Abstract
Highly pathogenic avian influenza (HPAI) H5N1 virus poses a major challenge to the poultry industry and human health in Egypt. Twenty one households and eight duck farms in Sharkia Province, Egypt were investigated for the presence of avian influenza virus (AIV) and/or duck hepatitis virus 1 (DHV-1). Mortality rates among the investigated farms and yards were, 18.9% (69/365) of native ducks, 60.9% (25/41) of Pekin ducks, 60.2% (6306/10473) of Muscovy ducks and 44.9% (1353/3015) of Mallard ducks. The RT-PCR revealed the circulation of HPAI-H5N1 virus (81/104) among the examined birds with a high percentage in Muscovy (83.7%) and Pekin (83.4%) ducks. Interestingly, co-infection of HPAI and DHV-1 viruses in three ducklings with age of 4–19 days was detected. Severe neurological signs with high mortality were observed in ducklings as early as 4 days of age. Influenza virus antigen was detected in the neurons and glial cells of the brain, hepatocytes, and the intestinal submucosal plexus. Although, genetic characterization of H5N1 isolates revealed HPAIV of clade 2.2.1.2, such increased mortalities and neurological signs regardless of the duck age might imply the natural selection of HPAI in ducks. Crucial monitoring of the disease situation in ducks is essential for the implementation of an effective prevention and control program.
Introduction
Avian influenza viruses (AIV) are classified into 16 and 9 subtypes based on the hemagglutinin (HA) and the neuraminidase (NA) glycoproteins respectively [Citation1]. Naturally infected aquatic birds play a critical role in widespread of these subtypes with a high likelihood of reassortment [Citation2,Citation3]. Influenza virus infection in wild ducks is ubiquitous and mostly asymptomatic [Citation4]. Formerly, the Asian lineage H5N1 HPAI viruses did not cause severe disease or mortality in ducks [Citation5,Citation6]. Nevertheless, since 2002, H5N1 HPAI viruses have been implicated in duck disease and mortality [Citation7–Citation9]. Further, domestic ducks are pivotal to the epidemiology and transmission pathway of H5N1 HPAI between wild waterfowl and domestic poultry [Citation10–Citation12]. The ability of domestic ducks in perpetuating H5N1 HPAI viruses raises the alarms to public health threat, hence the need for controlling their ongoing circulation and spread [Citation13].
The circulation of HPAI H5N1 viruses in Egypt since 2006 in backyard and poultry farms could exacerbate the lethality of ducks [Citation14]. The HPAI viruses spread among farms even under immune pressure produced by H5 virus vaccines, which might lead to their mutation and evolutions [Citation15]. Recent studies emphasized that ducks have been implicated in the dissemination and evolution of HPAI H5N1 viruses [Citation16–Citation18]. Duck hepatitis virus type 1 (DHV-1), is accountable for an acute highly contagious disease affecting young ducklings with a high mortality rate that could reach up to 95% [Citation19]. In Egypt, DHV-1 infection was firstly detected in 1969 [Citation20]. Recently, the virus maintained its incrimination in considerable mortalities, either in vaccinated or non-vaccinated ducks [Citation21–Citation23]. Although, AIV and DHV-1 natural co-infection is possible, there are no reports describing the clinical picture of such simultaneous infection. Hence, it is of interest to study HPAIV and DHV-1 in domestic ducks.
Materials and methods
.1 Virus isolation
One hundred and four ducks and ducklings of different breeds including; Native (n = 17), Pekin (n = 6), Muscovy (n = 43) and Mallard (n = 38) collected from 23 villages in Sharkia Province, Egypt during 2009–2017 were necropsied (). Samples from lung, trachea, brain and liver tissues were collected for virus isolation. Tissues were homogenized (1:10 w/v) in a sterile phosphate-buffered saline solution with antibiotics (Penstrept, Lonza) using a sterile mortar. The homogenate was centrifuged for 10 min at 3000 rpm. The supernatant was inoculated into the allantoic sac of five, 9–11-day-old embryonated chicken eggs (ECEs), which were then incubated at 37 °C for 5 days according to recommendations of the OIE [Citation24]. Subsequently, the allantoic fluids were harvested and tested by slide hemagglutination. The allantoic fluid showed hemagglutination was screened using Influenza type A antigen test kit (Flu detect™ test strip, Synobiotics Corporation, France).
able 1 Number of ducks in the backyard and commercial sector during outbreaks in Sharkia, Egypt during 2009–2017.
