Abstract
Centrocestus formosanus is a digenean that requires three host species to complete its life cycle. This study was conducted to observe the damage caused by two life stages of the C. formosanus on its host species. The snail Melanoides tuberculata was induced to shed cercariae by exposing to sunlight and specimens of koi carps were experimentally infected with cercariae. Gills of two infected fish were killed and fixed in Bouin’s solution daily for 21 days. Infected fish were continuously fed to a pond heron (Ardeola ralloides) for three weeks and therafter the bird was killed. Small intestine was resected as duodenum, jejunum, and ileum and fixed in formol saline. Gills and small intestine were prepared to study the histopathological damages. Flared opercula with protruding gills and increased respiratory rate were identified as the primary clinical signs of the fish. Encysted metacercariae were observed in the basal, middle and in the apical portion of the gills’ filaments and gradual distortions and extensive proliferation of the cartilage of the gills resulted in loss of the respiratory epithelium. A progression of fibroblast to chondroblast encapsulation of the parasite was observed in the gill of fish as a host response. The duodenum of the heron was severely infected with adult parasites than jejunum and ileum. Flukes were observed in the villi, mucosae, submucosae, and also in the tunica muscularis of the duodenum. In conclusion, this study revealed that the heavy infection of C. formosanus could cause severe pathological lesions in both koi carps and pond heron.
Introduction
Sri Lanka has gained a good reputation in the international market for exporting high-quality ornamental fish species [Citation1]. However, during culture practices, parasitic diseases harm the quality of fish on different levels. Certain parasites have the ability to reproduce rapidly to pathogenic levels and could cause high mortalities especially in young fish while some parasites have complex life cycles and they cannot reproduce in fish, but cause severe damages to fish even in lower numbers.
The heterophyid trematode Centrocestus formosanus (gill fluke) completes its life cycle using a snail and a fish as first and second intermediate hosts, respectively, and fish-eating birds or mammals as the definitive hosts [Citation2]. This fluke is native to Asia, but it has now been recorded from the United States and other parts of the world [Citation3–Citation6]. The metacercariae of C. formosanus encyst in the gills of many species of cultured fish and cause severe alterations in the architecture of gills, which could lead to respiratory difficulties, loss of production and death especially in young fish due to heavy infections [Citation7,Citation8]. Further, zoonotic cases due to species of the genus Centrocestus have been recorded from few Asian countries (C. armatus and C. formosanus in Korea, C. caninus in Thailand, C. kurokawai in Japan) as a result of eating raw or partially cooked freshwater fish, frogs and toads [Citation9–Citation11].
Among many parasite species, Centrocestus sp. have been recorded from many species of food fish, such as common carp (Cyprinus carpio L.), grass carp (Ctenopharyngodon Idella Valenciennes), silver carp (Hypophthalmichthys molitrix, Valenciennes), bighead carp (Aristichthys nobilis Richardson), catla (Catla catla Hamilton – Buchanan), Rohu (Labeo rohita Hamilton), Mrigal (Cirrhinus mrigala Hamilton) and common labeo (Labeo dussumieri Valenciennes), which were harvested from the mud ponds of fish culturing stations in Sri Lanka [Citation7,Citation12]. In addition, this parasite occurs in many ornamental fish species in this country, including koi carps and goldfish from mud ponds [Citation13,Citation14].
The previous studies conducted on Centrocestus sp. in Sri Lanka were primarily focused on documenting this parasite in many species of fish, and there is a dearth of information on the histopathological lesions caused by this parasite in both second-intermediate (fish) and definitive hosts (piscivorous birds). Therefore, the first objective of the current study was to determine the sequence of histopathological lesions caused by this parasite in the gills of the koi carp (C. carpio) and an additional objective was to determine the lesions produced by this parasitic infection in the small intestine of the pond heron (Ardeola ralloides).
Materials and methods
.1 Ethical approval
Ethical clearance for the study was obtained from the Institute of Biology (IOB), Sri Lanka (Permit No: ERC IOBSL136 11 15).
.2 Collection and maintaining the snails
The first intermediate host Melanoides tuberculata snails (>2cm in length) naturally infected with Centrocestus sp. were collected from two ornamental fish breeding centers in Sri Lanka: Rambadagalle in Northwestern province (GPS points: 7° 51′N; longitude-80° 50′E) and Ginigathhena in Central province (GPS points: 7° 00′N; longitude-80° 49′E). They were maintained in well-aerated tanks and fed with microalgae (Chlorella sp.).
