CRV) were shown to be safe even if administrated in 10× of the recommended doses in mice [Citation71].
At the time, plant based vaccines are under development. Trials are carried out to use some plants like tobacco, tomato, spinach, carrot, and maize for mass production of the target recombinant proteins with low cost. They are easy to produce and store. For antigen production, the alfalfa mosaic virus is used to express the rabies antigen protein. The modified virus is allowed to infect tobacco plants. The produced rabies antigen in the plant can be easily purified to be used in animal vaccination. In a similar manner, maize plants were treated to express the G protein of rabies virus. The obtained virus antigen offered 100% protection against challenge with bat rabies strains [Citation4].
A promising linear peptide of 29 amino acid structure derived from rabies glycoprotein protein is used to enable the entrance of rabies virus neutralizing single-chain antibody (ScFv) cross the blood brain barrier (BBB). This peptide has the ability to interact with the nicotinic acetylcholine receptors (nAchR) which enables the entrance of the antibodies in the muscle and nerve cells. A protein was constructed via the fusion of both ScFv and ScFv-29 A.A. peptide. This conjugated protein could be cloned and expressed in Tobacco related plant. The delivered data favor further development of this promising and economic approach to deal with pre- and post- exposures cases [Citation58]. Another carrier was also used to deliver the antibodies cross the BBB; namely the cholera toxin B subunit (CTB). It was used as a potent adjuvant. New generation of transgenic plants and recombinant plant viruses were developed for the production of oral vaccines. To achieve this goal, the alpha mosaic virus (AMV) coat protein is used as a carrier for N and G rabies virus proteins. The proteins are then expressed inside the plants by the tobacco mosaic virus (TMV) [Citation58,Citation70].
One of the main differences among virulent and avirulent rabies strains is the ability of avirulent strains to induce transiently open the BBB. The B cells cannot be observed in the brain during infection with encephalitic rabies strains, so that no neutralizing antibodies can be secreted in the CNS. Mutant strains like Pasteur strain is less pathogenic, however, it causes irreversible paralysis of limbs. The severe immune response in the CNS leads to damage and dysfunction of the CNS which ends with death of the case. The immune cells are sometimes incriminated in the spread of virus by carrying it from poorly innervated sites or infected transplanted organs to highly innervated LN or organs. The microglia cells present the viral antigens to the T cells. In turn, the T cells become activated through the effect of produced cytokines during the migration of the mononuclear cells in the CNS. Following their activation, the T cells begin to secrete IFN γ, which stimulates the microglia to secrete MHC class I and II resulting in the activation of an intracerebral inflammatory reaction. The body tries to control the inflammatory reaction by blocking the cytokines and the MHC I and II using different mediators as the prostaglandins and various INF types. The potent inflammatory reaction starts to cause damage to neurons in the CNS leading to the production of nitric oxide in the CNS. The production of nitric oxides causes neurotoxicity and leads to the accumulation of phagocytic microglia. The process ends with CNS dysfunction with a fatal end [Citation11,Citation38]. The T cells are inefficient in the elimination of the encephalitic strains of rabies which cause limited neuroinflammatory reactions, resulting in the absence of T cells in the CNS. However, T cells play a major role in the protection from infections caused by abortive strains due to their activation in the peripheries. The infected neurons upregulate the immune response which leads to the activation of programmed cell death of the migratory T cells [Citation11,Citation20]. Abortive strains cause high T cell activation in the periphery while the encephalitic strains induce little T cell activations [Citation38]. The IFN is required to allow the infected neurons to produce B7-H1 (which is an IFN-dependent immunoglobulin-like immune suppressive molecule). By so doing, the INF which usually supports the action of the immune system, supports the survival of infected neurons and consequently supports the rabies virus and its spread in the CNS. This may clarify why INF should not be used in the treatment of rabies. The rabies G protein stimulates survival signaling in the infected neurons to ensure their survival and prevents apoptosis [Citation11,Citation20].