.2 Reverse transcription and polymerase chain reaction
Viral nucleic acid was extracted from infected allantoic fluids, using Gene JET™ RNA purification kit (Fermentas, EU) as recommended by the manufacturer. The extracted RNA was reverse transcribed to cDNA using RevertAid™ H Minus First Strand cDNA synthesis kit (Fermentas, EU). The PCR was done using H5 [Citation25] and N1 [Citation26] gene specific primers. Ducklings under 3 weeks of age (n = 3) were tested forthe presence of RNA of DHV-1 in liver tissues using primers specific to amplify a region of the 3D gene [Citation27].
.3 HA gene sequencing
Three isolates were selected from three different houses representing difference in mortalities ranging from 2.5% in 2010 to 100% in 2011 for partial HA gene sequencing. The viral HA gene was amplified using HA gene-specific primers as described before by Njouom et al. [Citation25]. PCR products were purified after agarose gel electrophoresis using Gene JET™ Gel Extraction Kit (Fermentas, EU) as recommended by the manufacturer. Each purified PCR products was sequenced directly in both forward and reverse directions (Solgent Co. ltd. Korea). The HA gene nucleotide sequences of our isolates were available from GenBank accession numbers, JX520633 to JX520635.
.4 Multiple sequence analysis and phylogenetic tree
The gene and protein sequence were analyzed using DNASTAR program (Madison, WI, USA). A phylogenetic tree was constructed by neighbor-joining analysis of HA gene nucleotide alignments using MEGA5 (http://www.megasoftware.net). The internal branches reliability was assessed by 1000 bootstrap replications. The H5 designation used was based on the alignment with A/Goose/Guangdong/1/96 (H5N1) minus the 16 amino acids known as the HA signal peptide.
.5 Pathology
Different tissue samples were collected from necropsied birds and fixed in 10% formalin. Samples were processed and paraffin blocks were sectioned in duplicates and routinely stained with hematoxylin and eosin (H&E). Consecutive sections were immunohistochemically stained using monoclonal anti-influenza A nucleoprotein (ATCC HB-65 influenza A hybridoma cell line) and visualized by 3,3́diaminobenzidine tetrahydrochloride treatment using the Dako EnVision system (DakoCytomation Inc. USA). Sections were counterstained with hematoxylin and examined under the microscope. Positive binding signal appeared as brown precipitate [Citation28].
.6 Statistical analysis
Correlation between the age of the examined ducks and the clinical symptoms and lesions severity was calculated using Spearman’s rank (Rs) bivariate correlation analysis. The data were evaluated using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA).
Results
.1 Virus isolation and identification
The inoculated ECEs were examined and embryo mortality was recorded. Dead and hemorrhagic embryos were considered as possible proof for HPAIV infection. The tested harvested allantoic fluid revealed a positive hemagglutination activity in samples obtained from ducks that exhibited neurological signs (77.9%) and further confirmed by rapid kit and RT-PCR. Among the examined duck breeds, Muscovy and Pekin were highly susceptible with a percentage of 83.7% and 83.4%, respectively followed by Mallard (73.7%). Native breed represented the lowest percentage of 70.6%. Regarding DHV-1, an amplicon size of 467 bp was obtained confirming the presence of the virus in livers of ducklings (3/3) with a total percentage of 2.9% (3/104).
.2 Sequencing and phylogenetic analysis
Using primers specific for H5 and N1 gene, the isolates were confirmed to be H5N1 subtype and were further characterized by sequencing and BLAST analysis. The HA gene sequence comparison of the A/duck/Egypt/SHMK-2203/2010(H5N1), A/duck/Egypt/SHZA-6504/2011(H5N1) and A/duck/Egypt/SHZA-6605/2011(H5N1) showed that they shared 98.4–99.5% and 98.5–99.8% homology at the nucleotide and amino acid levels, respectively. Sequencing of the hemagglutinin gene segment of A/duck/Egypt/SHMK-2203/2010(H5N1) revealed that it contained multiple basic amino acids at the cleavage site (–PQGERRRKKR/GL–), while strains; A/duck/Egypt/SHZA-6504/2011(H5N1) and A/duck/Egypt/SHZA-6605/2011(H5N1) showed one amino acid substitutions in the cleavage site (–PQGEKRRKKR/GL–), (R325K).