.3 Screening of infected snails and collection of cercariae
Fifty snails were placed individually in small beakers containing 25 mL of de-chlorinated water and exposed to sunlight to induce the shedding of cercariae larvae and few drops of water from each beaker were observed under the microscope to confirm the shedding of larvae. Cercaria of Centrocestus sp. was identified using morphometric and morphological characters such as 263.07 μm in length with a heart-shaped body, two eyespots as well as single tailed and compared with published descriptions [Citation15,Citation16]. Among the 17 snails that released cercariae, 12 of them shed larvae of C. formosanus. The cercariae suspensions of C. formosanus were collected into a beaker and the density was estimated.
.4 Artificial infection of koi carp (Cyprinus carpio) from the cercariae
Matured Koi carps (Cyprinus carpio) up to 45 days were selected. Before the experiment, the fish were checked for external parasites and were treated with freshly prepared 2.5 ppm KMnO4 [Citation17] in order to clear the ectoparasites. All the experimental and control fish were fed twice a day with a commercial feed containing 35% protein and the temperature of the water was maintained at 29 °C. Three hundred fish (mean weight-3.0 ± 0.4 g, mean length-3.8 ± 0.5 cm) were kept individually in separate beakers with 300 mL of water. Approximately 200 cercariae were introduced to each beaker and after 2 h; the infected fish were stocked in two well-aerated treatment tanks (50 individuals per tank with 150L water). Furthermore, an extra tank was maintained as a control by stocking non-infected fish. The remaining infected fish (n = 200) were kept in a well-aerated tank as an extra stock.
.5 Observation of infected fish
The experimental tanks were inspected daily and the abnormal behavior and clinical signs of the fish were recorded.
.6 Chronological analysis of the damages caused by the metacercariae on the gills of fish
Wet mounts of the gills of some infected fish were prepared and observed by the microscope. Two infected fish from each treatment tank were killed daily for 21 days and the gills were fixed in Bouin’s solution to examine histopathology in order to determine the chronological lesions caused by the metacercariae.
.7 Infection of metacercariae to the pond heron (Ardeola ralloides)
To determine the damage caused in the small intestine of the definitive host by the adult fluke, the pond heron (A. ralloides) was selected as a possible natural definitive host of C. formosanus under Sri Lankan conditions. The wild caught heron was treated with Drontal plus® (a broad spectrum anthelmintic containing praziquantel, pyrantel, and febantel) to clear any intestinal parasitism, and the bird was housed in a cage and provided with fish and water ad libitum. Three days after the treatment, the bird was continuously fed with 28 days old metacercaria infected fish up to 3 weeks. The feces of the heron were observed daily under the microscope for the presence of the eggs of the parasite.
.8 Investigation of histopathological damage caused by adult fluke in the small intestine of the heron
After 3 weeks of experimental infection, the heron was dissected and the small intestine was resected into three parts such as the duodenum, jejunum, and ileum and fixed in formal saline for histopathology.
The fixed tissues (gills and small intestine) were dehydrated using a series of ethanol and embedded in wax. Thin sections of the above tissues were stained with Haemotoxylin and Eosin (H&E) and observed under the microscope and photographs of the lesions were taken.
Results
.1 Clinical signs of the infected fish
Highly infected fish showed restless behavior and often came to the water surface for air breathing. Increased respiratory rate as evidenced by the rapid rate of opercula movements, outwardly flared opercula flaps () and red colored inflamed gills were observed in heavily infected fish which died more readily than those fish in the control tank.
