Abstract
The efficacy of Vernonia amygdalina against chemical toxicity has attracted attention. The aim of this study was to evaluate the protective potentials of Vernonia amygdalina methanol extract (BLME) against petroleum toxicity. Thirty six male albino rats (Rattus norvegicus) were redistributed randomly into six groups of six rats each and fed with growers feed for a period of 30 days according to the following description: Group A = Feed; Group B = Feed + 100 mg kg−1 body weight of BLME; Group C = Feed + 200 mg kg−1 body weight of BLME; Group D = Feed (100 g Feed + 4 mL crude petroleum); Group E = Feed (100 g Feed + 4 mL crude petroleum) + 100 mg kg−1 body weight of BLME; Group F = Feed (100 g Feed + 4 mL crude petroleum) + 200 mg kg−1 body weight of BLME. Animals were sacrificed at the end of the experimental period and the serum and kidney were harvested for biochemical and histological analysis following standard procedures. The data generated were subjected to analysis of variance (ANOVA). The study revealed that crude petroleum stimulated alterations in kidney dysfunction makers: urea, creatinine and serum electrolytes which were significantly (P < 0.05) ameliorated by BLME administration relative to control. Oxidative stress markers, lipid peroxidation and enzymatic and non-enzymatic antioxidant profiles (MDA levels, GSH, Vitamin C. FRAP, CAT, SOD, GPx, GSTs) as well as oxidase enzymes (AO, SO, MO and XO) induced changes by crude petroleum were positively modulated by BLME administration. The study concluded that crude petroleum contaminated diets are injurious to animal health and BLME is able to prevent the renal dysfunction induced by crude petroleum contaminated diets.
Introduction
Crude petroleum is an unrefined petroleum hydrocarbon mixture which has from simple to complex structures such as resins, asphaltenes and others. Chemically, it is composed of hydrogen, carbon, sulphur, nitrogen, oxygen and metals [Citation1]. Polar compounds contained in crude petroleum contain heteroatoms of oxygen, nitrogen or sulphur and are ascertained by so many names, including heterocyclics, resins and NSO3 [Citation2]. Heterocyclic compounds of crude petroleum may be composed of metals in salts of carboxylic acids form or distinctively as porphyrin chelates or organo-metal complexes [Citation2,Citation3]. When it is refined, its contents entail different fractions which are majorly used as fuels. The nearly unavoidable importance of these fractions is what makes them in constant and daily contact with human such as petrol, diesel and lubricating oils for powering automobiles, kerosene for cooking, and heavy gas oils for tarring roads [Citation1]. Over the years, the increased trend in the domestic and industrial utilization of crude petroleum and its products has necessitated its concomitant exposure of humans and other animals to its high level risk [Citation4]. In certain cases, these products such as kerosene, diesel, and gasoline find itself into the food chain and gradually build up its negative effects in the tissues especially as it relates to nephrotoxicity [Citation5–Citation8].
In recent times, plants and plants-based materials are being tested as possible antidotes for crude petroleum toxicity in animals [Citation9,Citation10]. One important plant commonly grown in the tropical regions of the world with nutritional and health giving properties is bitter leaf (Vernonia amygdalina) [Citation11]. The medicinal properties of Vernonia amygdalina are highly documented by Ijeh and Ejike [Citation11]. It possesses anti-diabetic property, anthelmintic activities; antioxidant properties; hypolipidemic and anticancer activity [Citation11]. Also, the cathartic effect; abortifacient; antifertility; antimicrobial; antiplatelet and anticoagulant; antimalarial; hepatoprotective; analgesic activity; anti-inflammatory; anti-pyretic activity; antimutagenicity and effect on CD4+ cell count (HIV/AIDS) were also reported [Citation11]. Moreover, the safety of bitter leaf had been established through sole administration as well as administration in the presence of toxicants [Citation12–Citation14].
It is well established that crude petroleum and its fractions exposure has been severally implicated in their ability to alter the functionality of the kidney via the mechanistic increase in the concentrations of serum electrolytes, urea and creatinine and alteration in oxidative stress status [Citation1,Citation15,Citation16]. At the time of this investigation however, there was no documented evidence on the use of bitter leaf to prevent the renal damage induced by crude petroleum contaminated diets. The aim of this study was to evaluate the role of bitter leaf (Vernonia amygdalina) methanolic extract in prevention of renal toxicity induced by crude petroleum contaminated diet.