3.1.4 Adjuvants
In the veterinary field, many adjuvants are used to improve the immune response against weak antigens. The most commonly used adjuvant is the aluminum hydroxide. However, it induces serious side effects such as prolonged inflammation, severe irritation and probably necrosis at the site of injection, weak cellular immune response, and not effective in the production of antiviral response. Aluminum leads also to the production of homocytotropic antibodies (mainly IgE) which bind to the animal cells from which they originate [Citation72]. Modern alternative adjuvants were developed such as the nanoparticle adjuvants. The nanoparticles increase the immunogenicity of antigens and can trap the antigens or DNA until delivering them to the antigen presenting cells. They may be also coupled to the rabies glycoproteins to target the brain via the BBB. The use of silver green nanoparticles produced by Eucalyptus procera improved the immune response against inactivated rabies virus vaccine [Citation72–Citation74]. A new stable chemical adjuvant was developed which is an analog of a double-stranded RNA under the name (PIKA). PIKA interacts with toll-like receptor 3 (TLR3) responsible for the recognition of foreign antigens by the antigen-presenting cells (APCs) and stimulates the production of pro-inflammatory cytokines. By so doing, PIKA is able to stimulate both humoral and cellular immunity. The use of PIKA in combination of viral vaccines enables the protection of 70–80% of challenged animals, compared to 20–30% if PIKA was not applied [Citation75].
3.2 Treatment
Rabies virus (RABV) is a neurotropic virus that causes fatal disease. Although the course of the disease ends traditionally with the death of the case, many cases could survive the disease even after the occurrence of the clinical signs. This depends upon many factors related to the source of the virus (e.g. viruses delivered via bat bites are less virulent than dog bites), type of the virus (abortive strains are less virulent than encephalitic ones), and factors related to the host as the immune status and previous immunization. After exposure, the wound must be washed with soap, and the patient must receive passive and active immunization. The anti-sera must be injected at the site of bite because the virus persists at the site of inoculation for a time before get attached to the nerve ending. If the clinical signs appear, the patient must be kept in dark and quite place, must be supplied with oxygen, nutrients and energy. Additional treatment protocols are under investigation. They differ completely among different clinics. The administration of both vaccine (active immunization) and rabies immunoglobulins (passive immunization) must start as soon as possible following exposure to close the gap between the introduction of the virus and the production of rabies antibodies by the patient immune system. However, the administration of the immunoglobulin can be saved if the animal was previously vaccinated within a proper time [Citation40].
The absence of neuro-inflammatory reactions and keeping the BBB impermeable are key strategies in the pathogenesis of rabies infection. Since the BBB prevents the passage of antibodies from the peripheral circulation to the CNS, it is useless to treat the clinical patients with anti-rabies antibodies. Trials to induce controlled encephalitis to open the BBB bring the patients in high risk [Citation39]. Many therapeutical protocols were developed in the last decades through combining the immunization of the patients and the administration of anti-viral drugs or ketamine HCl. Brain cooling (Hypothermia) and the induction of drug-induced (therapeutic) coma were also recommended by some researchers. The induction of therapeutic coma aims to reduce the damage of neural cells due to the excessive stimulation caused by neurotransmitters. One of the most famous protocols is the Milwaukee protocol. This protocol was first tried on a 15 years old girl who survived the bat‘s bite with definite neurological sequelae [Citation5,Citation28,Citation39].The Milwaukee protocol involves the induction of coma, optimized oxygen supply and RBCs transfusion. Additional medication including ketamine, midazolam, heparin, the antiviral drug ribavirin, Amantadine, and high doses of benzodiazepines with supplemental barbiturates were given. No rabies vaccine or immunoglobulins were administrated in this protocol. The immune system of the girl developed its own antibodies over the time. However, this protocol failed to save the lives of other patients worldwide. The survival of the girl may also be attributed to the low virulence of the bat originating rabies viruses. The detailed protocol is described in Willoughby et al. [Citation26].