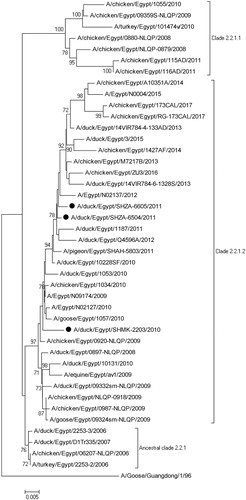
The HA gene of the duck AIV isolates was phylogenetically clustered with H5 HPAIVs in the clade 2.2.1.2 isolated from birds in backyard system, live bird market or human. The hemagglutinin gene of strains isolated during 2011 was found to be closely related to A/Egypt/N02137/2012(H5N1) isolated from human (). However, this cluster was phylogenetically distant from the viruses isolated from Egypt in 2006–2008. Comparative alignment of HA sequences of duck AIV showed that HA genes from three viruses shared a similarity of 95.1–99.6% with Egyptian AIV H5N1 subtypes (2006–2017).
.3 Clinical signs and pathology
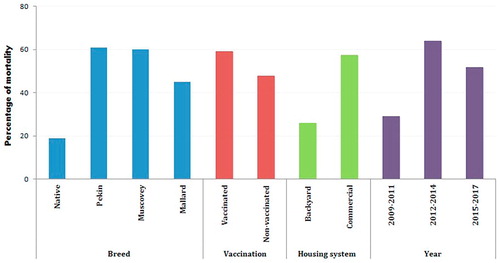
Sudden deaths and variable mortalities were recorded in examined ducks and were explored according to four factors: breed of birds, vaccination status against HPAIV, housing system and sampling years (). Regarding the breeds, the mortality rates were, 18.9% (69/365) of native ducks, 60.9% (25/41) of Pekin ducks, 60.2% (6306/10473) of Muscovy ducks and 44.9% (1353/3015) of Mallard ducks. Clinically, the ducks exhibited greenish diarrhea, neurological signs (81/104); ataxia, torticollis, circling, incoordination, inability to stand, () and a high mortality rate up to 100% in two of the examined houses (). Necropsy revealed meningeal hemorrhage and severely congested meningeal blood vessels (81/104) (). Congestion and few hemorrhagic patches were seen on the pancreas of twenty three ducks (23/104) and duodenal mucosa of three ducks (3/104). Petechial hemorrhages on the coronary fat and epicardium were seen (5/104). Hemorrhages on the liver were also observed (9/104). shows the clinical profile for the co-infected ducks with HPAIV and DHV-1.
able 2 The details of GenBank-published HPAIV strains isolated from ducks in Sharkia province, Egypt.
able 3 The clinical profile of HPAIV and DHV-1 co-infected ducks in Sharkia province, Egypt.
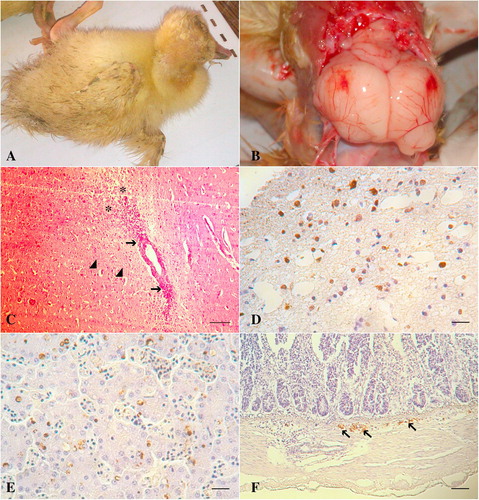
Histologically, spongiosis and vacuolation of the neuropil with activation of the glial cell were detected in the cerebral cortex. Perivascular lymphocytic cuffing of variable severities was seen in the brain of most of the examined ducks (). Multiple necrotic foci were observed in the pancreatic tissue. Immunohistochemistry was able to detect the AIV antigen in the neurons and glial cells of the brain (), hepatic and Küpffer cells () and neurons of the submucosal plexus of the intestines ().
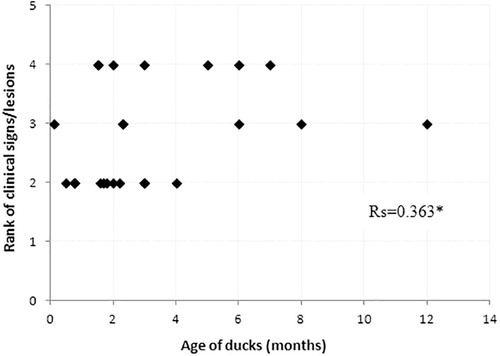
A Spearman’s rank correlation test showed no correlation between the age of the affected ducks and the severity of the clinical signs/lesions since the calculated Rs value reached 0.363 (). Nervous signs were observed in all ages of duck.