Materials and methods
.1 Materials
Matured bitter leaf (Vernonia amygdalina Del) was harvested from a farm at Abraka, Nigeria and was identified by Dr. Erhenhi A.H of Department of Botany, Delta state University, Abraka, Nigeria. The credentials of the leaf were corroborated at the Forestry Research Institute of Nigeria, Jericho Hill, Ibadan, Nigeria, where a specimen with the voucher number, F101863 was deposited at the herbarium. Thirty six male albino rats (Rattus norvegicus) were obtained from the animal house of the Faculty of Basic Medical Sciences; Delta State University, Nigeria. The rats were housed in a wooden cage and left to acclimatize for one week and were fed with grower’s mash throughout the acclimatization period. The grower’s mash used is a product of Rainbow Feed Limited and the composition as declared by the manufacturer is depicted in . All other reagents used for biochemical assay were of analytical grades.
able 1 Nutritional composition of growers mash used.
.2 Experimental design and treatment
After the period of acclimatization the rats were weighed and an average weight of 150 g–182 g was obtained. This followed the distribution of the animals randomly into six groups of six rats each and fed with growers feed for a period of 30 days according to the following description.
Group A = Feed
Group B = Feed + 100 mg kg−1 body weight of BLME
Group C = Feed + 200 mg kg−1 body weight of BLME
Group D = Feed (100 g Feed + 4 mL crude petroleum)
Group E = Feed (100 g Feed + 4 mL crude petroleum) + 100 mg kg−1 body weight of BLME
Group F = Feed (100 g Feed + 4 mL crude petroleum) + 200 mg kg−1 body weight of BLME
.3 Preparation of bitter leaves extract
The bitter leaves were washed and air dried at an open space within the laboratory confinement of the Department of Biochemistry, Delta State University, Abraka at room temperature for one week. At the end of the drying period, the bitter leaf was chopped off and macerated using a warren blender to a smooth dry powder and the extract of the bitter leaf obtained using methanol extraction technique as previously described by Okpoghono et al. [Citation10]. One hundred (100 g) of the powdered leaf was dissolved in 400 mL of methanol through sonication for 10 min, then filtered with Whatman No.1 using vacuum pump. The extract was then concentrated using rotary evaporator at 40–50 °C under reduced pressure to obtain the bitter leaf methanol extract (BLME). The extract was stored at −8°C until required.
.4 Administration of bitter leaves extract
The bitter leaf extract used for administration was freshly prepared at the point of administration by dissolving 20 g of the extract in 100 mL of distilled water to obtain 200 mg mL−1 out of which aliquots of the freshly dissolved extract was administered by gavage according to the rats body weight once daily. Rats in group A had no bitter leaf extract treatment. Rats in group B and C were fed with normal diet as those in group A, but were simultaneously treated with 100 mg kg−1 body weight of BLME and 200 mg kg−1 body weight of BLME; respectively. While rats in group D had crude petroleum contaminated diet without any treatment, whereas rats in groups E and F were fed with crude petroleum contaminated diet along with 100 mg kg−1 body weight of BLME and 200 mg kg−1 body weight of BLME mg of bitter leaf extract. All the treatments lasted for 30 days to allow for chronic exposure. These doses had been established to be clinically tolerable by experimental rats [Citation12].The rats in groups A and D were not administered the extracts while all animals were allowed free access to water. National research council guide for the care and use of laboratory animals was adhered to during the experiment [Citation17].
.5 Sample collection
At the end of the treatment period of 30 days, the animals were sacrificed by cervical decapitation on the 31st day after an overnight fast. Blood samples were collected immediately by cardiac puncture into sterile plain tubes and labeled. The blood samples collected were spurn using centrifuge at 3200g for 15 min and the serum collected for various biochemical assays. The kidneys also were harvested into labeled containers under cold conditions. Kidney wet tissue measuring 0.5g was homogenized in 9.0 mL of normal saline using pre-chilled mortar and pestle and the supernatant obtained was stored in the refrigerator and used for biochemical analysis following standard procedures within 48 h.