The administration of high doses of ketamine HCl aims to inhibit viral transcription. The admission of the synthetic antiviral preparation amantadine inhibits viral replication inside the cells without exerting any effect on viral adhesion or penetration of the virus [Citation37]. There is insufficient evidence to support the continued use of ketamine or amantadine for the therapy of rabies. Also the minocycline or corticosteroids should not be used in rabies therapy because of concerns about aggravating the disease. Steroids suppress the immune system and close the BBB leading to the reduction of antibodies and drugs entry to the brain. As a result, the patients die after a shorter incubation period. A variety of new antiviral agents are under development and evaluation, including favipiravir, RNA interference [Citation26,Citation37]. The use of therapeutic hypothermia by cooling the head and neck was also tested in order to reduce metabolism, inflammatory responses and other physiological activities in the brain [Citation37]. Meanwhile, the induction of the therapeutic coma aims to offer enough time for the immune system to produce anti rabies antibodies. However, as the viral phosphoprotein antagonizes the production of any intra-cerebral immune response, this approach for treatment failed to help patients to survive [Citation26,Citation32,Citation76–Citation79]. The use of Milkwaukee protocol could save the life of only 5 out of 44 patients. The rest of the patients died due to neurological immune reconstitution syndrome caused by the discontinuation of supplying potentially immunosuppressive drugs. The Milkwaukee protocol is no more recommended for the treatment of rabies [Citation37,Citation39]. Supplementation with antiviral drugs, which can cross the BBB, is important to prevent further spread of the virus in the CNS. There are many mechanisms used by the antiviral drugs to cross the BBB as the association with endogenous carriers able to cross BBB. One of the most commonly used drugs is the Ribavirin which induces mutation in the viral genome and prevents its replication [Citation80]. Most of recent protocols depend on the use of Ribavirin and interferon (IFN)-α for the treatment of rabies. Ribavirin is a RNA mutagen broad spectrum antiviral drug. It stimulates the innate immune response in the CNS and has, in addition, pro-inflammatory effect. While the interferon IFN-α reduces the virus spread from the site of entrance to the spinal cord. The administration of exogenous INF or INF inducers inhibits viral replication in experiments done with tissue culture and animal model. The use of new antiviral preparations such as Favipiravir is under investigation [Citation26,Citation37]. However, Ribavirin and interferon-α showed disappointing results when applied for the treatment of rabies in many clinics [Citation37]. The administration of interferon and interferon inducers is debated. Interferons are immunomodulatory substances which have an antiviral effect and capable of modifying the immune response in the CNS [Citation81].
A new approach for the treatment of rabies was successfully developed through molecular engineering of rabies antibodies to offer a protective level of passive immunotherapy. The new antibody in made of a fused molecule of a trivalent single chain Fv specific for binding rabies virus glycoprotein with the bacteriophage T4 trimerization domain of the fibritin (foldon) to enable its production in bacterial cells [Citation82–Citation84].