Discussion
Previous studies concluded that H5N1 HPAI viruses are constantly evolving and changing their pathogenicity in ducks [Citation17,Citation29]. In this study, ducks naturally infected with H5N1 HPAI showed a characteristic clinical picture for AIV infection with 100% mortality. The genomes of AIVs are not stable in duck populations, being continually reshuffled by reassortment [Citation30]. Based on the phylogenetic analysis of the HA gene sequences, the viruses cluster into group 2.2.1.2. These viruses were associated with high morbidity and mortality rates in ducks. Meanwhile, AIV isolated from ducks in 2011 have a close relationship with A/Egypt/N02137/2012(H5N1) isolated from human. The H5N1 virus has become enzootic in Egypt with a high number of H5N1 outbreaks among poultry and cases among human [Citation31].
In naturally infected free-living birds, the outcome of the HPAIV infection could be influenced by various factors including age of bird, amount and route of viral exposure, course of infection, concurrent infections, level of virus replication in tissues, levels of immunity, population density and environmental factors [Citation32–Citation34]. In the previous surveillance for AIV in Egypt during 2010–2012, the highest percentage of AIV detected was among turkeys and ducks that appeared to be healthy [Citation35]. In our study, severe neurological signs with high mortality (62%) were observed in ducklings as early as 4 days of age suggesting increased pathogenicity of HPAI in ducks regardless of their age. Similar findings were suggested in a study on AIV infection in pigeons [Citation36]. In contrary, a lower mortality rate among non-vaccinated ducks of 28.5 and 40% in Mallard and Muscovy ducks respectively was recorded [Citation37]. In this study, variable mortalities (2.5–100%) in single and mixed virus infections were recorded. The ability of the virus to cause a wide range of such variations may be attributed to the age of the bird, concurrent infection with other pathogens, bird status and environmental factors [Citation32–Citation34]. Furthermore, a high mortality rate (47%) was recorded in ducks infected with HPAIV [Citation38]. Generally, those aggressive outbreaks, close relationship with human AIV isolates and natural selection of the virus, raise the alarms to human infection. The mortality rates were lower in native breed indicating that they can relatively tolerate the infection. Even though, it does not change the pantropism of HPAIV in the affected birds.
Systemic infection and nervous disorders were obvious in all examined duck breeds regardless of their age. This study showed that AIV antigen was detected in the nuclei of neurons and glial cells which points to virus replication in the brain. The highest percentage of ducks (77.9) with encephalitis, in combination with high levels of virus nucleoprotein as detected by IHC, confirms the viral neurotropism as previous studies showed [Citation34,Citation39]. In contrary, these findings are different from previous research stating that minimal or no lesions and viral staining were observed in the brain, even though viral replication was displayed in ducks experimentally infected with A/Thailand PB/6231/04(H5N1) [Citation40]. The aforesaid variation may be attributed to the age of ducks, virus strain and route of infection. The severe lesions in the lungs, brain, liver, pancreas, and intestines detected histologically and confirmed AIV antigens by IHC explain the high mortality rate in the affected birds. These findings are consistent with the previous results [Citation41,Citation42]. Detection of virus nucleoprotein in the peripheral nervous system is not well documented in birds with natural H5N1 infection [Citation34]. Nevertheless, in this study, the virus nucleoprotein was detected in the submucosal plexus of the intestines. Moreover, the presence of viral antigen in multiple tissues of infected ducks pinpoints the pantropism of HPAI H5N1 viruses.
Conclusions
In this investigation, the clinical manifestations, and laboratory tests indicated that the morbidity and mortality observed among the examined ducks were caused by H5N1 HPAIV alone or in combination with DHV-1. Consequently, experimental pathogenicity of single and co-infection with both viruses is necessary to clarify the role of each component in exacerbation of the disease condition among ducks. Since severe neurological dysfunction and the highest AIV infection (83.7%) were recorded in Muscovy ducks, it is meaningful to carry out a study for monitoring this breed for HPAIV infection. In general, our results indicated that HPAI-H5N1 virus is circulating in Egypt among backyard domestic ducks causing severe disease pattern in ducklings with up to 100% mortality. The spread of H5N1 within domestic ducks could result in economic losses. Furthermore, a close genetic relationship between the isolated H5N1 viruses from backyard birds and humans pose a great risk to public health.
Competing interests
All authors declare that they have no conflicts of interest.