.6 Biochemical analysis
Determination of serum kidney function profiles were done using commercial Teco diagnostic kits for potassium ion, sodium ion, calcium ion, chloride ion, bicarbonate ion and creatinine (Cr) while Randox diagnostic kit was used for the determination of urea. The following standard methods were employed for the assay of biochemical indices. Lipid peroxidation (MDA) was determined according to the method of Gutteridge and Wilkins [Citation18], in a reaction based on the reaction of malondialdehyde (MDA) with thiobabituric acid (TBA) to form a MDA – TBA adduct that absorbs light strongly at 532 nm. Aldehyde oxidase activity was determined by the method of Johns [Citation19] in a reaction based on the oxidation of benzaldehyde to benzoate using 2,6-dichloroindolephenol (DCIP) as the electron acceptor. The activity of the enzyme is given in units per gramme tissue and one unit is the amount of enzyme that produces one micromole of benzoate per minute. Sulphite oxidase activity was determined by the method of Macleod et al. [Citation20] based on the oxidation of sulphite to sulphate by the enzyme using ferricyanide as electron acceptor. The activity of the enzyme was expressed in units per gramme tissue and one unit represents the amount of the enzyme that reduces one micromole of ferricyanide per minute. Monoamine oxidase activity was determined by the method of McEwen [Citation21] based on the oxidative deamination of benzylamine to benzaldehyde. The activity of the enzyme is expressed in units per gramme tissue and one unit of the enzyme is defined as the amount of enzyme that is required for the production of one micromole of benzaldehyde per minute. The activity of xanthine oxidase was determined by the method of Stirpe and Della Corte [Citation22] using xanthine as the substrate and oxygen as electron acceptor. The enzyme activity was expressed as units per gramme tissue where each unit is the amount of the enzyme that produces one micromole of uric acid. Ferric-reducing antioxidant power (FRAP) assay was based on the formation of an intense blue colour complex formed when ferric tripyridyltriazine complex is reduced to the ferrous form giving a chromophore that absorbs maximally at 540 nm [Citation23].The method of Ellman [Citation24] was used for the assay of reduced glutathione while assay for vitamin C employed the method reported by Achuba [Citation25] based on the use of 2,6-dichlorophenol-indophenol (DCIP) and iodine as a titrant. Superoxide dismutase (SOD) activity was determined based on its ability to inhibit the oxidation of epinephrine by superoxide anion. One unit of superoxide dismutase activity is calculated as the amount of enzyme required for 50% inhibition of the oxidation of epinephrine to adrenochrome at 480 nm per min [Citation26]. Catalase activity was determined by the method of Rani et al. [Citation27] based on the fact that catalase breaks down hydrogen peroxide to give oxygen that oxidizes potassium dichromate and the activity was expressed in terms of moles of H2O2 consumed/min. The oxidation of chromate gives a chromophore that absorbs maximally at 610 nm. Glutathione-s-transferase activity was assayed spectrophotometrically at 340 nm by measuring the rate of l-chloro-2, 4-dinitrobenzene conjugation with reduced glutathione as a function of time according to the established method of Habig [Citation28]. The activity of glutathione peroxidase was determined based on the reduction of hydrogen peroxide to the corresponding stable alcohol and water using glutathione as the reducing reagent. The activity was expressed in terms of μmol of glutathione utilized/minute/mg protein [Citation29].
.7 Histological examination
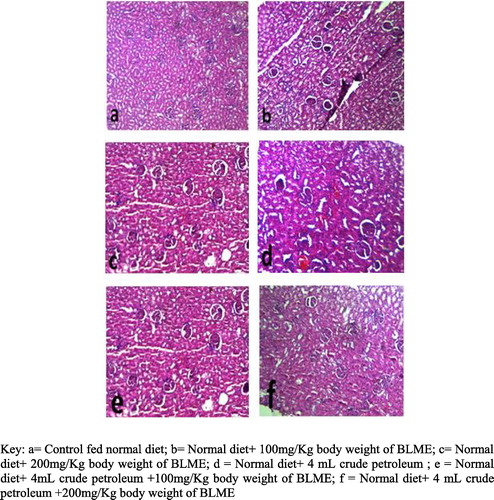
A known portion of the kidney, of each rat was harvested and fixed in 10% formol saline for 48 h and processed for paraffin wax embedding with an automatic tissue processor by dehydrating through 70%, 90%, 95% and two changes of absolute ethanol for 90 min each. Clearing was achieved through two changes of xylene for 2 h each; and infiltrating with two changes of paraffin wax for 2 h. Sections were cut at 5 μm with a rotary microtome. The sections were stained by haematoxylin and eosin (H and E) using the method of Al-Attar et al. [Citation30], examined and photographed using a light microscope.