Shivasharanappa and his team studied the effect of the induction of TLR-3 receptors and their associated cytokines (IL1-α, TNF-α, and IFN-α) in the treatment of clinical cases of rabies. The aim of the study was to investigate the effect of giving exogenous cytokines to influence the endogenous cytokines. This approach of treatment could mild the symptoms, delay the death and increase the number of survivals [Citation85,Citation86]. The development of NanoLuc luciferase (NanoLuc) molecules enabled screening of intracellular molecules which can inhibit RABV infection. The NanoLuc molecules are small luminescent proteins (19 kDa), which are very stable and ATP independent. The development of recombinant RABV-NanoLuc viruses enables the exact screening of the intracellular changes following RABV infection, which in turn, enables us from developing new tools to inhibit RABV multiplication and spread [Citation87]. Another receptor subunit peptides (nicotinic acetylcholine receptor alpha 1; nAChR a 1) play also a major role in the attachment of the RABV to the neurons. Blocking the receptors, which are usually present at the neuromuscular junction, results in the inhibition of viral replication and exhibits therefore an anti-viral activity [Citation88,Citation89]. Other unconventional approaches were also developed to treat rabies such as switching off the viral genes expression through the use of silencing (siRNA) preparations. siRNA targeting the rabies gene N were much more efficient than those targeting the gene L. Different artificial microRNAs (amiRNAs) are now under development. Multiple amiRNAs against various genetic variants which can be given in one shot were also developed. However, the presence of unknown genetic variations among wild type isolates limits the use of this approach. Kumar et al. developed a new assay to deliver the siRNA directly to the CNS with the aid of a small peptide of 29 A.A. This peptide is a part of the rabies G protein which binds to the nicotinic acetylcholine receptors of the CNS and by so doing, it enables the passage of the siRNA across the BBB [Citation90]. Another promising approach for the treatment of rabies is under investigation. The concept is based on the blockage of the viral exist from infected cells by the interference with the Nedd4-PPxY interaction and PPxY-dependent budding [Citation37].
Finally, rabies is the most fatal known infectious disease worldwide. Although the disease was neglected due to many reasons, the international community gave attention to the disease in the last years. The international health organizations (WHO, OIE and FAO) work together hand in hand to eradicate the disease by the year 2030. They adapted different strategies to achieve this goal mainly through intensive vaccination for domestic and wild carnivore. New generation rabies vaccines provide safe, economic and highly efficient alternatives to conventional vaccines. Edible vaccines (plant based vaccines) show promising results despite technological obstacles [Citation91,Citation92].
4 Conclusions
Rabies is one of the most serious but neglected tropical diseases. The present review presented the international distribution of rabies at the time, various routes of transmission, and the advances in its prevention and therapeutic concepts. New rabies vaccines were developed based on the developing recombinant DNA technology and Nano-technology. The newly developed vaccines are safer, more economic and enable efficient rabies control among both domestic and wild animals compared to the conventional ones. By so doing, the rabies free countries can easily maintain their fragile status as rabies free. The developed assays support the huge running projects since 2015 aiming to completely eradicate rabies in the year 2030.
Competing of interest
The author declares no competing interests.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- A.DeviatkinA.LukashevRecombination in the rabies virus and other lyssavirusesInfect Genet Evol60201897102
- D.PunguyireA.Osei-TutuE.AleserT.LetsaLevel and pattern of human rabies and dog bites in Techiman Municipality in the Middle Belt of Ghana: a six year retrospective records reviewPan Afr Med J282017281
- N.A.CeballosS.V.MorónJ.M.BercianoNovel Lyssavirus in bat, SpainEmerg Infect Dis192013793795
- A.MetlinL.PaulinS.SuomalainenE.NeuvonenS.RybakovV.MikhalishinCharacterization of Russian rabies virus vaccine strain RV-97Virus Res1322008242247
- A.FooksA.BanyardD.HortonN.JohnsonL.McElhinneyA.JacksonCurrent status of rabies and prospects for eliminationLancet384201413891399
- G.AmarasingheN.Aréchiga-CeballosA.BanyardC.BaslerS.BavariA.BennettTaxonomy of the order Mononegavirales: update 2018Arch Virol2018 doi.https://doi.org/org/10.1007/s00705-018-3814-x
- C.TroupinL.DacheuxM.TanguyC.SabetaH.BlancCh.BouchierLarge-Scale Phylogenomic Analysis reveals the complex evolutionary history of rabies virus in multiple carnivore hosts. Parrish C, edPLoS Pathog122016e1006041
- A.Velasco-VillaM.R.MauldinM.ShiL.E.EscobarN.F.Gallardo-RomeroI.DamonThe history of rabies in the Western HemisphereAntiviral Res1462017221232
- Blaisdell J. A frightful, but not necessarily fatal, madness: rabies in eighteenth-century England and English North America [PhD Thesis]. USA: Iowa State University; 1995. http://lib.dr.iastate.edu/rtd/11041.