.2 Wet mounts of the gills of infected koi carps
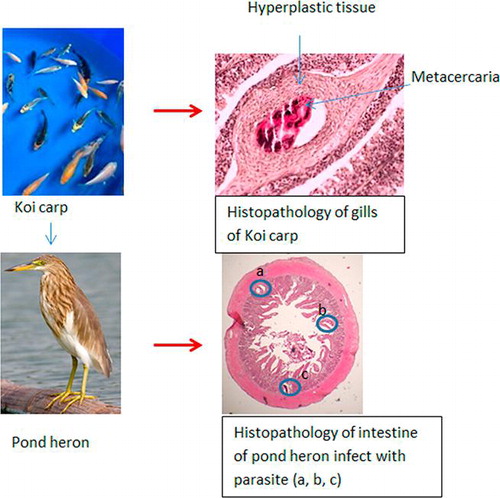
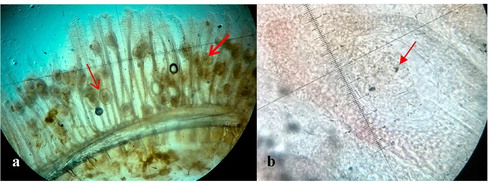
Microscopic examination of the wet mounts of infected gills revealed many metacercariae encysted along the entire length of the gill filaments and few of them were found to be encysted on the gill arch (). Infected metacercarial cysts found in gills were ranged from 10 to 20 in fish showed clinical signs and they were 20–25 cysts in dead fish. The cyst wall surrounding the metacercariae consisted of concentric layers of cells and two eyespots were visible in the metacercariae ().
.3 Histopathological changes caused by the metacercariae on gills of koi carp
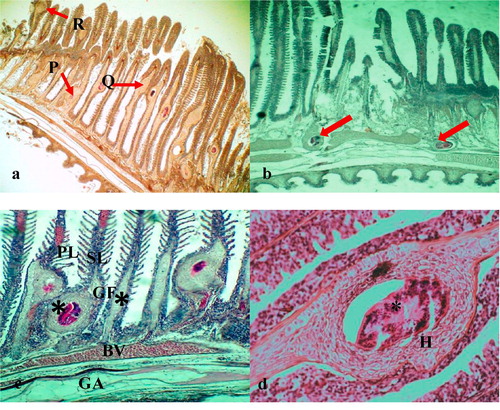
The encysted metacercariae were observed at different sites of the gill filaments such as base, middle and apical portions () and the majority of them were found adjacent to the cartilage of gill filaments and on the gill arch (). Overall, the gill architecture was found to be altered by the cartilage proliferation, epithelial hyperplasia followed by the fusion of primary lamellae, increased number of mucous cells, fusion of secondary lamellae and clubbing of gill filaments (). The metacercaria was encapsulated by a thin, non-cellular homogeneous and retractile cyst wall, which was surrounded by layers of chondroblasts or chondrocytes of host origin (). The eosinophilic chondroblast or chondrocyte layers around the parasitic cyst consisted of granulated nuclei and varied in size. The cells adjacent to the parasite were flattened while the cells at the periphery were hypertrophied ().
.4 Sequence of chronological damage caused by the metacercaria stage on gills of koi carp
Cercariae were found to be attached to the cartilaginous rod after 2 h of infection. Mild hemorrhage and oedema with few necrotic cells were seen around the parasite (). At 15 and 24 h post-infection, hemorrhage, oedema, inflammatory cells and fibroblast-like cells were observed around the parasite ( & ). An organized encapsulation of the parasite by 2–3 layers fibroblast-like cells was apparent by 48 h ().
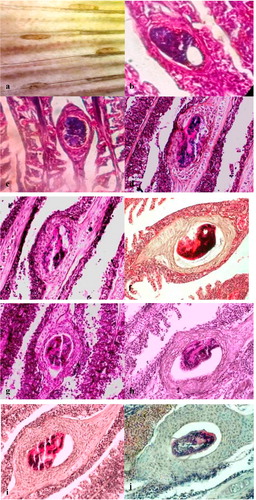
By 72 h of infection, there were up to 3 layers of eosinophilic and rectangular cells surrounding the cyst (). The thickness of the encapsulation around the parasite had increased up to 3–4 layers of the cell by the 4th day post-infection (). The outermost cells were flattened while the innermost cells were cuboidal to rectangular in shape. After 5 days post-infection, the encapsulation was increased up to 4 layers with basophilic cells with a large nucleus (), and the number of cell layers was gradually increased up to 4- 5 by 7 days ().
Thickness of the cyst was increased up to 8–10 cell layers by 10 days post-infection (). The cell layers were primarily cuboidal shaped chondroblasts or chondrocytes, which were surrounded by flattened fibroblast-like cells. More than 8 cell layers were observed in the cyst at 21 days post-infection (). Some hyperplastic cysts were observed without the parasite ().