.8 Statistical analysis
Analysis of data was carried out using the single Factor analysis of Variance (ANOVA) with the aid of the Statistical Package for the Social Sciences version 17 (SPSS 17). Post hoc analysis (comparisons across Groups) was done using Bonferroni at P < 0.05 level of significance.
Results
As shown in (), results showed that treatment of rats with100 mg kg−1 and 200 mg kg−1 body weight of BLME caused a significant (P < 0.05) reduction in serum creatinine and compared to normal control rats in group A and rats fed crude petroleum contaminated diets without treatment (group D). Rats fed with crude petroleum contaminated diets and treated with 100 mg kg−1 and 200 mg kg−1 body weight of BLME in groups E and F showed significant decrease in creatinine concentration compared to rats maintained on crude petroleum contaminated diet without any treatment (group D). However, administration of BLME did not significantly (P < 0.05) alter urea, sodium ion, potassium ion, calcium ion. chloride ion and bicarbonate ion levels in rats fed with crude petroleum contaminated diet without BLME treatment (group D).
able 2 Effect of bitter leaf extract on serum markers of kidney function of rats fed crude petroleum contaminated diet.
The administration of BLME to rats fed with crude petroleum contaminated diet did not reduce lipid peroxidation in the kidney of rats (). However, BLME administration enhanced the activities of the oxidase enzymes (AO, SO MO and XO) significantly (P < 0.05) across all groups compared to rats in control group (group A) and rats fed with crude petroleum treated diet only (group D).
able 3 Effect of bitter Leaf extract on lipid peroxidation and oxidative enzyme profile of rats fed crude petroleum contaminated diet.
Treatment of rats with various doses of BLME did not alter the level of glutathione but significantly improved the levels of the other non-enzymatic antioxidants in the kidney of rats fed crude petroleum contaminated diet (). The ferric reducing antioxidant power (FRAP) and vitamin C in rats fed contaminated diets and treated with 100 mg kg−1 and 200 mg kg−1 (groups E and F) were significantly (P < 0.05) higher compared to the untreated rats (group D). The increase in the levels of these antioxidants was more pronounced at 200 mg kg−1 body weight treatment.
able 4 Effect of bitter leaf extract on levels of non-enzymatic antioxidant profile in the kidney of rats fed crude petroleum contaminated diet.
Results presented in shows the consequence of BLME treatment on the activities of the enzymatic antioxidants: SOD, CAT, GSTs and GPx in rats fed with crude petroleum adulterated diet. The inclusion of crude petroleum in diet caused significant (P < 0.05) reductions in the activities of the enzymes in rats fed with adulterated diet without BLME treatment (group D) compared to the activity in control rats (group A) that were given untainted diets. However, treatment with 100 mg kg−1 and 200 mg kg−1 body weight of BLME (groups E and F) improved the activities of the enzymes compared to the activities in normal control (group A). Moreover, administration of 200 mg kg−1 body weight of BLME enhanced the enzyme activities more than 100 mg kg−1 body weight of BLME
able 5 Effects of bitter leaf on enzymatic antioxidant profile in the kidney of rat fed crude petroleum contaminated diet.
The preventive attributes of BLME on the histopathological impact of crude petroleum contaminated diets in the kidney of rats are presented in . There were very clear and visible glomeruli renal tubules and renal arteries in all groups. However, there were observed significantly visible distortions of kidney architecture signaled by early stage of tissue necrosis and inflammation in the kidney of rats in group C which were fed uncontaminated diets but treated with 200 mg kg−1 body weight of BLME. Also rats fed contaminated diets without treatment showed more visible stages of tissue necrosis and blood coagulations while rats in group E had slight visible signs of necrosis, the rats in group F were observed to have a normal kidney without necrosis indicating possible prevention of the necrosis observed in other groups.