Acknowledgments
We would like to thank the Veterinary and Biomedical Sciences Department, Animal Disease Research and Diagnostic Laboratory, SDSU, Brookings, USA for providing the immunohistochemistry equipment and reagents.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- R.A.FouchierV.MunsterA.WallenstenT.M.BestebroerS.HerfstD.SmithCharacterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gullsJ Virol79200528142822
- H.ChenG.J.D.SmithS.Y.ZhangK.QinJ.WangK.S.LiH5N1 virus outbreak in migratory waterfowlNature4362005191192
- A.S.LipatovV.A.EvseenkoH.L.YenA.V.ZaykovskayaA.C.DurimanovS.I.ZolotykhInfluenza (H5N1) viruses in poultry, Russian Federation, 2005–2006Emerg Infect Dis132007539546
- D.E.SwayneD.A.HalvorsonInfluenzaY.M.SaifJ.R.GlissonA.M.FadlymL.R.McDougaldL.NolanDiseases of poultry2008IowaBlackwell, Ames153184
- D.J.AlexanderG.ParsonsR.J.ManvellExperimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quailAvian Pathol151986647662
- L.E.L.PerkinsD.E.SwaynePathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeonsAvian Dis4620025363
- J.BinghamD.J.GreenS.LowtherJ.KlippelS.BurggraafD.E.AndersonInfection studies with two highly pathogenic avian influenza strains (Vietnamese and Indonesian) in Pekin ducks (Anas platyrhynchos), with particular reference to clinical disease, tissue tropism and viral sheddingAvian Pathol382009267278
- Y.TangP.WuD.PengX.WangH.WanP.ZhangCharacterization of duck H5N1 influenza viruses with differing pathogenicity in mallard (Anas platyrhynchos) ducksAvian Pathol382009457467
- O.GuionieC.Guillou-CloarecD.CourtoisB.S.BougeardM.AmelotJestin V. Experimental infection of Muscovy ducks with highly pathogenic avian influenza virus (H5N1) belonging to clade 2.2Avian Dis542010538547
- H.ChenG.DengZ.LiG.TianY.LiP.JiaoThe evolution of H5N1 influenza viruses in ducks in southern ChinaPNAS, USA10120041045210457
- K.S.LiY.GuanJ.WangG.J.SmithK.M.XuL.DuanGenesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern AsiaNature4302004209213
- D.J.Hulse-PostK.M.Sturm-RamirezJ.HumberdP.SeilerE.A.GovorkovaS.KraussRole of domestic ducks in the propagation and biological evolution of highly pathogenic H5N1 influenza viruses in AsiaPNAS, USA10220051068210687
- J.K.KimN.J.NegovetichH.L.ForrestR.G.WebsterDucks: the ‘‘Trojan horses’’ of H5N1 influenza. Influenza and Other RespirViruses32009121128
- M.AlyA.ArafaM.HassanEpidemiological findings of outbreaks of disease caused by highly pathogenic H5N1 avian influenza virus in poultry in Egypt during 2006Avian Dis522008269277
- E.M.AbdelwhabA.A.SelimA.ArafaS.GalalW.H.KilanyM.K.HassanCirculation of avian influenza H5N1 in live bird markets in EgyptAvian Dis542010911914
- M.S.IbrahimY.WatanabeH.F.EllakanyA.YamagishiS.SapsutthipasT.ToyodaHost-specific genetic variation of highly pathogenic avian influenza viruses (H5N1)Virus Genes422011363368
- J.L.WasilenkoA.M.ArafaA.A.SelimM.K.HassanM.M.AlyA.AliPathogenicity of two Egyptian H5N1 highly pathogenic avian influenza viruses in domestic ducksArch Virol15620113751
- H.A.KaoudH.A.HusseinA.R.El-DahshanH.S.KaliefaM.A.RohaimCo-circulation of avian influenza viruses in commercial farms, backyards and live market birds in EgyptInt J Vet Sci Med22014114121
- C.H.TsengN.J.KnowlesH.J.TsaiMolecular analysis of duck hepatitis virus type 1 indicates that it should be assigned to a new genusVirus Res1232007190203
- E.R.M.RefaieStudies on some diseases of ducklings with special reference to salmonellosis and duck viral hepatitisVet Med J Giza161969312
- H.A.A.BayoumieL.K.Abd EL-SamieMolecular characterization of a duck virus hepatitis isolate isolated from Sharkia governorateAssiut Vet Med J6120155665
- A.M.ErfanA.A.SelimM.K.MoursiS.A.NasefE.M.AbdelwhabEpidemiology and molecular characterisation of duck hepatitis A virus from different duck breeds in EgyptVet Microbiol1772015347352
- M.N.HassaanA.M.ShahinA.A.M.EidIsolation and molecular identification of duck hepatitis A virus in Sharkia GovernorateZag Vet J4620188895
- OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Chapter 2.3.4). http://www.oie.int/en/international-standard-setting/terrestrial-manual/access-online/. Accessed 25 May 2013; 2012.