.5 Histopathological changes caused by the adult stage of the parasite in the small intestine of pond heron (Ardeola ralloides).
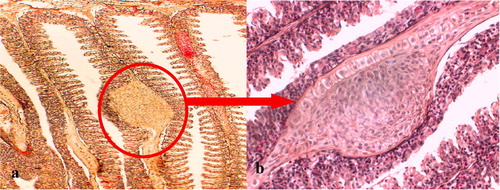
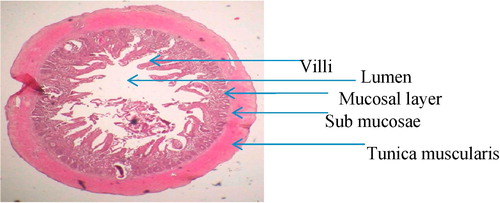
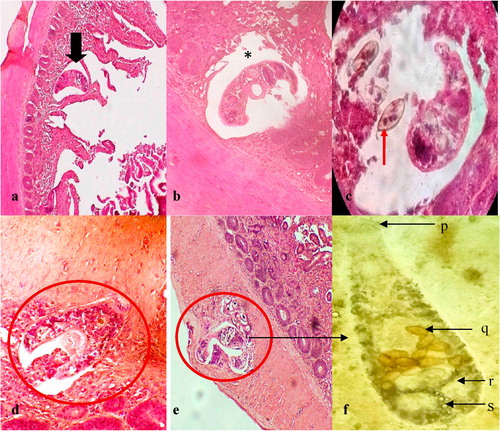
While examining the histological sections of the duodenum, jejunum, and ileum, it was apparent that more flukes were found in the duodenum than in the ileum. The main lesions observed in the duodenum included fusion of the villi ( & ), hyperplasia of the crypt epithelium and epithelial damage around the site of worm attachment. Flukes with intrauterine eggs were observed in the submucosal layer of the duodenum ( & ). Flukes were seen attached to the mucosae of the intestinal crypts by using their oral sucker. Moreover, they were found deeply invaded into the submucosa and tunica muscularis ( & ). The adult fluke collected from the small intestine was small, pyriform, consisted of 32 oral spines, with X-shaped excretory bladder, and the eggs with latticed design ().
Discussion
Many studies have been carried out in different countries, including Sri Lanka to document lesions caused by the metacercariae of C. Formosanus in the gills of the second intermediate host [Citation7,Citation18–Citation21]. However, the information on the chronological damage caused in the gills of fish by C. formosanus is scant, which precludes the meaningful comparison of this study with similar studies done on this parasite. This study indicated that the chronological lesions observed in the gills were related to the invasion and encystment of the parasite in the gill lamellae followed by the host responses to the parasite.
Previous studies documented the occurrence of hemorrhages in the gills of fish infected with metacercariae [Citation19,Citation22]. Similarly, the hemorrhage observed in this study within hours after infection could be due to the mechanical damage of gill capillaries by the invading parasite. Oedema and infiltration of leucocytes observed in the gills were probably due to the onset of inflammatory reactions in the host in response to the injury. After the invasion of the bronchial tissues, the parasite was found to be adjacent to the cartilage of primary gill lamellae within two hours of infection and the parasites were surrounded by a few layers of host cells within 24 h post-infection ( & ). The host cell layer tencircled the parasite increased in thickness with time and gradually transformed into chondroblasts or chondrocytes (). Blazer and Gratzek [Citation18] have observed the chronological damage caused in the gills of guppies (Poecilia reticulata) and goldfish (Carassius auratus) by an unidentified metacercaria for 6 days, and their findings generally corroborate with this study. The hyperplasia of the cartilage cells around the parasite observed in this study is probably a defense mechanism of the host against the parasite as suggested in earlier studies [Citation8,Citation18,Citation20,Citation21]. The encystment of the metacercariae in the gill filaments and gill arches caused severe distortion of the gill architecture and reduction of the respiratory surface, which may explain the respiratory signs, such as air-breathing and increased respiratory rate of fish observed in this study.