Discussion
The deleterious effects of crude petroleum to the kidney had been well elucidated by earlier studies [Citation8,Citation13]. Likewise, the distortion of metabolic stability through the consumption of petroleum tainted diets has been reported by Okpoghono et al. [Citation10].This study reports similar dysfunction of the kidney owing to intake of crude petroleum tainted diets evidenced by rising serum creatinine and urea in rats not treated with any dose of the BLME but fed crude petroleum tainted diets (). Rising serum creatinine and urea is an established indicator of poor glomerular filtration and has been established as a significant clinical marker for kidney dysfunction and loss of kidney integrity [Citation8,Citation31]. The present study, although observed no significant rise in serum sodium ion concentrations, there were significant increases in both serum potassium ion concentration and calcium ion concentration owing to consumption of petroleum tainted diets (). These results are in agreement with the studies of Orisakwe et al. [Citation15] and Uboh et al. [Citation16]. The observed high levels of calcium and potassium ions have been reported to be linked with the disruption of certain ion pumps and transmembrane ATPases owing to increased lyses within the kidney and the liver [Citation32,Citation33]. The study also observed a reduction of chloride ion concentration in rats owing to crude petroleum contamination of the diets relative to the control was not in agreement with those reported in the study of Ita and Edagha [Citation34] which reported increased serum chloride ions due to oral consumption of crude petroleum. Although the treatment of rats fed tainted diets indicated observed reduction in the serum calcium, potassium and chloride ion concentration relative to those not treated, it is indicative that the BLME extract had some level of efficacy in balancing the observed distortion in electrolyte derangement.
A significant observation of this study was the ability of the BLME at both doses to reduced serum electrolyte concentrations (Na+, K+, Ca+ and HCO3−) relative to the normal control which implies that the BLME may have the capability of improving glomerular filtration even in the absence of toxicants (). This observation, however is not in agreement with studies done with other plant extracts such as Aframomum sceptrum and Aframomum malagueta which showed elevation of serum and renal electrolytes (Na+, K+ and HCO3−) relative to control [Citation31,Citation35]. The renal improvement potential possessed by the BLME in the absence of any toxicant and in the presence of the toxicological effects of crude petroleum tainted diets may have been conferred on the BLME due to its high antioxidant defence capacities as reported by Ijeh and Ejike [Citation11]. They are said to have a rich potential for antioxidants such as flavonoids, alkaloids and polyphenols.
Tissue lipid peroxidation has been identified as one significant marker of oxidative stress status of any organism that has been associated with the deleterious effects of petroleum contamination in plants and animals [Citation36,Citation37]. The process of lipid peroxidation, often times preceded by disruption of the natural antioxidant defence, which in several cases are signalled by the depletion of both enzymatic and non-enzymatic antioxidants [Citation38]. Increase in lipid peroxidation is closely linked with the induction of cytosolic enzymes such as the oxidases (sulphite, aldehyde, xanthine and monoamine) needed for the clearance of increasing sulfoxides, N-oxides, and aromatic nitro compounds and 1,2-benzisoxazole derivatives; that are associated with environmental toxicants [Citation39]. In this study, the administration of BLME to rats fed with crude petroleum tainted diet gave rise to increased lipid peroxidation and the concomitant increase in the activities of oxidative enzymes: sulphite oxidase, aldehyde oxidase, monoamine oxidase and xanthine oxidase relative to control (). More so, reduction in lipid peroxidation at a dose of 100 mg kg−1 body weight of BLME and concomitant increase in the activities of oxidative enzymes across the two doses () were observed. This may be due to the preventive effects of BLME against crude petroleum nephrotoxicity. Although the administration of BLME at the 200 mg kg−1 dosage to rats fed tainted diet indicated increased MDA level relative to rats not treated with BLME, this may be indicative of the negative effects of high dose of plant extracts which was submitted by Ogbeke et al. [Citation31] that when plant extracts are administered in the right dose it will remain effective in conferring protection to tissues in situations of metabolic stress but when administered in the wrong dosage however, it ends up in the continued induction of metabolic stress. The positive effects of BLME observed at the 100 mg Kg−1 body weight on petroleum tainted diets are similar to those conferred on petroleum induced nephropathy in the study of Achuba and Ogwumu [Citation7]. The BLME which is said to be a rich source of catechin and several polyphenols and essential mineral elements and remains significant in the enhancement of vascular nitric oxides (NO) activity which is significant for the mechanistic control of oxidative stress and several signal transduction pathways [Citation40].