- Y.WangSh.GuoResearch progress of rabies vaccineJ Appl Virol122012
- R.SinghK.SinghS.CherianM.SaminathanS.KapoorR.ManjunathaRabies – epidemiology, pathogenesis, public health concerns and advances in diagnosis and control: a comprehensive reviewVet Q372017212251
- J.BronnertH.WildeV.TepsumethanonB.LumlertdachaTh.HemachudhaOrgan transplantations and rabies transmissionJ Trav Med142007177180
- G.GongalaS.MudhusudanabM.SudarshancB.MahendradTh.HemachudhaeH.WildeeWhat is the risk of rabies transmission from patients to health care staff?Asian Biomed62012937939
- D.HicksA.FooksN.JohnsonDevelopments in rabies vaccinesClin Exp Immunol1692012199204
- CDC Report 2017. Wildlife reservoirs for rabies. Downloaded at https://www.cdc.gov/rabies/exposure/animals/wildlife_reservoirs.html.
- P.MshelbwalaA.OgunkoyaB.MaikaiDetection of rabies antigen in the saliva and brains of apparently healthy dogs slaughtered for human consumption and its public health implications in Abia State, NigeriaISRN Vet Sci2013 468043
- H.WertheimT.NguyenK.NguyenM.de JongW.TaylorT.LeFurious rabies after an atypical exposurePLoS Med620093
- U.SipahioğluS.AlpautTransplacental rabies in humansMikrobiyol Bul1919859599
- Ch.AguèmonA.TarantolaE.ZoumènouS.GoyetP.AssoutoS.LyRabies transmission risks during peripartum–Two cases and a review of the literatureVaccine34201617521757
- P.UllasA.DesaiSh.MadhusudanaRabies DNA vaccines: current Status and future worldJ Vacc220123645
- FAO Report 2017. FAO, OIE and WHO enlarge their collaboration commitment to face health challenges downloaded at: http://www.fao.org/Ag/againfo/home/en/news_archive/2017_%20Tripartite_strategic_document.html.
- WHO Report 2017. Towards a rabies-free world as unparalleled global initiative gets underway downloaded at: http://www.who.int/neglected_diseases/news/WRD_2017_%20Press_release/en/.
- S.GribenchaAbortive rabies in rabbits and white rats infected intracerebrallyArch Virol491975317322
- L.GribanovaS.GribenchaG.Mal'kovI.BarinskiĭBiological properties of variants of the rabies street virusVopr Virusol331988201206
- A.GalelliL.BaloulM.LafonAbortive rabies virus central nervous infection is controlled by T lymphocyte local recruitment and induction of apoptosisJ Neurovirol62000359372
- R.WilloughbyK.TievesG.HoffmanN.GhanayemC.Amlie-LefondM.SchwabeSurvival after treatment of rabies with induction of comaN Engl J Med352200525082514
- B.TianM.ZhouY.YangL.YuZ.LuoD.TianLab-attenuated rabies virus causes abortive infection and induces cytokine expression in astrocytes by activating mitochondrial antiviral-signaling protein signaling pathwayFront Immunol820182011
- A.JacksonCurrent and future approaches to the therapy of human rabiesAntiviral Res9920136167
- G.IurasogRabies with incubation of 19 years and 6 monthsMicrobiol111966543
- A.GardnerAn unusual case of rabiesLancet21970523
- G.BaerThe natural history of rabies2nd ed.2000CRC PressBoston USA 9780849367601
- N.JohnsonA.FooksColl K.McRe-examination of human rabies case with long incubation, AustraliaEmerg Infect Dis14200819501951