Although the piscivorous birds are the main definitive host of Centrocestus sp., only a few studies had been carried out to document the lesions caused by this parasite in birds. In one study, Nath [Citation23] used pigeons as definitive hosts to document the pathological responses against C. formosanus.
The main lesions observed in the duodenum of the experimentally infected A. ralloides were the fusion of the villi, hyperplasia of the crypt epithelium and epithelial damage around the site of worm attachment ( & ). The adult C. formosanus has been found in all layers of the small intestine, such as mucosa, submucosa and tunica muscularis, of pond heron.
Studies conducted on the damage caused by C. armatus, C. caninus, C. formosanus in the intestine of Rattus novegicus suggested that intestinal lesions in the host may both depend on the species of the parasite and the host [Citation24–Citation26]. Chai et al. [Citation27] have reported the deep invasion of a heterophyid trematode Metagonimus yokogawai into the submucosal layer in mice. Africa et al. [Citation28] recorded presence of the eggs of heterophyid flukes (Haplorchis sp., Stellantchasmus sp. and Procerovum sp.) in the heart and spinal cord of human due to deeper penetration and subsequent spread of the flukes to internal organs. The present study revealed the deep invasion of adult C. formosanus up to tunica muscularis layer of the small intestine of the pond heron.
The observed adult parasite morphology was consistent with the findings of earlier studies and confirmed as C. formosanus [Citation16,Citation29]. According to data and observations gathered from the current study, it was experimentally proved that C. formosanus could be survived successfully in the small intestine of the pond heron (A. ralloides), a common bird species live associated with water bodies in Sri Lanka. Therefore, it might be a potential definitive host for C. formosanus under local conditions.
Conclusions
A progression of fibroblast to chondroblast encapsulation of the parasite was observed in the gills of the fish as a host response. Adult flukes were observed in the villi, mucosae, submucosae and also in the tunica muscularis of the duodenum of pond heron. This study revealed that the heavy infection of C. formosanus could cause severe pathological lesions in both koi carps and pond heron. The findings of the present study are important for understanding the severity of the damage caused by the C. formosanus in cultural systems that assists in early detection and control through proper treatment methods.
Acknowledgment
This study was self financed and basic facilities for research were provided by the Department of Zoology, Faculty of Science, and the Faculty of Fisheries and Marine Sciences & Technology, University of Ruhuna, Sri Lanka.
Competing interests
All authors declared that they have no conflicts of interest.
Notes
Peer review under responsibility of Faculty of Veterinary Medicine, Cairo University.
References
- S.GunasekaraThe export of indigenous freshwater fish species from Sri LankaJ WNPS242007713
- Yamaguti S. Systema Helminthum. The digenetic trematodes of vertebrates, Interscience, New York, Druzno lake Part- I, Acta Parasite Pole 1958;6: pp. 1–64.
- J.Y.ChaiS.Woon-MokY.Tai-SoonS.E.KeeseonM.Duk-YoungY.L.MiCentrocestus formosanus (Heterophyidae): human infections, and the infection source in Lao PDRJ Parasitol992013531536
- M.FoojanM.J.HannahW.K.PerB.KurtImport of exotic and zoonotic trematodes (Heterophyidae: Centrocestus sp.) in Xiphophorus maculatus: implications for ornamental fish import control in EuropeActa Parasitol592014276283
- D.KrailasS.NamchoteT.KoonchornboonW.DechruksaD.BoonmekamTrematodes obtained from the thiarid freshwater snail Melanoides tuberculata (Müller, 1774) as vector of human infections in ThailandZoosyst Evol9020145786
- H.A.PintoV.L.T.MatiA.L.MeloMetacercarial Infection of wild Nile tilapia (Oreochromis niloticus) from BrazilSci World J2014201417