As regards antioxidant defence, this study indicated a significant (P < 0.05) increase in FRAP in rats fed with crude petroleum tainted diets and treated with BLME (). Also, significant (P < 0.05) depletion in GSH relative to normal control and rats not exposed to tainted diets but treated with BLME was also observed (). Increased FRAP level is a significant indicator of an improved antioxidant capacity and the presence of elements that have the ability to neutralize the negative effects of increasing peroxyl and alkoxyl radicals which constitute significant reducing agents and oxygen quenchers [Citation41]. GSH on the other hand is an important antioxidant in the scavenging of the reactive oxygen species and peroxides [Citation42,Citation43]. Reduced glutathione (GSH) was significantly depleted in the tissues of rats exposed to crude petroleum tainted diets relative to control but increased relative to the rats fed tainted diets without treatment. This observed trend is in agreement with those reported by Azeez et al. [Citation1], Alisi et al. [Citation44] and Odewabi et al. [Citation45] who used animal models as well as gasoline station attendants exposed to petroleum fumes in their studies. An insight to the justification of increased GSH levels in treated rats relative to untreated rats has been earlier reported in the study of Huang et al. [Citation46] who stated that there is an increase in the levels of tissue glutathione during early stages of tissue regeneration. It is important to note that matured bitter leaf has been reported to have a level of glutamic acid, one of the amino acids essential for the synthesis of glutathione [Citation47,Citation48]. GSH has also been identified as a significant factor in the maintenance of the concentrations of exogenous antioxidants such as vitamins C and E in their reduced (active) forms. This may be a justification for the observed increase in the concentrations of vitamin C in the kidney of rats fed tainted diets and treated with BLME relative to those not treated after been exposed to tainted diets
The results of the enzymatic antioxidants indicated a significant reduction in the activities of SOD, CAT, GSTs and GPx in untreated rats relative to control, however, the eventual enhancement of these enzymes’ activities by BLME in the tissues of rats exposed to petroleum tainted diets and untainted diets relative to the untreated rats gives credence to the antioxidative properties possessed by the bitter leaf () and its capabilities to enhance the enzymatic antioxidant defence mechanistic activities as reported by Iwalokun et al.[Citation49]. Also this study recorded the ability of BLME to confer protection on the kidney ultra-structure due to the negative effects of petroleum tainted diets. The consumption of petroleum tainted diets has been previously reported to induce tissue injury by Achuba and Ogwumu [Citation7]; and Okpoghono et al. [Citation10]. In line with their studies which utilized palm oil and Monodora myristica, the administration of BLME was able to enhance the gradual healing and clearance of the observed tissue necrosis and inflammation in the kidney. This observation is also given credence by the wound healing properties of BLME earlier reported by Kambizi and Afolayin [Citation50]. As presented by the histopathological examination (), rats treated with both doses of BLME were observed to show visible regeneration and clearance of the observed tissue necrosis in rats not treated with BLME
Conclusions
This study indicated that the consumption of crude petroleum contaminated diets induced kidney damage and consequent malfunction. However, the administration of bitter leaf extract offered defence against the induced negative effects of crude petroleum and ameliorated and restored the lost renal function capabilities by conferring protection on tissue ultra-structure.
Competing interests
There is no competing interest to declare
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- O.M.AzeezR.E.AkhigbeC.N.AnigboguOxidative status in rat kidney exposed to petroleum hydrocarbonsJ. Nat Sci Biol Med42013149154 10.4103/0976-9668.107280
- Achuba FI, Peretiomo – Clarke BO, Okolie TC. Oxidative stress in the brain of rabbits with petroleum- induced hypoglycaemia. Biol Lett 2005;42:33-9.