- R.NjouomJ.T.AubinA.L.BellaB.M.DemsaP.RouquetB.GakeHighly pathogenic avian influenza virus subtype H5N1 in ducks in the Northern part of CameroonVet Microbiol1302008380384
- K.TsukamotoT.AshizawaK.NakanishiN.KajiK.SuzukiM.ShishidoUse of reverse transcriptase PCR to subtype N1 to N9 neuraminidase genes of avian influenza virusesJ Clin Microbiol47200923012303
- M.C.KimY.K.KwonS.J.JohJ.H.KwonJ.H.KimS.J.KimDevelopment of one-step reverse transcriptase-polymerase chain reaction to detect duck hepatitis virus type 1Avian Dis512007540545
- L.E.L.PerkinsD.E.SwaynePathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous speciesVet Pathol382001149164
- D.Q.PhuongN.T.DungP.H.JørgensenK.J.HandbergN.T.VinhJ.P.ChristensenSusceptibility of Muscovy (Cairina moschata) and mallard ducks (Anas platyrhynchos) to experimental infections by different genotypes of H5N1 avian influenza virusesVet Microbiol1482011168174
- V.G.DuganR.ChenD.J.SpiroN.SengamalayJ.ZaborskyE.GhedinThe evolutionary genetics and emergence of avian influenza viruses in wild birdsPLoS Pathog42008 e1000076
- G.KayaliA.KandeilR.El-SheshenyA.S.KayedA.M.MaatouqZ.CaiAvian influenza A (H5N1) virus in EgyptEmerg Infect Dis222016379388
- M.J.Pantin-JackwoodD.E.SwaynePathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducksAvian Dis512007250259
- D.KalthoffA.BreithauptJ.P.TeifkeA.GlobigT.HarderT.C.MettenleiterPathogenicity of highly pathogenic avian influenza virus (H5N1) in adult mute swansEmerg Infect Dis14200812671270
- C.BröjerE.O.ÅgrenH.UhlhornK.BernodtT.MörnerD.S.JanssonPathology of natural highly pathogenic avian influenza H5N1 infection in wild tufted ducks (Aythya fuligula)J Vet Diagn Invest212009579587
- G.KayaliA.KandeilR.El-SheshenyA.S.KayedM.M.GomaaA.M.MaatouqActive surveillance for avian influenza virus, Egypt, 2010–2012Emerg Infect Dis202014542551
- S.M.G.MansourR.M.ElBakreyH.AliD.E.B.KnudsenA.A.M.EidNatural infection with highly pathogenic avian influenza virus H5N1 in domestic pigeons (Columba livia) in EgyptAvian Pathol432014319324
- I.T.HagagS.M.G.MansourZ.ZhangA.A.H.AliE.M.IsmaielA.A.SalamaPathogenicity of highly pathogenic avian influenza virus H5N1 in naturally infected poultry in EgyptPLoS One102015 e0120061
- N.HaiderK.Sturm-RamirezS.U.KhanM.Z.RahmanS.SarkarM.K.PohUnusually high mortality in waterfowl caused by highly pathogenic avian influenza A (H5N1) in BangladeshTransbound Emerg Dis642017144156
- J.D.BrownD.E.StallknechtJ.R.BeckD.L.SuarezD.E.SwayneSusceptibility of North American ducks and gulls to H5N1 highly pathogenic avian influenza virusesEmerg Infect Dis12200616631670
- M.J.Pantin-JackwoodD.L.SuarezE.SpackmanD.E.SwayneAge at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5N1 viruses in ducksVirus Res1302007151161
- M.VascellariA.GranatoL.TrevisanL.BasilicataA.ToffanA.MilaniPathologic findings of highly pathogenic avian influenza virus A/Duck/Vietnam/12/05 (H5N1) in experimentally infected pekin ducks, based on immunohistochemistry and in situ hybridizationVet Pathol442007635642
- M.J.Pantin-JackwoodM.Costa-HurtadoE.ShepherdE.DeJesusD.SmithE.SpackmanPathogenicity and transmission of H5 and H7 highly pathogenic avian influenza viruses in mallardsJ Virol90201699679982