- N.JohnsonK.MansfieldNunez A.HicksD.HealyS.BrookesInflammatory responses in the nervous system of mice infected with a street isolate of rabies virusDev Biol13120086572
- S.ShankarA.MahadevanS.SapicoM.GhodkirekarR.PintoS.MadhusudanaRabies viral encephalitis with probable 25 year incubation period!Ann Indian Acad Neurol152012221223
- A.Velasco-VillaS.A.ReederL.A.OrciariP.A.YagerR.FrankaJ.D.BlantonEnzootic rabies elimination from dogs and reemergence in wild terrestrial carnivores, United StatesEmerg Infect Dis14122008 Dec18491854 10.3201/eid1412.080876
- Y.LiuS.ZhangX.WuJ.ZhaoY.HouF.ZhangFerret badger rabies origin and its revisited importance as potential source of rabies transmission in Southeast ChinaBMC Infect Dis2010
- C.AppolinarioA.JacksonAntiviral therapy for human rabiesAntivir Ther202015110
- M.LafonEvasive strategies in rabies virus infectionAdv Virus Res7920113353
- Terryn S. Development and evaluation of antiviral immunoglobulin single variable domains for prophylaxis of rabies in mice [PhD Thesis]. Belgium: Ghent University; 2016a. Available at http://users.ugent.be/~ddmeulen/WWW/resources/Thesis-Sanne-Terryn.pdf.
- S.TerrynA.FrancartH.RommelaereC.StortelersS.Van GuchtPost-exposure Treatment with anti-rabies VHH and vaccine significantly improves protection of mice from lethal rabies infectionPLoS Negl Trop Dis1020168
- J.McGettiganExperimental rabies vaccines for humansExpert Rev Vaccine9201011771186
- R.RappuoliInner Workings: 1885, the first rabies vaccination in humansPNAS111201412273
- C.FermiÜber die Immunisierung gegen Wutkrankheit. (Immunisation against rabies)Z Hyg Infectionskrankh581908233276
- D.SempleThe preparation of a safe and efficient antirabic vaccineSci Mem Med Sanit Dept India441911131
- H.KoprowskiH.CoxStudies on chick embryo adapted rabies virus: I. Culture characteristics and pathogenicityJ Immunol601948533554
- E.FuenzalidaR.PalaciosJ.M.BorgonoAnti-rabies antibody responses in man to vaccine made from infected suckling-mouse brainsBull WHO301964431436
- M.TulluS.RodriguesM.MuranjanS.BavdekarJ.KamatP.HiraNeurological complications of rabies vaccinesIndian Pediat402003150154
- R.KisslingGrowth of rabies virus in non-nervous tissueProc Soc Exp Biol Med981958223225
- R.KisslingD.ReeseAnti-rabies vaccine of tissue culture originJ Immunol911963362368
- T.WiktorM.FernandesH.KoprowskiCultivation of rabies virus in human diploid cell strain WI-38J Immunol931964353366
- A.KondoGrowth characteristics of rabies virus in primary chicken embryo cellsVirol271965199204
- A.FlamandP.CoulonF.LafayC.TuffereauAvirulent mutants of rabies virus and their use as live vaccineTrends Microbiol11993317320
- C.JalletY.JacobC.BahloulA.DringsE.DesmézièresN.TordoChimeric lyssavirus glycoproteins with increased immunological potentialJ Virol731999225233
- S.PatialV.ChaturvediA.RaiM.SainiR.ChandraY.SainiVirus neutralizing antibody Response in mice and dogs with a bicistronic DNA vaccine encoding rabies virus glycoprotein and canine Parvovirus VP2Vaccine25200740204028