- R.AlmedaZ.WambaughZ.WangC.HyattZ.LiuE.J.BuskeyInteractions between zooplankton and crude oil: Toxic effects and bioaccumulation of polycyclic aromatic hydrocarbonsPLoS One862013 e67212 10.1371/journal.pone.0067212
- F.E.UbohS.U.UfotE.U.EyongComparative effect of withdrawal from exposure on gasoline and diesel induced nephrotoxicity in male albino wistar ratsJ Clin Toxicol32013170 10.4172/2161-0495.1000170
- S.D.RamachandranP.V.HodsonC.W.KhanK.LeeOil dispersant increases PAH uptake by fish exposed to crude oilEcotoxicol Environ Safety592004300308
- R.AlmedaZ.WambaughC.ChaiZ.WangZ.LiuE.J.BuskeyEffects of crude oil exposure on bioaccumulation of polycyclic aromatic hydrocarbons and survival of adult and larval stages of gelatinous zooplanktonPLoS One82013 e74476 10.1371/journal.pone.0074476
- F.I.AchubaM.D.OgwumuPossible protective role of palm oil and beef liver on the kidney and liver of wistar albino rats fed diesel contaminated dietBiokemistri262014124129
- F.I.AchubaC.C.NwokogbaEffects of honey supplementation on hydrocarbon-induced kidney and liver damage in wistar albino ratsBiokemistri2720155055
- F.I.AchubaL.A.UboguB.O.EkuteMoringa oleifera attenuates crude oil contaminated diet induced biochemical effects in wistar albino ratsUK J Pharm Biosci420167077 10.20510/ukjpb/4/i5/126494
- J.OkpoghonoF.I.AchubaB.O.GeorgeProtective effect of Monodora myristica extracts on crude petroleum oil contaminated catfish (Clarias gariepinus) diet in ratsInt J Vet Sci Med62018117122 10.1016/j.ijvsm.2018.03.006
- I.I.IjehC.E.C.C.EjikeCurrent perspectives on the medicinal potentials of Vernonia amygdalina DelJ Med Plants Res5201110511061
- H.E.KadiriProtective effect of Vernonia amygdalina (bitter leaf) extract on rats exposed to cyanide poisoningBiokemistri292017126131
- M.S.AbebeG.GebruToxic effect of Vernonia amygdalina Delile on blood parameters and histopathology of liver and kidney in ratsGlobal Med Plants Res12015001008
- O.LolodiG.E.EriyamremuEffect of Methanolic extract of Vernonia amygdalina (Common Bitter Leaf) on lipid peroxidation and antioxidant enzymes in rats exposed to cycasinPak J Biol Sci162013642646 10.3923/pjbs.2013.642.646
- E.OrisakweA.A.NjanO.J.AfonneD.D.AkumkaV.N.OrishO.O.UdemezueInvestigation into the nephrotoxicity of Nigerian bonny light crude oil in albino ratsInt J Environ Res Public Health12004106110
- F.E.UbohM.I.AkpanabiatuJ.I.NdemY.AlozieP.E.EbongComparative nephrotoxic effect associated with exposure to diesel and gasoline vapours in ratsJ Toxicol Environ Health Sci.120096874
- NRC (National Research Council)Guide for the care and use of laboratory animals8th ed.2011Institute of Laboratory Animal Resources, National Academy Press
- J.M.C.GutteridgeC.WilkinsCopper dependent hydroxyl radical damage to ascorbic acid formation of thiobarbituric acid reactive productsFEBS Lett1371982327340
- Johns DG.Humanliver aldehyde oxidase: differential inhibition of oxidation of charged and uncharged substratesJ Clin Invest46l196714921505
- R.M.MacleodW.FarkasI.FridovichP.HandlerPurification and properties of hepatic sulphite oxidaseJ Biol Chem236196118411846
- C.M.McEwenMonoamine oxidase (human serum or plasma)S.P.ColowickN.O.KaplanMethods in Enzymology1971Academic PressNew York692693
- F.StirpeE.Della CorteRegulation of rat liver xanthine oxidase. Conversion in vitro of the enzyme activity from dehydrogenase (type D) to oxidase (type O)J Biol Chem244196938553863
- N.O.SoniAntioxidant assay in vivo and vitroInt J Phytopharmacol520145158
- G.C.EllmanTissue sulflydryl groupsArch. Biochem. Biophys.8219597077
- F.I.AchubaAfrican land snail Achatina marginatus, as bioindicator of environmental pollutionNorth West J Zool41200815
- H.P.MisraI.FridovichThe role of superoxide ion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutaseJ Biol Chem247197231703175
- P.RaniU.K.MeenaJ.KarthikeyanEvaluation of antioxidant properties of berriesIndian J Clin Biochem191994103110