- L.K.S.W.BalasuriyaA study on the metacercarial cysts of a Centrocestus sp. (Digenia: Heterophylidae) occurring on the gills of cultured fishes in Sri LankaJ Inland Fish41988310
- J.MitchellR.M.OverstreetA.E.GoodwinT.M.Brandt“Spread of an exotic fish-gill trematode: a far-reaching and complex problemFisheries30820051116
- T.KurokawaOn a new species of Stamnosoma found from the manTokyo Iji Shinshi591935293298 (in Japanese)
- S.HongH.WooJ.ChaiS.W.ChungS.LeeB.S.SeoStudy on Centrocestus armatus in KoreaKorean J Parasitol2619885560
- J.WaikagulT.WongsarojP.RadomyosV.MeesomboonR.PraewanichP.JongsuksuntikulHuman infection of Centrocestus caninus in ThailandSoutheast Asian J Trop Med Public Health281997831835
- N.D.WimalawickramaA.PathirathneEvaluation of the effects of infestation by trematodes and Lernaea on Catla catla, an Indian carp cultured in Sri LankaSri Lanka J Aq Sci1020058596
- I.D.S.I.P.ThilakaratneG.RajapakshaA.HewakoparaRajapakse RPVJ, Faizal ACM. Parasitic infections in freshwater ornamental fish in Sri LankaDis Aquat Organ542003157162
- B.G.D.SumuduniD.H.N.MunasingheW.P.R.ChandrarathnaS.DeN.J.AmarasingheRelationship between condition factor and external parasite density of goldfish (Carrasius auratus (Linnaeus, 1758)) and Koi carp (Cyprinus carpio (Linnaeus, 1758)) during dry and wet seasonsInt J Curr Res6201482828285
- L.E.HernandezM.T.DiazA.K.BashirullahDescription of different developmental stages of Centrocestus sp. (Nishigori, Digenea: Heterrophyidae)Rev Cient200361924285292
- F.YousifM.AyoubM.TadrosS.E.I.BardiciThe first record of Centrocestus formosanus (Nishigori 1924) Digenea Heterophidae in EgyptJ Exp Parasitol16820165661
- E.J.NogaFish Disease Diagnosis and Treatment2000Iowa State University Press1st ed.
- V.S.BlazerJ.B.GratzekCartilage proliferation in response to metacercarial infections of fish gillsJ Comp Pathol951985273280
- A.J.MitchellA.E.GoodwinM.J.SalmonT.M.BrandtExperimental infection of an exotic heterophyid trematode, Centrocestus formosanus, in four aquaculture fishesN Am J Aquac6420025559
- E.GjurcevicZ.PetrinecZ.KozaricS.KuzirV.Gjurcevic KanturaM.VucemiloMetacercariae of Centrocestus formosanus in goldfish (Carassius auratus L.) imported into CroatiaHelminthologia442007214216
- A.RezaieZ.T.DezfulyM.MesbahA.RanjbarHistopathologic report of infestation by Centrocestus formosanus in Iranian grass carp and common carpIran J Vet Sci Tech920174953
- E.M.Velez-HernandezF.Constantino-CasasL.J.Garcia-MarquezD.Osorio-SarabiaGill lesions in common carp, Cyprinus carpio L., in Mexico due to the metacercariae of Centrocestus formosanusJ Fish Dis211988229232
- D.NathPathology of Centrocestus formosanus (Nishigori, 1924) infection in experimental pigeonIndian J Anim Sci421972952954
- S.SaenphetC.WongsawadK.SaenphetA.RojanapaibulP.VanittanakomJ.ChaiChronological observations of intestinal histopathology in rats (Rattus norvegicus) infected with Centrocestus caninusSoutheast Asian J Trop Med Public Health3720066973
- S.J.HongJ.H.HanC.K.ParkS.Y.KangIntestinal pathologic findings at early stage infection by Centrocestus armatus in albino ratsKorean J Parasitol351997135138
- V.L.T.MatiH.A.PintoA.L.MeloExperimental infection of Swiss and AKR/J mice with Centrocestus formosanus (Trematoda: Heterophyidae)Rev Inst Med Trop Sao Paulo552013133136
- J.Y.ChaiJ.KimS.H.LeeInvasion of Metagonimus yokogawai in to the sub mucosal layer of the small intestine of immune suppressed miceKorean J Parasitol331995313321
- C.M.AfricaW.De LeonE.Y.GarciaVisceral complications in intestinal heterophyidiasis of manActa Med Philipp (Monographic series)11940132
- C.WongsawadP.WongsawadS.AnuntalabhochaiJ.Y.ChaiK.SukontasonOccurrence and molecular identification of liver and minute intestinal flukes metacercariae in freshwater fish from Fang-Mae Ai Agricultural Basin, Chiang Mai province, ThailandAsian Biomed7201397104