- A.PintoA.Reyes-SandovalH.ErtlChe- mokines and TRANCE as genetic adjuvants for a DNA vaccine to rabies virusCell Immun2242003106113
- Z.ChenM.ZhouX.GaoG.ZhangG.RenC.GnanaduraiA novel rabies vaccine based on a recombinant parainfluenza virus 5 expressing rabies virus glycoproteinJ Virol87201329862993
- D.YangH.KimK.LeeJ.SongThe present and future of rabies vaccine in animalsClin Exp Vaccine Res220131925
- W.PhoolcharoenC.PrehaudC.van DolleweerdL.BothA.da CostaM.LafonEnhanced transport of plant-produced rabies single-chain antibody-RVG peptide fusion protein across an in cellulo blood–brain barrier devicePlant Biotech15201713311339
- R.AmannJ.RohdeU.WulleD.ConleeR.RaueO.MartinonA new rabies vaccine based on a recombinant Orf virus (Parapoxvirus) expressing the rabies virus glycoproteinJ Virol87201316181630
- J.MakiA.GuiotM.AubertB.BrochierF.CliquetC.HanlonOral vaccination of wildlife using a vaccinia–rabies-glycoprotein recombinant virus vaccine (RABORAL V-RG®): a global reviewVet Res48201757
- M.ArtoisC.GuittreI.ThomasH.LebloisB.BrochierJ.BarratPotential pathogenicity for rodents of vaccines intended for oral vaccination against rabies: a comparisonVaccine101992524528
- A.VosA.NeubertO.AylanP.SchusterE.PommereningT.MullerAn update on safety studies of SAD B19 rabies virus vaccine in target and non-target speciesEpidemiol Infect1231999165175
- P.HostnikE.Picard-MeyerD.RihtaricI.ToplakF.CliquetVaccine-induced rabies in a red fox (Vulpes vulpes): isolation of vaccine virus in brain tissue and salivary glandsJ Wildl Dis502014397401
- F.CliquetM.AubertElimination of terrestrial rabies in Western European countriesA.SchudelM.LombardControl of infectious animal diseases by vaccination2004KargerBasel185204
- Ph.MählF.CliquetA.GuiotE.NiinE.FournialsN.Saint-JeanTwenty year experience of the oral rabies vaccine SAG2 in wildlife: a global reviewVet Res45201477
- J.CennaM.HunterG.TanA.PapaneriE.RibkaM.SchnellReplication-deficient rabies virus–based vaccines are safe and immunogenic in mice and nonhuman primatesJ Infect Dis15200912511260
- T.MasataniM.OzawaK.YamadaN.ItoM.HorieA.MatsuuContribution of the interaction between the rabies virus P protein and I-kappa B kinase ∊ to the inhibition of type I IFN induction signallingJ Gen Virol972016316326
- A.Velasco-VillaL.EscobarA.SanchezA.Gallardo-RomeroF.Vargas-PinoV.Gutierrez-CedilloEmerson successful strategies implemented towards the elimination of canine rabies in the western hemisphereAntivir Res1432017112
- Meslin F. Oral vaccination of dogs against rabies. WHO Report, 2007. Downloaded at: http://www.who.int/rabies/resources/guidelines%20for%20oral%20vaccination%20of%20dogs%20against%20rabies_with%20cover.pdf.
- WHO Report 1998: Field application of oral rabies vaccines for dogs: WHO/EA4C/ZDI/98. I5 Distr.: General original: English Report of a WHO Consultation organized in collaboration with the Office International des Epizooties (OIE). downloaded at: http://www.who.int/rabies/en/Field_application_for_oral_rabies_vaccines_for_dogs.pdf.