- W.H.HabigM.J.PabstW.B.JakobyGlutathione -s-transferases: first enzymic step in mercapturic acid formationJ Biol Chem249197471307139
- M.R.KhanW.RizviR.A.KhanS.SheenCarbon tetrachloride induced nephrotoxicity in rats: protective role of Digera muricataJ Ethnopharmacol12220099199
- A.M.Al-AttarA.A.AlrobaiD.A.AlmalkiProtective effect of olive and juniper leaves extracts on nephrotoxicity induced by thioacetamide in male mice SaudiJ Biol Sci2420171522 10.1016/j.sjbs.2015.08.013
- G.I.OgbekeB.O.GeorgeP.C.Ichipi-IfukorAframomum sceptrum modulation of renal function in monosodium glutamate (MSG) induced toxicityUK J Pharm Biosci420165460
- S.O.ItaE.O.AlukoD.E.AtangA.B.AntaiE.E.OsimVitamin C or E supplementation ameliorates Nigerian Bonny light crude oil induced erythrocytes haemolysis in male wistar ratsBiochem. Mol. Biol120134451 10.12966/bmb.10.01.2013
- S.GowdaP.B.DesaiS.S.KulkarniV.V.HullA.A.K.MathS.N.VernekarMarkers of renal function testsN Am J Med Sci.22010170173
- S.O.ItaI.A.EdaghaRenal protective effect of antioxidant vitamins C and E against crude oil -induced nephrotoxicityMerit Res J Med Med Sci42016425431
- A.UnuebhoU.InegbeneborEffect of aqueous extract of alligator pepper (Aframomum malegueta) on serum electrolytesInt J Herbs Pharmacol Res4201526
- L.A.NwaoguG.O.C.OnyezeEffect of chronic exposure to petroleum pollution on oxidative stress parameters and histology of liver tissues of native fowl (Gallus domesticus)Int J Biochem Res Rev42014233242
- F.I.AchubaM.O.Ja-anniEffect of abattoir waste water on metabolic and antioxidant profiles of cowpea seedlings grown in crude oil contaminated soilInt J Recycl Organic Waste Agric720185966
- A.AhmadM.M.HossainU.SinghalN.IslamComparative study of marker of oxidative stress among normotensive, pre-hypertensive and hypertensive subjectsBiomed Res242013491495
- R.S.KadamK.R.IyerIsolation of liver aldehyde oxidase containing fractions from different animals and determination of kinetic parameters for benzaldehydeIndian J. Pharm Sci7020088588
- Y.CurinR.AndriantsitohainaPolyphenols as potential therapeutical agents against cardiovascular diseasesPharmacol Rep57Suppl200597107
- B.BirasurenN.Y.KimH.L.JeonM.R.KimEvaluation of the antioxidant capacity and phenolic content of Agriophyllum pungens seed extracts from MongoliaPreventive Nutr Food Sci182013188195 10.3746/pnf.2013.18.3.188
- A.PompellaA.VisvikisA.PaolicchiV.TataA.CasiniThe changing faces of glutathione, a cellular protagonistBiochem Pharmacol66200314991503
- N.CoutoN.MalysS.GaskellJ.BarberPartition and turnover of glutathione reductase from Saccharomyces cerevisiae: A proteomic approachJ Proteome Res12201328852894
- C.S.AlisiA.O.OjiakoC.G.OsuagwuG.O.OnyezeResponse pattern of antioxidants to lipid peroxide concentration in carbon tetrachloride-induced hepato-toxicity is tightly logistic in rabbitsEur J Med Plants12011118129
- A.O.OdewabiO.A.OgundahunsiM.OyalowoEffect of exposure to petroleum fumes on plasma antioxidant defense system in petrol attendantsBr J Pharm Toxicol520148387
- Z.Z.HuangC.ChenZ.ZengH.YangJ.OhL.ChenMechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver regenerationFASEB J1520011921
- A.SodamadeProximate analysis, mineral content, amino acid composition and functional properties of Vernonia amygdalina vegetable Leaf protein ConcentratesGreener J Agric Sci32013204210
- L.TangW.WangW.ZhouK.ChengY.YangM.LiuThree- pathway combination for glutathione biosynthesis in saccharomyces cerevisiaeMicrob Cell Fact142015139150 10.1186/s12934-015-0327-0
- B.A.IwalokunB.U.EfededeJ.A.Alabi-SofundeT.OdualaO.A.MagbagveolaA.I.AkinwandeHepatoprotective and antioxidant activities of Vernonia amygdalina on acetaminophen-induced hepatic damage in miceJ Med Food92006524530
- L.KambiziA.J.AfolayanAn ethnobotanical study of plants used for the treatment of sexually transmitted diseases (njovhera) in Guruve District, ZimbabweJ Ethnopharmacol77200159