- P.KumarB.KumarV.AnnapurnaT.KrishnaS.KalyanasundaramP.SureshNonclinical toxicology study of recombinant-plasmid DNA anti-rabies vaccinesVaccine5200627902798
- V.AsgaryA.ShoariF.Baghbani-AraniS.ShandizM.KhosravyA.JananiGreen synthesis and evaluation of silver nanoparticles as adjuvant in rabies veterinary vaccineInt J Nanomed11201635973605
- Y.LiuR.HuangL.HanW.KeK.ShaoL.YeBrain-targeting gene delivery and cellular internalization mechanisms for modified rabies virus glycoprotein RVG29 nanoparticlesBiomat25200941954202
- M.MasseriniNanoparticles for brain drug deliveryISRN Biochem2013 238428
- Y.ZhangSh.ZhangW.LiY.HuJ.ZhaoF.LiuA novel rabies vaccine based-on toll-like receptor 3 (TLR3) agonist PIKA adjuvant exhibiting excellent safety and efficacy in animal studiesVirol4892016165172
- C.McKimmieN.JohnsonA.FooksJ.FazakerleyViruses selectively upregulate Toll-like receptors in the central nervous systemBiochem Biophys Res Commun3362005925933
- Z.WangL.SarmentoY.WangX.LiV.DhingraT.TseggaiAttenuated rabies virus activities, while pathogenic rabies virus evades the host innate immune responses in the central nervous systemJ Virol7920051255412565
- K.BrzozkaS.FinkeK.ConzelmannInhibition of interferon signaling by rabies virus phosphoprotein P: activation-dependent binding of STAT1 and STAT2J Virol80200626752683
- R.McDermidL.SacingerB.LeeJ.JohnstoneR.GibneyM.JohnsonHuman rabies encephalitis following bat exposure: failure of therapeutic comaCMAJ1782008557561
- C.HuangZ.LiY.HuangG.ZhangM.ZhouQ.ChaiEnhancement of blood-brain barrier permeability is required for intravenously administered virus neutralizing antibodies to clear an established rabies virus infection from the brain and prevent the development of rabies in miceAntivir Res1102014132141
- D.SilinO.LyubomskaF.ErshovV.VFrolovG.KutsynaSynthetic and natural immunomodulators acting as interferon inducersCurr Pharml Des15200912381247
- H.LiangG.HuX.XueL.LiX.ZhengY.GaoSelection of an aptamer against rabies virus: a new class of molecules with antiviral activityVirus Res184C2014713
- I.TurkiA.HammamiH.KharmachiM.MousliEngineering of a recombinant trivalent single-chain variable fragment antibody directed against rabies virus glycoprotein G with improved neutralizing potencyMol Immunol5720146673
- X.WuT.SmithR.FrankaM.WangW.CarsonC.RupprechtThe feasibility of rabies virus-vectored immunocon- traception in a mouse modelTrials Vaccinol320141118
- N.ShivasharanappaR.SinghK.SinghR.SinghB.MadhuRabies virus induces expression of TLR-3 and its associated cytokines in Swiss-albino miceJ Vet Sci Photon1152014384394
- S.MehtaS.RoyS.MukherjeeN.YadavN.PatelA.ChowdharyExogenous interferon prolongs survival of rabies infected miceVir Dis262015163169
- N.BouteP.LoweS.BergerM.MalissardA.RobertM.TesarNanoLuc Luciferase – A multifunctional tool for high throughput antibody screeningFron Pharmacol7201627
- P.AninditaM.SasakiH.NoboriA.SatoM.CarrN.ItoGeneration of recombinant rabies viruses encoding NanoLuc luciferase for antiviral activity assaysVirus Res2152016121128
- B.SajjanarS.SaxenaD.BishtA.SinghG.Manjunatha-ReddyR.SinghEffect of nicotinic acetylcholine receptor alpha 1 (nAChR a 1) peptides on rabies virus infection in neuronal cellsNeuropeptides5720165964
- P.KumarH.WuJ.McBrideK.JungM.KimB.DavidsonTransvascular delivery of small interfering RNA to the central nervous systemNature520073943
- E.LaereA.LingY.WongR.KohM.LilaS.HusseinPlant-based vaccines: production and challengesJ Botany2016111 ID 4928637
- M.ŁuckaT.KowalczykJ.SzemrajT.SakowiczPlants as an alternative source of therapeutic proteinsPostepy Hig Med Dosw222015362373
